Abstract
Varicocele repair in adolescent remains controversial. Our aim is to identify and combine clinical trials results published thus far to ascertain the efficacy of varicocelectomy in improving testis volume and semen parameters compared with nontreatment control. A literature search was performed using Medline, Embase and Web of Science, which included results obtained from meta-analysis, randomized and nonrandomized controlled studies. The study population was adolescents with clinically palpable varicocele with or without the testicular asymmetry or abnormal semen parameters. Cases were allocated to treatment and observation groups, and testis volume or semen parameters were adopted as outcome measures. As a result, seven randomized controlled trials (RCTs) and nonrandomized controlled trials studying bilateral testis volume or semen parameters in both treatment and observation groups were identified. Using a random effect model, mean difference of testis volume between the treatment group and the observation group was 2.9 ml (95% confidence interval [CI]: 0.6, 5.2; P< 0.05) for the varicocele side and 1.5 ml (95% CI: 0.3, 2.7; P< 0.05) for the healthy side. The random effect model analysis demonstrated that the mean difference of semen concentration, total semen motility, and normal morphology between the two groups was 13.7 × 106 ml−1 (95% CI: −1.4, 28.8; P = 0.075), 2.5% (95% CI: −3.6, 8.6; P= 0.424), and 2.9% (95% CI: −3.0, 8.7; P= 0.336) respectively. In conclusion, although varicocelectomy significantly improved bilateral testis volume in adolescents with varicocele compared with observation cases, semen parameters did not have any statistically significant difference between two groups. Well-planned, properly conducted RCTs are needed in order to confirm the above-mentioned conclusion further and to explore whether varicocele repair in adolescents could improve subsequently spontaneous pregnancy rates.
Keywords: adolescent, meta-analysis, semen analysis, testis, varicocele
INTRODUCTION
Fewer topics in urology have been as controversial as the effect of varicocelectomy on male infertility, especially when it comes to adolescent varicocele. Varicocele has been traditionally treated with surgery such as high retroperitoneal ligation and microsurgical varicocelectomy while embolization has also been applied as an alternative therapy.1 However, whether a causal relation exists between varicocele in adolescents and their future infertility has been questioned, since varicocele is present in many men with normal fertility.2 Moreover, one recent cohort investigated the effects of treatment for varicocele in boys aged 12 to 17 years old to determine their chance of paternity later in life. It had not been proved that the presence of varicocele during adolescence influenced later fertility or paternity.3 So, the controversy remains over adolescents who will benefit from varicocelectomy.
Although some meta-analysis and prospective studies have clearly demonstrated that varicocele repair is associated with a significant improvement in testis volume and semen parameters, the nonrandomized controlled trial (NRCT) research designs without observation and healthy groups limit their level of evidence.4,5 Herein, we reviewed and made a new meta-analysis of the randomized controlled trial (RCT) and NRCTs available in the literature in an attempt to ascertain the efficacy of varicocelectomy in improving testis volume and semen parameters compared with no treatment and gain insights into this issue.
MATERIALS AND METHODS
Literature search and article selection
We included studies that were published as complete reports, addressed the treatment of varicocele in adolescents, and contained an assigned control group. In the present meta-analysis, we analyzed RCTs (Evidence level 2b) and NRCTs (Evidence level 3b).6 Although RCTs remain to be a reference standard, some investigators have recommended using NRCTs in a meta-analysis, as long as the studies have been carefully evaluated for potential bias.7 A literature search was performed using Medline, Embase and Web of Science with the aid of an external professional statistician on January 22, 2014. Key words, used in different combination, were adolescent/adolescence, puberty/pubertas, varicocele/varicocelectomy, infertility/subfertility, testicles/testis, pregnancy/pregnant and semen/sperm. Search limits were set for the English language and human species. The retrieved articles were viewed by TZ and WZ independently. Reference lists of retrieved articles as well as relevant review articles were also studied. Finally, trials that reported testis volume or semen parameters in adolescents as an outcome measure and presented data separately for treated and untreated groups were chosen and included by consensus (Figure 1).
Figure 1.
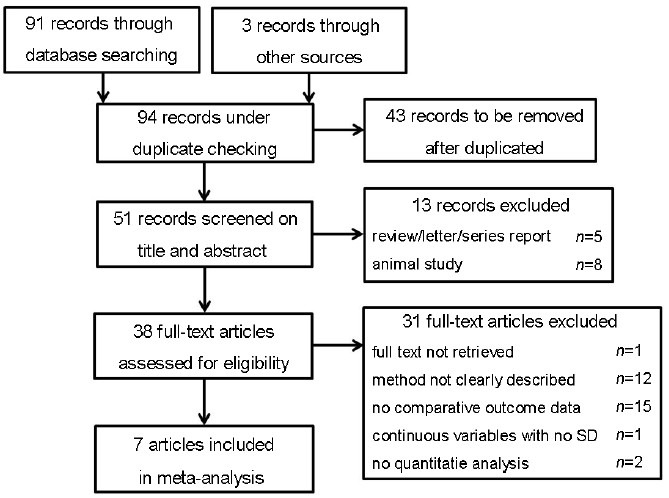
Flow diagram of search strategy.
Inclusion and exclusion criteria
The subjects were adolescents (aged from 9 to 21 years old) who underwent varicocelectomy for unilateral palpable varicocele definitely diagnosed by physical examinations and Doppler ultrasound, with or without the testicular asymmetry or abnormal semen parameters. Studies that involved bilateral and subclinical varicocele were excluded, in that we expected the study to focus on the effect of varicocelectomy on bilateral testis volume and semen parameters of patients with unilateral palpable varicocele. The treatment methods included transcatheter embolization, open ligation, and microsurgical varicocelectomy, and the postoperative testicular volume of both sides was estimated and recorded. The collection and evaluation criteria for semen quality followed World Health Organization (WHO) indications were followed for collection and evaluation criteria for semen quality. Based on the criteria of included studies, patients in the observation group were adolescents of the same age group with unilateral palpable varicocele.
Evaluation of methodological quality
The methodological quality of the studies was evaluated based on the Jadad scale for RCTs8 and the Newcastle-Ottawa Scale (NOS) for NRCTs.9
Statistical analysis
The measures of treatment effect were combined as a weighted average of the individual study measures. Statistical significance was set at α= 0.05. The Q statistic was used to test between study homogeneity: homogeneity was rejected when the Q statistic P < 0.10. Depending on whether the homogeneity was accepted or rejected, we used the fixed or random effect model to calculate the combined mean difference in outcome measures and the 95% confidence interval (CI). The statistical analyses were conducted using Stata version. 12.0 statistical software (StataCorp., College Station, TX, USA).
RESULTS
A total of seven trials have been included in our study, composing of three RCTs10,11,12 and four NRCTs13,14,15,16 (Table 1). The methodological quality of included studies was relatively high for three of the nonrandomized studies (NOS: 7 of 9 points and 6 of 9 points) and medium for one (NOS: 5 of 9 points), whereas the three RCTs were medium quality (Jadad scale: 3 of 5 points), which was mainly attributed to the no blind methods.
Table 1.
Summary of comparative studies
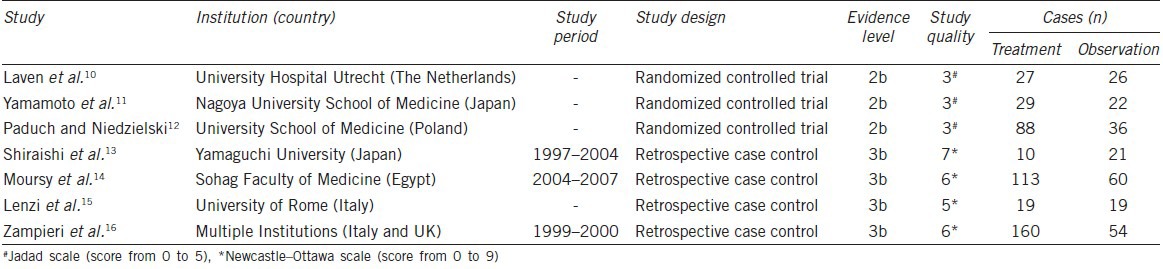
A total of 446 cases in the treatment group and 238 cases in the observation group were compared in our meta-analysis. The baseline patient characteristics of included studies had been carefully evaluated for potential bias (Table 2). The follow-up periods in all studies were at least 1-year, ensuring the average age at the time of the reexamination was 18 years old. Furthermore, the varicocele grades in all studies were evaluated according to the system of Dubin and Amelar.17 Except for the one of Shiraishi et al.13 that focused on the grade 1 varicocele, most studies invested the effects of varicocelectomy on grades 2 and 3 varicoceles.
Table 2.
Summary of baseline patient characteristics of comparative studies

Of the seven trials, three RCTs10,11,12 and two NRCTs13,14 compared testis volume outcomes of varicocele side after varicocelectomy with those without treatment in adolescents (Figure 2). These researches involved 432 cases (267 to treatment and 165 to observation). However, the Q statistic P < 0.001, indicating the nonhomogeneity of the studies (heterogeneity: I2 = 92%; P < 0.001). Using the random effect model, the combined difference was 2.9 ml (95% CI: 0.6, 5.2; P < 0.05), indicating that improvement in effect of varicocelectomy compared to observation was statistically significant. At the same time, testis volume outcomes of the healthy side were measured and compared between the two groups (Figure 3). And the significant difference of 1.5 ml (95% CI: 0.3, 2.7; P < 0.05) of testis volume between varicocele repair and observation had been cleared after conducting a meta-analysis using the random effect model (heterogeneity: I2 = 68%; P = 0.012).
Figure 2.
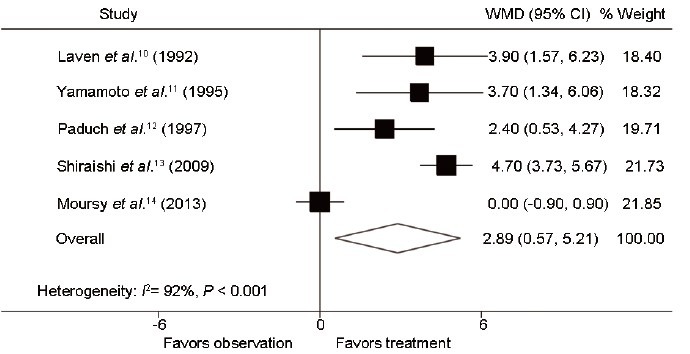
Forest plot comparing testis volume of varicocele side between varicocelectomy and observation. The combined testis volume of varicocele side was improved in the treatment group compared with the observation group.
Figure 3.
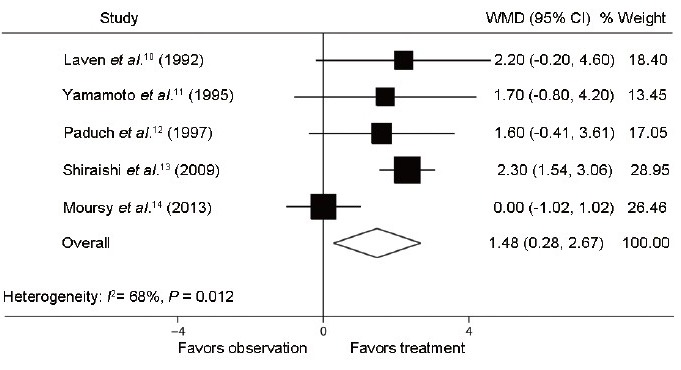
Forest plot comparing testis volume of healthy side between varicocelectomy and observation. The combined testis volume of healthy side was improved in the treatment group compared with the observation group.
Furthermore, the following semen parameter variables were recorded: semen concentration (million ml−1), total semen motility (%), and normal morphology (%). We identified four trials composed of two RCTs10,11 and two NRCTs,15,16 which respectively reported on semen concentration after varicocele repair compared to observation (Figure 4). The mean difference in semen concentration in these studies ranged from −5.0 to 27.6 million ml−1. The Q statistic P = 0.008, indicating nonhomogeneity of the studies (heterogeneity: I2 = 74%; P = 0.008). The random effect model combined difference in semen concentration between the two groups was 13.7 million ml−1 (95% CI: −1.4, 28.8; P = 0.075), indicating that postoperative semen concentration in treatment group did not show significant difference compared with that in observation group. Four identical trials were analyzed for reporting on percent total semen motility and normal morphology after varicocele repair compared to observation (Figure 5 and Figure 6). The mean difference in total semen motility ranged from − 4.0% to 9.4%. The Q statistic P = 0.015, indicating nonhomogeneity of the studies (heterogeneity: I2 = 71%; P = 0.015). The random effect model combined difference in total semen motility between the two groups was 2.5% (95% CI: −3.6, 8.6; P = 0.424). The mean difference in percent normal morphology ranged from −3.0% to 9.6%. The Q statistic P = 0.023, indicating nonhomogeneity of the studies (heterogeneity: I2 = 68%; P = 0.023). The random effect model combined difference in percent normal morphology between two groups was 2.9% (95% CI: −3.0, 8.7; P = 0.336). The results of combined percent total semen motility and normal morphology, similar to the combined semen concentration, suggested that the varicocelectomy for adolescents had no significant improvement on semen parameters compared to observation cases.
Figure 4.
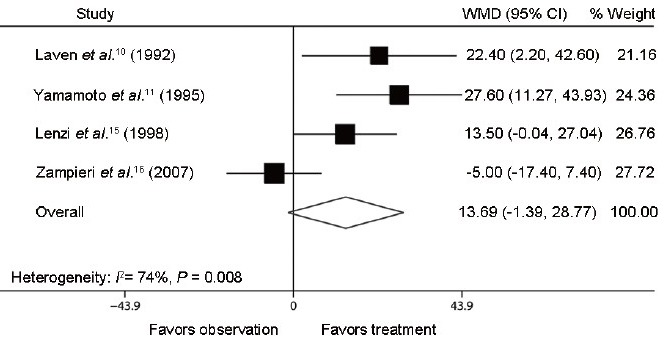
Forest plot comparing semen concentration between varicocelectomy and observation. The combined semen concentration shared no significant difference between treatment group and observation group.
Figure 5.
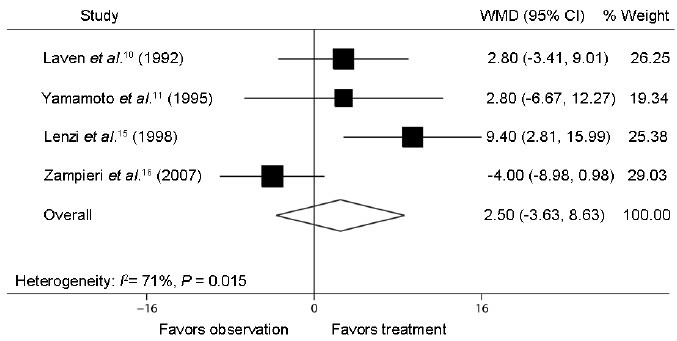
Forest plot comparing percent total semen motility between varicocelectomy and observation. The combined percent total semen motility shared no significant difference between treatment group and observation group.
Figure 6.
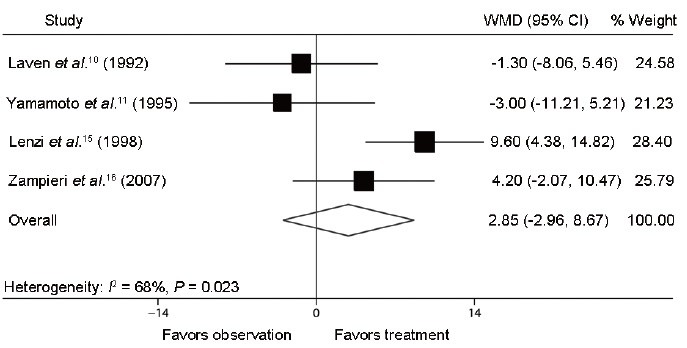
Forest plot comparing percent normal morphology between varicocelectomy and observation. The combined percent normal morphology shared no significant difference between treatment group and observation group.
DISCUSSION
Prior to our study, there was only one meta-analysis concerning the effect of varicocelectomy on adolescent varicocele. After comparing the testis volume before and after surgery, it was revealed the advantage of surgical treatment on reducing testicular hypotrophy when the discrepancy is above 10%.4 But the authors did not suggested additional prospective and controlled studies, which were necessary to elucidate the treatment of adolescents with varicocele. We perform a new meta-analysis of the available RCTs and NRCTs, suggesting that varicocelectomy for adolescents may realize the improvement of the testis volume of both sides, but has no significant improvement effect on semen parameters compared with observation cases, indicating routinely treating varicocele in adolescents seems ill-advised.
Until date, there have been no determined parameters that can predict the future fertility outcomes of adolescents with varicocele. In a large cohort, Zampieri and Cervellione18 observed that all cases of spontaneous testicular vein reflux caused by varicocele were associated with testicular hypotrophy. Hence, testis volume is commonly used as a predictor for assessing whether surgical management is indicated. Controversy still exists when it comes to the interrelation between preoperative patient age and postoperative improvement in testis volume. Cayan et al.19 included 39 boys of 11 to 19 years old in their study and found out the mean improvement in testis volume was statistically significantly higher in boys younger than 14 years old. In contrast, Decastro et al.20 concluded the age at surgery was not significantly associated with subsequent improvement in testis volume.
However, it should be noted firstly that anywhere from 50% to 80% of male patients with varicocele never have problems with fertility,21 and no study has demonstrated a correlation between loss of testicular volume and later fertility status in adolescents with varicocele. Secondly, testicular asymmetry in adolescents with varicocele can worsen, remain unchanged or ameliorate during follow-ups. Some adolescents with varicocele and considerable testicular size discrepancy manifest significant testicular “catch-up” growth during continued physiologic development. Preston et al.22 demonstrated significant catch-up growth (volume differential <20%) in 7 of 14 patients with a testicular hypotrophy of >20% who were managed conservatively, suggesting that testis developed at different rates during adolescence and that prophylactic varicocele repair might expose many subjects to the unnecessary risks of surgery. Kolon et al.23 observed similar phenomenon that 71% of testicular size disproportion (volume differential > 15%) in adolescent varicocele resolved spontaneously in a 2 years follow-up period. Finally, although there is often a rapid increase of testis volume in adolescence after varicocele correction as proved by the selected studies, it has been theorized that testicular catch-up growth after varicocelectomy could be edema secondary to severing of lymphatics during the procedure.24 It raises the question whether testis volume could be applied as a unique or key measure to select adolescent patients receiving treatment or evaluate the effect of varicocele repair in adolescents.
Recently, semen parameter as an important measurement to evaluate the fertility status of varicocele patients has been published. In a prospective study, Guzick et al.25 suggested subfertility for semen characteristics such as a semen concentration of <13.5 million ml−1, motility <32%, and normal morphology <9%. In addition, they found that the percentage of normal semen morphology was the most powerful discriminator between the fertile and infertile populations. In contrast, Nallella et al.26 reported that semen concentration and motility were superior indicators to the percentage of normal morphology for differentiating between fertile and infertile men. Furthermore, Bonde et al.27 found that the probability of conception increased with an increasing semen concentration up to 40.0 million ml−1. Despite the different ranges proposed for various semen characteristics to distinguish between fertile and infertile men, a common observation in all the above-mentioned studies was that better pregnancy outcomes were associated with better semen parameters. So in this study the combined semen parameters including semen concentration, total semen motility, and normal morphology were reasonable to evaluate the effect of varicocele repair on adolescent patients. Thus, our study showed that varicocelectomy improved bilateral testis volume in adolescents with varicocele compared with observation cases, while it might not help increasing their future chance of paternity in that semen parameters had no statistically significant difference between two groups. Recently, the new parameters of evaluating varicocelectomy outcomes, such as seminal DNA fragmentation index and reactive oxygen species, may prove useful.28 However, the preoperative cut-off values for these parameters predicting positive varicocelectomy outcomes or even future fertility are still undetermined, and they also have their share of criticism.29
When it comes to the sources of heterogeneity of combined semen parameters, the appropriate length of time required following varicocelectomy for semen parameters evaluation has not been well established. This factor was considered by Al Bakri et al.30 who concluded in their recent study that the best semen quality recovery occurred after 3 months from surgery and then did not improve further. Based on the result, the semen samples in the treatment group were collected at least 1-year after surgery. Furthermore, there has been no standard for adolescent semen parameters, so all of these studies awaited semen analysis studies in both treatment and observation groups till the patients turned 18 years of age. However, due to the huge span of the years when these selected trials were under way, the semen parameters were assessed using varying criteria (Laven et al.10 and Yamamoto et al.11 based on WHO standard of 1987 while Zampieri et al.16 based on WHO standard of 1999), which could not be avoided and might lead to bias. Besides, our meta-analysis included data from a number of different treatment methods with transcatheter embolization, high retroperitoneal ligation and microsurgical varicocelectomy, which were not stratified according to the different approaches adopted to treat the varicocele. In summary, the trials included in the meta-analysis had certain heterogeneity in terms of patient dropout rates, preoperative varicocele grades, sample assessment criteria and treatment methods.
Previous meta-analysis and systematic reviews of adolescent varicocele studies did not include controlled trials, and several investigators found methodologic defects in these studies that might lead to the biased results.3,31 RCT studies allow a more unbiased analysis of the effects of varicocele repair on testis volume and semen parameters, but the available number of prospective RCTs that evaluate the effect of varicocele repair on adolescent later infertility has been limited. One possible reason is associated with the difficulty in enrollment, considering patients refusing to be randomized to the no-treatment group. Except the study from Paduch and Niedzielski,12 which used a postponement-of-treatment design. In their trial, boys assigned to the treatment group were scheduled to undergo varicoceletomy at the earliest possible date and controls were scheduled for surgery more than 12 months after study entry, and only data from the period before treatment in the controls had been included. Perhaps, there is also a publication bias in series that are available and referenced in published literature. Because only a few available RCTs met our inclusion criteria, combining the data from NRCT and RCTs was considered the only way of reaching a logical conclusion, although it might be controversial to some. Otherwise, in this study there is another limitation of no meta-analysis about the differential change of semen parameter between pre- and post-operation because of limited data. Of note, Laven et al.10 and Yamamoto et al.11 have referred to improving postoperative sperm concentration compared with preoperation. However, even after 1-year follow-up in both studies, there was no differential semen parameter between untreated group and healthy control. The above outcome further suggests that only observation without surgery will not deteriorate the semen parameter in adolescents with varicocele.
Generally, it seems that the varicocelectomy in adolescents may have improvement effect on testis volume compared to the observation group, but is not associated with improvement in semen concentration as well as total semen motility and normal morphology. However, there is still insufficient evidence at present to demonstrate a beneficial effect of varicocele repair in adolescents on their future spontaneous pregnancy rates, and the mere presence of a varicocele is insufficient to recommend repair treatment. The need persists for well-planned, properly conducted RCTs to explore the optimal selection criteria for intervention in the adolescents who will have subsequent problems with fertility, which can avoid unnecessary operation complications caused by overtreatment, and can save medical resources and increase economic efficiency.
AUTHOR CONTRIBUTIONS
TZ and WZ conceived this study, carried out the searching and drafted the manuscript. QC and LL participated in the article screening, performed the statistical analysis. HC and CLX checked the data. GHC and YHS contributed to the design of this study and provided proposals for the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This work was funded by the 1255 Fund (CH125510101) of Changhai Hospital, Second Military Medical University, Shanghai, China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Mehta A, Goldstein M. Microsurgical varicocelectomy: a review. Asian J Androl. 2013;15:56–60. doi: 10.1038/aja.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evers JL, Collins JA. Assessment of efficacy of varicocele repair for male subfertility: a systematic review. Lancet. 2003;361:1849–52. doi: 10.1016/S0140-6736(03)13503-9. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert G, Orye C, De Win G. Pubertal screening and treatment for varicocele do not improve chance of paternity as adult. J Urol. 2013;189:2298–303. doi: 10.1016/j.juro.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Li F, Chiba K, Yamaguchi K, Okada K, Matsushita K, et al. Effect of varicocelectomy on testicular volume in children and adolescents: a meta-analysis. Urology. 2012;79:1340–5. doi: 10.1016/j.urology.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Raheem OA. Surgical management of adolescent varicocele: systematic review of the world literature. Urol Ann. 2013;5:133–9. doi: 10.4103/0974-7796.115728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips B, Ball C, Sackett D. Oxford Centre for Evidence-Based Medicine – Levels of Evidence. Centre for Evidence-Based Medicine. [Last accessed on 2014 Jan 22; Last updated on 2009 Mar 1]. Available from: http://www.cebm.net/index.aspx?o=1025 .
- 7.Williams JK. Understanding evidence-based medicine: a primer. Am J Obstet Gynecol. 2001;185:275–8. doi: 10.1067/mob.2001.116740. [DOI] [PubMed] [Google Scholar]
- 8.Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999;20:448–52. doi: 10.1016/s0197-2456(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 9.Wells G, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta analyses. Ottawa Hospital Research Institute. [Last accessed on 2014 Jan 22]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 10.Laven JS, Haans LC, Mali WP, te Velde ER, Wensing CJ, et al. Effects of varicocele treatment in adolescents: a randomized study. Fertil Steril. 1992;58:756–62. doi: 10.1016/s0015-0282(16)55324-2. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Hibi H, Katsuno S, Miyake K. Effects of varicocelectomy on testis volume and semen parameters in adolescents: a randomized prospective study. Nagoya J Med Sci. 1995;58:127–32. [PubMed] [Google Scholar]
- 12.Paduch DA, Niedzielski J. Repair versus observation in adolescent varicocele: a prospective study. J Urol. 1997;158:1128–32. doi: 10.1097/00005392-199709000-00111. [DOI] [PubMed] [Google Scholar]
- 13.Shiraishi K, Takihara H, Matsuyama H. Effects of grade 1 varicocele detected in the pediatric age-group on testicular development. J Pediatr Surg. 2009;44:1995–8. doi: 10.1016/j.jpedsurg.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Moursy EE, ElDahshoury MZ, Hussein MM, Mourad MZ, Badawy AA. Dilemma of adolescent varicocele: long-term outcome in patients managed surgically and in patients managed expectantly. J Pediatr Urol. 2013;9:1018–22. doi: 10.1016/j.jpurol.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Lenzi A, Gandini L, Bagolan P, Nahum A, Dondero F. Sperm parameters after early left varicocele treatment. Fertil Steril. 1998;69:347–9. doi: 10.1016/s0015-0282(97)00474-3. [DOI] [PubMed] [Google Scholar]
- 16.Zampieri N, Corroppolo M, Zuin V, Cervellione RM, Ottolenghi A, et al. Longitudinal study of semen quality in adolescents with varicocele: to treat or not? Urology. 2007;70:989–93. doi: 10.1016/j.urology.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 17.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–9. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 18.Zampieri N, Cervellione RM. Varicocele in adolescents: a 6-year longitudinal and followup observational study. J Urol. 2008;180:1653–6. doi: 10.1016/j.juro.2008.03.114. [DOI] [PubMed] [Google Scholar]
- 19.Cayan S, Akbay E, Bozlu M, Doruk E, Erdem E, et al. The effect of varicocele repair on testicular volume in children and adolescents with varicocele. J Urol. 2002;168:731–4. doi: 10.1016/s0022-5347(05)64735-0. [DOI] [PubMed] [Google Scholar]
- 20.Decastro GJ, Shabsigh A, Poon SA, Laor L, Glassberg KI. Adolescent varicocelectomy – is the potential for catch-up growth related to age and/or Tanner stage? J Urol. 2009;181:322–7. doi: 10.1016/j.juro.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Pryor JL, Howards SS. Varicocele. Urol Clin North Am. 1987;14:499–513. [PubMed] [Google Scholar]
- 22.Preston MA, Carnat T, Flood T, Gaboury I, Leonard MP. Conservative management of adolescent varicoceles: a retrospective review. Urology. 2008;72:77–80. doi: 10.1016/j.urology.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Kolon TF, Clement MR, Cartwright L, Bellah R, Carr MC, et al. Transient asynchronous testicular growth in adolescent males with a varicocele. J Urol. 2008;180:1111–4. doi: 10.1016/j.juro.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 24.Kocvara R, Dolezal J, Hampl R, Povýsil C, Dvorácek J, et al. Division of lymphatic vessels at varicocelectomy leads to testicular oedema and decline in testicular function according to the LH-RH analogue stimulation test. Eur Urol. 2003;43:430–5. doi: 10.1016/s0302-2838(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 25.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–93. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 26.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–34. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–7. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 28.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–36. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 30.Al Bakri A, Lo K, Grober E, Cassidy D, Cardoso JP, et al. Time for improvement in semen parameters after varicocelectomy. J Urol. 2012;187:227–31. doi: 10.1016/j.juro.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 31.Fine RG, Poppas DP. Varicocele: standard and alternative indications for repair. Curr Opin Urol. 2012;22:513–6. doi: 10.1097/MOU.0b013e328358e1a4. [DOI] [PubMed] [Google Scholar]


