Abstract
Resistance to sodium carbonate extraction is regarded as a canonical way to distinguish integral membrane proteins (MPs) from other membrane‐associated proteins. However, it has been observed that carbonate extraction releases some mitochondrial integral MPs. Here, by analyzing both artificially designed and native mitochondrial inner MPs containing transmembrane domains (TMDs) of different hydrophobicities, we show that carbonate treatment can release moderately hydrophobic TMDs from the mitochondrial inner membrane. These results suggest that resistance and sensitivity to carbonate extraction may be interpreted with caution when analyzing the nature of mitochondrial inner MPs.
Keywords: membrane protein, carbonate extraction, integral membrane, mitochondria
Introduction
Membrane proteins (MPs) are ubiquitous in both eukaryotic and prokaryotic cells, making up ∼20–30% of the total proteins.1 They are not only abundant but carry out diverse and essential functions, for example, in regulating the transport of molecules and the flow of information across the cell membranes.
MPs can be either peripheral or integral. Peripheral MPs crowd the surface of the membrane through electrostatic, hydrogen‐bonding or hydrophobic interactions with lipid head groups or other integral MPs and can be detached from the membrane by relatively mild treatments such as changing the ionic strength or pH in the buffer. However, some proteins at the surface that are bound to the membrane via modification with fatty acids (e.g., GPI‐anchored and lipid‐linked proteins) can be released from the membrane by treatment with lipases. Integral MPs are firmly embedded in the lipid bilayer by hydrophobic interactions between the hydrocarbon chains of lipids and the hydrophobic domains of proteins, and can only be removed by solubilization with detergents.
Sodium carbonate extraction was first introduced as a way to isolate intracellular/organellar membranes.2 By suspending purified fractions of rat liver endoplasmic reticulum (ER), peroxisomes or mitochondria in sodium carbonate buffer, the membranes were converted to membrane sheets retaining only integral MPs and lipid‐anchored proteins. The efficiency of this method relies on the alkaline pH that decreases noncovalent protein‐protein interactions, releasing loosely attached peripheral MPs without disrupting the integrity of the membranes.2, 3, 4 Since then, sodium carbonate has been widely accepted as the method to distinguish peripheral MPs from integral MPs.
The mitochondrial inner membrane (IM) is densely packed with proteins with ∼75:25 protein:lipid ratio.5 Previous reports on the insertion of proteins into the mitochondrial IM revealed that mitochondrial IM proteins are less hydrophobic than the bacterial or ER MPs.6 Membrane insertion of mitochondrial IM proteins via the TIM23 complex using model transmembrane domains (TMDs) showed that the hydrophobicity of the TMDs is not the only determinant for membrane insertion, but that charged flanking residues can strongly influence on membrane insertion.7
The unique protein‐lipid environment of the mitochondrial IM may therefore condition the effect of sodium carbonate extraction on separating peripheral from integral mitochondrial IM proteins. Indeed, mitochondrial single‐spanning IM proteins with a moderately hydrophobic TMD, such as Dld1p and Mgm1p, were shown to be sensitive to carbonate extraction and released from the membrane.8, 9, 10, 11
With the aim of exploring the physico‐chemical properties of mitochondrial IM proteins, in this study, we have systematically analyzed the susceptibility of both artificial and natural mitochondrial IM TMDs to sodium carbonate extraction. Our results show a correlation between the hydrophobicity of the TMD and the susceptibility to carbonate extraction, indicating that mitochondrial IM proteins harboring moderately hydrophobic TMDs can be released by carbonate extraction.
Results
Experimental setup and calculation of m‐ratio
Mgm1p is a dynamin‐like GTPase that is involved in mitochondrial IM fusion in yeast. It undergoes a unique topogenesis that generates two isoforms; membrane anchored l‐Mgm1p and soluble s‐Mgm1p.12 In our previous study, we replaced the first hydrophobic segment of Mgm1 by a series of 19‐residue model segment (H‐segment) composed of n Leu and 19‐n Ala residues, and determined membrane insertion efficiency of the H‐segment into the mitochondrial IM by measuring the relative amounts of l‐Mgm1p compared with s‐Mgm1p.7 We verified that, for the H‐segment to be anchored in the IM, the segment should be either be sufficiently hydrophobic (n > 6) or contain charged flanking residues. The hydrophobicity of the first TMD of Mgm1p influences the balance between the two isoforms, that is, higher n (lower ΔG app) leads to formation of more l‐Mgm1p; the number of Leu residues required for 50% retention of the H‐segment in the IM was found to be n = 5‐6. Additionally, the Mgm1p carrying the H‐segment of low hydrophobicity (n = 1–3) could still produce mostly l‐Mgm1p when the charged flanking residues (N‐terminal KKPK and/or the C‐terminal KPKK or DPDD) are present. Hence, charged flanking residues may play a more important role in anchoring the TMD presumably via interaction between lipid head groups and/or membrane potential.
We therefore tested the effect of sodium carbonate extraction on Mgm1p variants containing different H‐segments that mainly generate the l‐Mgm1p. [Fig. 1(A,B)]. Since the m‐AAA protease in the IM recognizes Mgm1p with the modified H‐segment and dislocates it from the IM,13 we transformed various Mgm1p constructs into the Δyta10 strain where Yta10p, a component of m‐AAA is missing. Mitochondria from yeast cells carrying each Mgm1p variant were isolated and subjected to carbonate extraction [Fig. 1(C)]. Samples in the pellet and supernatant fractions were separated on the SDS‐gel and Western blotting was followed to detect Mgm1p. The band intensities of the l‐Mgm1p isoforms from the pellet and supernatant fractions were measured and accounted as 100% total.
Figure 1.
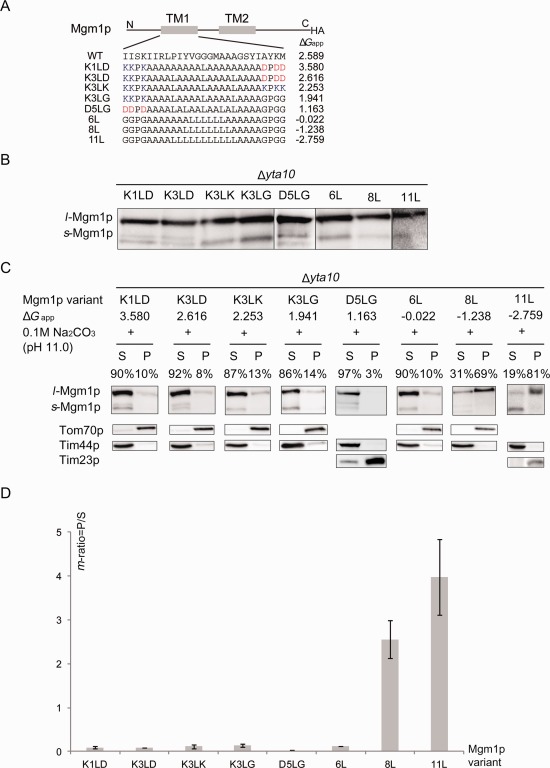
Carbonate extraction of Mgm1p variants. A: Schematic drawing of Mgm1p variant. The first TMD of Mgm1p (TM1) was replaced by an H‐segment containing 19 amino acid residues. ΔG app for membrane insertion of each construct is present as calculated in Hessa et al.19 Hydrophobicity increases as ΔG app decreases. B: Expression of Mgm1p variants. Mitochondria expressing Mgm1p variants in Δyta10 strain were isolated and subjected to Western blot analysis. All constructs express a long Mgm1p isoform (l‐Mgm1p) as a major form. C: Western blot analysis of Mgm1p variants with different hydrophobicity (ΔG app). Mitochondria expressing Mgm1p variants in Δyta10 strain were isolated and subjected to 0.1M sodium carbonate, pH 11.0 treatment for 30 min. Each protein was detected by anti‐HA antibody and its m‐ratio was calculated by dividing the l‐Mgm1p band intensity in the pellet fraction by that of the supernatant. Tim44p is a control for soluble protein (detected only in supernatant) and Tom70p and Tim23p are for membrane‐bound proteins (detected mostly in pellet). S, supernatant; P, pellet. Constructs are shown in the order of the number of Leu residues in the H‐segment: hydrophobicity increases as ΔG app decreases from K1LD to 11L. Percentages of l‐Mgm1p isoform in the pellet and supernantant are presented. D: The m‐ratio of each variant is shown in bar graph. The average of results from two independent experiments is shown with standard deviations.
To develop an unbiased analysis, we calculated the ratio of the l‐Mgm1p band intensity of the pellet and supernatant samples, denoted as membrane ratio (m‐ratio). The band intensity of l‐Mgm1p in the pellet was divided by that of the supernatant. The m‐ratio near 1 indicates that ∼50% of l‐Mgm1p molecules are released from the membrane by carbonate extraction and thus, m‐ratio over 1 marks more l‐Mgm1p molecules found in the pellet whereas m‐ratio below 1 means more l‐Mgm1p molecules in the supernatant.
Effects of carbonate extraction of Mgm1p with the H‐segment of varying hydrophobicity
We wondered whether the l‐Mgm1p isoforms resulted from Mgm1p variants carrying considerably low hydrophobic H‐segments (n = 1–3) flanked by charged residues (K—D, D—G) are more sensitive to carbonate extraction than those carrying sufficiently hydrophobic H‐segments (n = 6–11). Purified yeast mitochondria expressing these Mgm1p constructs were treated with 0.1M sodium carbonate at pH 11.0. l‐Mgm1p isoforms from less hydrophobic Mgm1p variants (n = 1–6) were mostly found in supernatant while those of hydrophobic constructs (n = 8–11) were found in pellet fractions [Fig. 1(C)]. Interestingly the 6Leu/13Ala segment was mostly found in the supernatant, which corresponds to the number of Leu residues required for 50% retention in the IM in the wild type.7
The m‐ratio in this case is the relative l‐Mgm1p band intensity from the pellet and supernatant samples. An m‐ratio over 2 was determined for 8L and 11L constructs whereas it was below 1 for 1L‐6L constructs, indicating that l‐Mgm1p was mainly released in supernatant by carbonate treatment for these latter constructs [Fig. 1(D)]. More s‐Mgm1p isoforms were found in the samples from isolated mitochondria compared to whole cell lysates, which we suspect that some l‐Mgm1p isoforms were converted to s‐Mgm1p during the mitochondria preparation [Fig. 1(C)].
Mitochondrial IM proteins with moderately hydrophobic TMD are more sensitive to carbonate extraction
To verify whether the sensitivity of mitochondrial single‐spanning MPs carrying the moderately hydrophobic TMDs to carbonate extraction is a general phenomenon, various mitochondrial IM protein‐Mgm1p fusions (MFPs) obtained in a previous study [Fig. 2(A)] (Table 1)14 were subjected to carbonate extraction. This set of fusion proteins were expressed in yeast and the membrane insertion of the TMDs was verified by the formation of membrane anchored Mgm1‐fusion protein (L‐MFP). Mitochondria were purified and subjected to sodium carbonate treatment. The m‐ratio was calculated by measuring the ratio of the band intensity present in the pellet versus the one in the supernatant.
Figure 2.
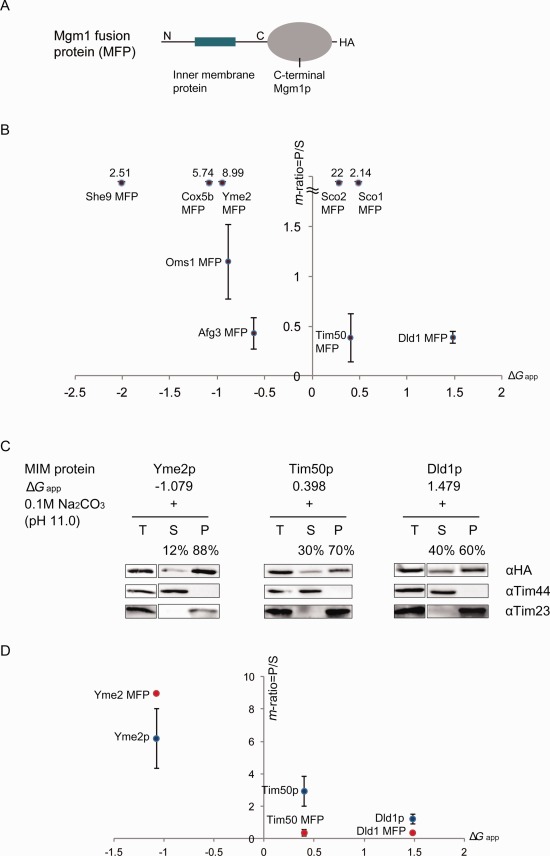
Carbonate extraction of mitochondrial inner MPs with moderately hydrophobic TMD. A: Schematic drawing of Mgm1‐fusion protein (MFP). The C‐terminus of Mgm1p starts from the Ser residue at position 117. B: Relationship between TMD hydrophobicity and m‐ratio of MFPs. Mitochondria expressing MFPs in the W303‐1α were isolated and subjected to 0.1M sodium carbonate treatment for 30 min. Each m‐ratio was calculated by dividing membrane anchored Mgm1‐fusion protein band intensity of pellet by that of supernatant. C: Western blot analysis of three integral MPs Dld1p, Tim50p, and Yme2p with different hydrophobicity (ΔG app). Mitochondria expressing inner MPs were isolated and subjected to 0.1M sodium carbonate, pH 11.0 treatment for 30 min, followed by ultracentrifugation and SDS‐PAGE. Each protein was detected by anti‐HA antibody and its m‐ratio was calculated by dividing the band intensity of Dld1p, Tim50, or Yme2p in the pellet by that of supernatant. Tim44 is a control for soluble protein (detected only in supernatant) and Tim23 is for membrane‐bound protein (detected only in pellet). T, total membrane; S, supernatant; P, pellet. Percentages of each protein in the pellet and supernatant are presented. D: The m‐ratio of full‐length proteins (blue circless) and corresponding Mgm1‐fusion versions (red circles) were plotted with respect to their corresponding TMD hydrophobicity (ΔG app). The average of data obtained from two independent experiments are shown with standard deviations.
Table 1.
Information of Yeast Mitochondrial Inner MPs Fused with the C‐Terminal Domain of Mgm1p
| IM protein | Length (aa) | Predicted TM sequence | ΔG app | Average of MFP m‐ratio |
|---|---|---|---|---|
| She9p | 456 | TWGTFILMGMNIFLFIVLQLLL | −1.985 | 2.51 |
| Cox5bp | 126 | FITKGVFLGLGISFGLFGLVRLL | −1.163 | 5.74 |
| Yme2p | 384 | TRIAIPVLFALLSIFAVLVF | −1.079 | 9.00 |
| Oms1p | 143 | MTKYMIGAYVIFLIYGLFFTKKL | −0.89 | 1.15 |
| Afg3pa | 160 | FANTMFLTIGFTIIFTLLT | −0.619 | 0.43 |
| Sco2p | 115 | RWKATIALLLLSGGTYAYL | 0.023 | 22.0 |
| Tim50p | 151 | YANWFYIFSLSALTGTAIYMAR | 0.398 | 0.39 |
| Sco1p | 108 | FSTGKAIALFLAVGGALSYFF | 0.479 | 2.14 |
| Dld1p | 377 | WLKYSVIASSATLFGYLFA | 1.479 | 0.39 |
Afg3p used in this study contains only the first TMD, corresponding to Yta10(1TM) in the previous study.14
We plotted the m‐ratio of MFPs in function of the hydrophobicties of their TMDs (Table 1) [Fig. 2(B)]. MFPs with TMDs with lower ΔG app (<−0.7) showed m‐ratio over 1 whereas those with higher ΔG app showed m‐ratio below 1. Interestingly, Sco1 MFP and Sco2 MFP showed a higher m‐ratio even though their hydrophobicities were relatively low. Sco1p and Sco2p are known to aid the incorporation of COX subunits I and II.15 We speculate that Sco1 and Sco2 may be associated with their partner proteins in the process of complex formation, which may prevent their membrane release by carbonate extraction.
In case the C‐terminal domain of Mgm1p might influence the sensitivity to carbonate extraction, we prepared full‐length Yme2p, Tim50p, and Dld1p without the C‐terminal domain of Mgm1p. Isolated mitochondria expressing these proteins were treated with sodium carbonate and the m‐ratio of each protein was calculated [Fig. 2(C,D)]. For Tim50p and Yme2p, the majority were detected in the pellet fraction with the calculated m‐ratio of 3.00 and 6.20, respectively. Dld1p, which has a less hydrophobic TMD, was found almost equally in both supernatant and pellet (m‐ratio of 1.22) [Fig. 2(C)].
The calculated m‐ratios of corresponding fusion proteins are 8.99 for Yme2 MFP and 0.39 for both Dld1 MFP and Tim50 MFP. Although the order of m‐ratio value was slightly different between the full‐length proteins (Yme2p > Tim50p > Dld1p) and the MFPs (Yme2p > Tim50p = Dld1p), the decreasing trend was maintained [Fig. 2(D)]. In fact, it verifies the earlier results that Dld1p and Mgm1p are sensitive to carbonate extraction and are released from the membrane.8, 9, 10, 11
Overall, these results show that single‐spanning MPs carrying moderately hydrophobic TMD can be released when subjected to sodium carbonate extraction.
Discussion
The sodium carbonate extraction has been widely used as a canonical method to separate integral from peripheral MPs. However, it has been observed that some mitochondrial integral MPs are released under alkaline condition provided by sodium carbonate.8 In this report, by analyzing a set of mitochondrial inner MPs with controlled hydrophobicity, we show that carbonate extraction releases inner MPs with moderately hydrophobic TMDs.
Mitochondrial MPs tend to have less hydrophobic TMDs compared with the MPs targeted to the secretory pathway through the ER.6, 16 Earlier works have shown that these moderately hydrophobic mitochondrial MPs can be anchored in the IM via the charged residues flanking the TMD, presumably by interacting with lipid head groups and/or membrane potential.17 In turn, this set of MPs may be more susceptible to carbonate extraction compared to those carrying more hydrophobic TMDs. Thus, our results suggest that although sodium carbonate extraction is useful to determine hydrophobic integral MPs, when analyzing moderately hydrophobic mitochondrial MPs, the data should be interpreted with caution.
Materials and Methods
Plasmid construction and yeast transformation
pHP84MGM1HA H‐segments, Mgm1p fusion protein plasmids were obtained from the previous studies.7, 14 Genes encoding mitochondrial protein, Yme2p, Tim50p, and Dld1p were amplified by PCR using genomic yeast DNA prepared from W303‐1α (MAT α, ade2, can1, his3, leu2, trp1, and ura3) and cloned into pHP84HA vector. All plasmids were prepared by homologous recombination in yeast as previously described in Kim et al.18 Mgm1p variants were expressed in Δyta10 strain (MAT a, ade2‐1, his3‐11,15, yta10::HIS3MX6, trp1‐1, leu2,112, and ura3‐52), where the Yta10p subunit of the m‐AAA protease is deleted. MFP and Yme2HA, Tim50HA, and Dld1HA constructs were transformed into W303‐1α.
Isolation of yeast mitochondria and sodium carbonate extraction and Western blot analysis
Yeast transformants carrying each test gene were grown in 1 L of –Leu medium containing glucose (2% w/v) at 30°C up to OD600 1‐2. Yeast mitochondria were isolated as previously described.14 40 μg of mitochondria were resuspended and incubated in 100 μL of cold 0.1 M Na2CO3 (pH 11.0) for 30 min on ice. The suspensions were ultracentrifuged at 4°C for 30 min at 90,000 g in Beckman polycarbonate tubes in a TLA 110 rotor. Supernatants and pellets were incubated in 500 μL of 12.5% TCA for at least 15 min on ice, followed by centrifugation for 15 min at 28,000 g at 4°C. The samples were washed with 500 μL of 100% acetone, subsequently dried for 5 min in the room temperature. The dried pellet obtained from acetone wash was incubated in 35 μL of 1× SDS‐PAGE sample buffer for 3 min at 95°C and loaded onto the 6.5 and 12.5% SDS‐gels. Proteins of interest were detected by anti‐HA antibodies. Tim44p was used as a marker for peripheral (supernatant) MP whereas Tim23p or Tom70p were used as integral (pellet) MP controls, detected with anti‐Tim44, Tim23, and Tom70 antibodies, respectively, kindly provided by Professor Toshiya Endo (Kyoto Sangyo University, Kyoto, Japan).
Acknowledgments
Authors thank the laboratory members for critical reading of the manuscript. The authors declare that there are no conflicts of interest.
References
- 1. Wallin E, von Heijne G (1998) Genome‐wide analysis of integral MPs from eubacterial, archaean, and eukaryotic organisms. Protein Sci 7:1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fujiki Y, Hubbard AL, Fowler S, Lazarow PB (1982) Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol 93:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujiki Y, Fowler S, Shio H, Hubbard AL, Lazarow PB (1982) Polypeptide and phospholipid composition of the membrane of rat liver peroxisomes: comparison with endoplasmic reticulum and mitochondrial membranes. J Cell Biol 93:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hack E, Lin C, Yang H, Horner HT (1991) T‐URF 13 protein from mitochondria of Texas male‐sterile maize (Zea mays L.): Its purification and submitochondrial localization, and immunogold labeling in Anther tapetum during microsporogenesis. Plant Physiol 95:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheffler IE (2007) Mitochondria. New York: Wiley. [Google Scholar]
- 6. von Heijne G (1986) Why mitochondria need a genome. FEBS Lett 198:1–4. [DOI] [PubMed] [Google Scholar]
- 7. Botelho SC, Ostererg M, Reichert AS, Yamano K, Bjorkholm P, Endo T, von Heijne G, Kim H (2011) TIM23‐mediated insertion of transmembrane alpha‐helices into the mitochondrial IM. Embo J 30:1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herlan M, Vogel F, Bornhovd C, Neupert W, Reichert AS (2003) Processing of Mgm1 by the rhomboid‐type protease Pcp1 is required for maintenance of mitochondrial morphology and of mitochondrial DNA. J Biol Chem 278:27781–27788. [DOI] [PubMed] [Google Scholar]
- 9. Wong ED, Wagner JA, Gorsich SW, McCaffery JM, Shaw JM, Nunnari J (2000) The dynamin‐related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J Cell Biol 151:341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Messerschmitt M, Jakobs S, Vogel F, Fritz S, Dimmer KS, Neupert W, Westermann B (2003) The IM protein Mdm33 controls mitochondrial morphology in yeast. J Cell Biol 160:553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sesaki H, Southard SM, Yaffe MP, Jensen RE (2003) Mgm1p, a dynamin‐related GTPase, is essential for fusion of the mitochondrial outer membrane. Mol Biol Cell 14:2342–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herlan M, Bornhovd C, Hell K, Neupert W, Reichert AS (2004) Alternative topogenesis of Mgm1 and mitochondrial morphology depend on ATP and a functional import motor. J Cell Biol 165:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Botelho SC, Tatsuta T, von Heijne G, Kim H (2013) Dislocation by the m‐AAA protease increases the threshold hydrophobicity for retention of transmembrane helices in the IM of yeast mitochondria. J Biol Chem 288:4792–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park K, Botelho SC, Hong J, Osterberg M, Kim H (2013) Dissecting stop transfer versus conservative sorting pathways for mitochondrial IM proteins in vivo. J Biol Chem 288:1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krummeck G, Rodel G (1990) Yeast SCO1 protein is required for a post‐translational step in the accumulation of mitochondrial cytochrome c oxidase subunits I and II. Curr Genet 18:13–15. [DOI] [PubMed] [Google Scholar]
- 16. von Heijne G (1990) The signal peptide. J Membr Biol 115:195–201. [DOI] [PubMed] [Google Scholar]
- 17. Osterberg M, Calado Botelho S, von Heijne G, Kim H (2011) Charged flanking residues control the efficiency of membrane insertion of the first transmembrane segment in yeast mitochondrial Mgm1p. FEBS Lett 585:1238–1242. [DOI] [PubMed] [Google Scholar]
- 18. Kim H, Park H, Montalvo L, Lennarz WJ (2000) Studies on the role of the hydrophobic domain of Ost4p in interactions with other subunits of yeast oligosaccharyl transferase. Proc Natl Acad Sci USA 97:1516–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hessa T, Meindl‐Beinker NM, Bernsel A, Kim H, Sato Y, Lerch‐Bader M, Nilsson I, White SH, von Heijne G (2007) Molecular code for transmembrane‐helix recognition by the Sec61 translocon. Nature 450:1026–1030. [DOI] [PubMed] [Google Scholar]


