Abstract
The involvement of epithelial-to-mesenchymal transition (EMT) in metastasis has long been under debate. Recent efforts to probe the occurrence and functional significance of EMT in clinical samples and animal models have produced exciting but sometimes conflicting findings. The diversity of EMT underlies the challenge in studying its role in metastasis.
Keywords: Epithelial-to-mesenchymal transition (EMT), cancer metastasis, plasticity, in vivo tracing
The metastatic dissemination of epithelial tumors has been commonly thought to rely on epithelial-to-mesenchymal transition (EMT), wherein carcinoma cells lose their polarity and cell-cell adhesions to gain fibroblast-like properties, such as migration and invasion [1, 2]. However, the pathological importance of EMT during metastasis has been a subject of debate, in large part due to the difficulty to observe EMT as it occurs in vivo. Mesenchymal tumor cells, even if they are derived from epithelial tumors through EMT, often cannot be distinguished from the stromal cells, most of which are of the mesenchymal lineage. Furthermore, EMT is often considered as a transient process and tumor cells need to undergo the reverse process, mesenchymal-to-epithelial transition (MET), in order to successfully colonize a distant organ [3]. Recent studies have used multiplex tumor and EMT marker analysis to reveal abundant proportion of circulating tumor cells manifesting different degrees of mesenchymal features, and fluctuation of such populations in different cancer subtypes and in response to treatments [4]. In vivo lineage marking experiments in mouse models similarly revealed the occurrence of EMT to different degrees depending on the initiating oncogenes [5]. These studies provided supporting evidence for the in vivo occurrence of EMT, and underscored the complexity of the process in the context of tumor heterogeneity.
Recently, two research groups employed lineage marking and conditional knockout strategies in mouse models of breast and pancreatic cancers to further study the contribution of EMT to metastasis [6, 7]. Discoveries from both groups appeared to contradict the prevailing hypothesis that EMT is required for metastasis. To track EMT events in vivo, Fischer and colleagues established a triple-transgenic mouse model, in which the PyMT oncogene is expressed in mammary epithelial cells to induce spontaneous breast cancer, and RFP is permanently switched to GFP when Cre recombinase expression is activated under the promoter of Fsp1 (fibroblast specific protein 1), a gene highly expressed in the fibroblasts [6]. Surprisingly, the majority of cells in the primary tumor and lung metastasis are RFP+, suggesting the absence of Fsp1 promoter activation, and by extension, EMT. Similar results were observed in the MMTV-Neu model or when the Vimentin promoter was used. The researchers further showed that inhibition of EMT by overexpressing miR-200 did not affect the number of lung metastasis formed. In the second study, Zheng and co-workers created tissue-specific knockout of Snail or Twist, two EMT-inducing transcription factors, in spontaneous pancreatic adenocarcinoma mouse models to inhibit EMT. Despite the loss of Snail or Twist, the incidence of metastasis remained similar [7].
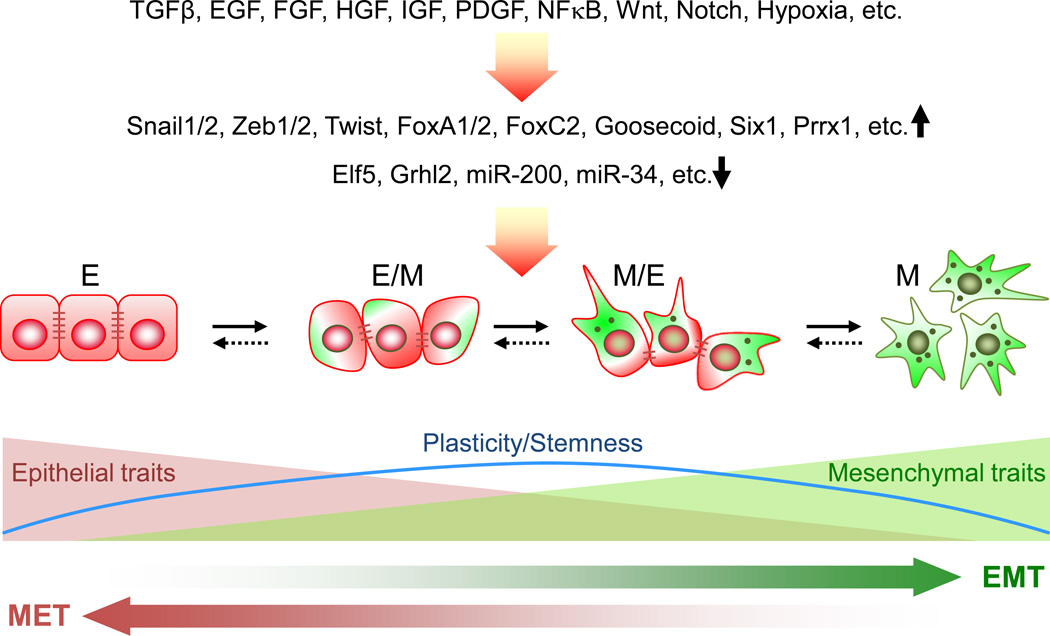
Together, these two studies provide intriguing evidence opposing the occurrence and functional importance of EMT in metastasis. However, the potential limitations in these studies require careful evaluation. EMT, which can be triggered by multiple mechanisms, is orchestrated by a complex network of transcription factors, epigenetic regulators and non-coding RNAs [8] (Figure 1). Most importantly, EMT in the context of malignant tumor is not a binary process, but instead includes a spectrum of intermediate states that have different degrees of simultaneous expression of epithelial and mesenchymal markers, some of which likely evade the detection by the lineage marking method used in the study. Furthermore, it has been suggested that those partial EMT states are associated with higher aggressiveness and stemness compared with the complete epithelial or mesenchymal states, and tumor cells with both epithelial and mesenchymal features are likely to be highly proficient in collective cell movement, an effective way of metastatic dissemination that does not require fibroblast-like single cell mobility [9]. Therefore, tracing cells based on the expression of a single gene as in Fischer et al.’s study may not fully capture all the ongoing EMT events, particularly those partial EMT cells. Indeed, as demonstrated by Fischer et al., a prolonged treatment of cultured PyMT cells with TGF-β, a strong EMT inducer, only induced a modest increase in the Fsp1-Cre driven RFP to GFP color switch (from 15% GFP+ cells to ~22%), indicating that this reporter system might miss a substantial portion of partial EMT cells that did not have sufficient Fsp1 promoter activity, but may be functionally important in driving metastasis. In an extreme scenario, if the GFP+ cells represented those that had transitioned to the farthest end of the mesenchymal spectrum and become fixed in a terminally differentiated state, they may have lost the critical cellular plasticity as well as tumor initiating properties needed for cancer metastasis [10].
Figure 1. The EMT spectrum.
EMT can be triggered by a number of different initiating signals and downstream regulators. During this process, epithelial cells transition to different extents toward the fully mesenchymal phenotype by progressively acquiring mesenchymal features while losing epithelial traits. The intermediate or partial EMT states, in which cells express both epithelial and mesenchymal markers, have been suggested to exhibit higher plasticity and stemness [9], although more extensive research is needed to validate the pathological relevance of the hypothesis. The levels of expression of epithelial adhesion molecules, such as E-cadeherin, and mesenchymal markers, such as Fsp1 and Vimentin, are represented by numbers of brown lines and green dots, respectively.
Beyond the dynamics of EMT process, it is also worth noting that diverse cancer subtypes may exhibit varying degrees of EMT and employ different mechanisms for metastatic dissemination. Studies on circulating tumor cells (CTCs) in human breast cancer patients demonstrated that CTCs from lobular breast cancer patients were predominantly epithelial, while those from HER2+ and triple negative subtypes were mostly mesenchymal [4]. Both PyMT and Neu mouse models develop luminal breast cancer with more epithelial traits [11]. In these models, the highly proliferative epithelial cancer cells may invade through collective migration or passive shedding, which relies less on extreme mesenchymal properties. Although complete EMT events may be relatively rare in these tumors, partial EMT may still occurs and such “dual-character” partial EMT cells could still promote metastasis by enhancing collective cell move, or by cooperating with the epithelial-like cells to promote various steps of metastasis [10, 12]. A similar lineage tracking experiment showed readily detectable EMT in c-Myc driven mouse models with both basal-like and luminal features [5]. Future research should explore the occurrence of EMT in tumors with basal or mixed features, such as Brca1fl/fl;TgMMTV-Cre;p53+/−, or TgC3(1)-Tag mammary tumor models, and employ additional markers of EMT in order to obtain a more comprehensive view of the prevalence of EMT in a wide spectrum of cancer types.
Previous gain-of-function studies have helped establish the importance of EMT in metastasis, although arguments were made that such models may be artificial and did not accurately reflect the natural process of cancer progression. Interpreting findings by the loss-of-function approach, such as conditional knockout of EMT master transcriptional factors Twist and Snail [7], also need to apply a measure of caution. It is necessary to fully demonstrate the effect of such genetic manipulation on the EMT spectrum in order to distinguish a partial or complete ablation of EMT, as loss of one EMT driver can be compensated by an alternative EMT-promoting pathway. Thus, the lack of effect on metastasis after genetic knockout of one factor may not necessarily nullify the involvement of EMT in metastasis.
Despite these limitations and caveats, these two recent papers illustrated the complex role of EMT in tumor progression and the need to carefully dissect its dynamics and involvement in metastasis. Among the studies on the diverse signaling pathways, transcription factors, epigenetic regulators and non-coding RNAs involved in EMT, few have investigated their temporal and spatial expression during metastasis. Such knowledge is of critical importance for designing more sensitive and robust tracing systems or conditional expression models to precisely dissect the dynamics and functional importance of epithelial-mesenchymal plasticity during cancer progression, metastasis and response to treatment. In parallel, comprehensive single cell analysis of clinical specimens will provide complementary insights into the clinical involvement of EMT in cancer progression, which will facilitate the development of EMT-targeting strategies in cancer therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends in cell biology. 2015;25:675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brabletz T, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai JH, et al. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu M, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trimboli AJ, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer research. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- 6.Fischer KR, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng X, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nature reviews. Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 9.Jolly MK, et al. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Frontiers in oncology. 2015;5:155. doi: 10.3389/fonc.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celia-Terrassa T, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. The Journal of clinical investigation. 2012;122:1849–1868. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herschkowitz JI, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome biology. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuji T, et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer research. 2008;68:10377–10386. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]