Abstract
Most members of the p53 family of transcription factors form tetramers. Responsible for determining the oligomeric state is a short oligomerization domain consisting of one β‐strand and one α‐helix. With the exception of human p53 all other family members investigated so far contain a second α‐helix as part of their tetramerization domain. Here we have used nuclear magnetic resonance spectroscopy to characterize the oligomerization domains of the two p53‐like proteins from the tunicate Ciona intestinalis, representing the closest living relative of vertebrates. Structure determination reveals for one of the two proteins a new type of packing of this second α‐helix on the core domain that was not predicted based on the sequence, while the other protein does not form a second helix despite the presence of crucial residues that are conserved in all other family members that form a second helix. By mutational analysis, we identify a proline as well as large hydrophobic residues in the hinge region between both helices as the crucial determinant for the formation of a second helix.
Keywords: p53, p73, p63, oligomerization domain, tetramerization, Ciona intestinalis, NMR structure
Short abstract
PDB Code(s): 2MW4
Introduction
The p53 family has a long and complex evolutionary history.1, 2 The origin of this highly important protein family reaches back about one billion years of evolution. A p53 ancestor gene is first clearly observed in the modern‐day descendants of the early metazoan sea anemone. In mammals this protein family comprises three different proteins, p53, p63, and p73.3, 4 All three are products of gene duplication events and subsequent specialization during vertebrate evolution starting from a single genomic copy. The original function of the founding member of this family of transcription factors is most likely monitoring the genetic integrity of germ cells.4, 5 In mammals this function is carried out by the family member p63,5 which is highly expressed in oocytes in a closed, inhibited conformation.6 After the development of renewable tissue, control of epithelial stem cells emerged, again carried out by p637, 8, 9 and finally tumor suppression, carried out by p7310 and in particular by p53.11
Common to all family members is an N‐terminal transactivation domain, a central DNA‐binding domain (DBD) and a C‐terminal oligomerization domain that regulates the oligomeric state of the protein. Some family members also have isoforms with a SAM domain12 and C‐terminal domains regulating the transcriptional activity.13 Of all domains the DBD is the most conserved one and new family members are often identified via their sequence identity in the DBD. The oligomerization domain shows a lower degree of conservation and a higher level of structural diversity. Usually, family members form tetramers in their active state, with the dimeric p53 protein from Caenorhabditis elegans, Cep‐1, being the exception.14 The basic architecture of the oligomerization domain can be described as a dimer of dimers.15, 16 Each monomer contributes one β‐strand and one α‐helix. Two monomers form the primary dimer via an intermolecular anti‐parallel β‐sheet that is stabilized by antiparallel hydrophobic helix packing. The tetramer is composed of two such primary dimers, which pack against each other with their helices, resulting in a close to orthogonal arrangement with D2 symmetry. Deviations from this basic design exist for example in the oligomerization domains of insects, where structure determination of the oligomerization domain of the p53 form from Drosophila melanogaster has revealed that every secondary structure element is doubled.14 However, significant differences between the oligomerization domains of the mammalian family members have been identified as well. While p53 shows the architecture described above, both p63 and p73 contain an additional C‐terminal helix that stabilizes the tetrameric state.17, 18 From an evolutionary view point p73 seems to be more closely related to p63 than to p53 and the presence or absence of this second helix in the tetramerization domain—as well as the presence of a SAM domain—seems to be an evolutionary marker. To distinguish the two different types of architecture, we call the basic p53‐like design an oligomerization domain (OD) and the p63/p73‐like structure containing a second helix per monomer a tetramerization domain (TD).
To obtain further insight into the structural requirements for the formation of a second helix and the evolution of the p53 family, we investigated the architecture of the TDs of the p53 family members from Ciona intestinalis (C.int.). This organism belongs to the tunicates (or urochordates), a group that represents the closest living relatives of vertebrates (Fig. 1). The fast embryogenesis—fertilization to the free‐swimming tadpole takes only about 18 h—and its compact genome with only roughly 160 million base pairs has made C.int. a model organism to investigate the genetic control mechanisms regulating development in early embryogenesis.19 The genomes of C.int. and of the very closely related species Ciona savignyi (C.sav.) each contain two genes encoding proteins belonging to the p53 family.20, 21 Phylogenetic analysis revealed that they resulted from a duplication event independent of the duplications that led to the p53 family in vertebrates. Consequently, they form an independent monophyletic group and this makes the vertebrate p53, p63, and p73 proteins co‐orthologous to any of the two Ciona homologs, which are themselves paralogous to one another. According to this relationship, the Ciona proteins were named p53/p73‐a and p53/p73‐b. The observation that the gene encoding C.int. p53/p73‐b lacks any introns suggests retro‐transposition as the mechanism most likely associated with the lineage‐specific duplication.20 While in consequence only a single C.int. p53/p73‐b protein is expressed, five different isoforms of C.int. p53/p73‐a have been identified on transcript level (Fig. 2).
Figure 1.
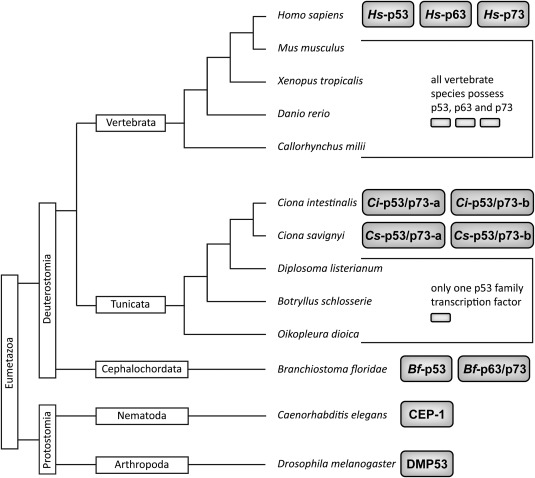
Taxonomy tree of the subkingdom eumetazoa within the animal kingdom with some sample species from different subphyla. Subkingdom (Eumetazoa) and superphyla (Deuterostomia, Protostomia) are indicated in boxes with vertical orientation. The subphyla are shown in boxes with horizontal orientation. Species names are listed with italic lettering. The protein names of p53 family transcription factors are indicated in gray boxes.
Figure 2.
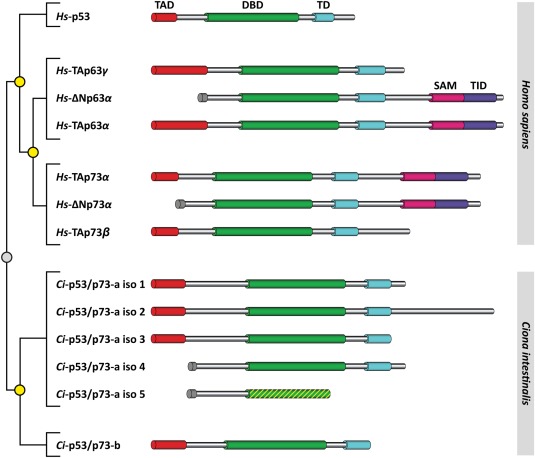
Phylogenetic tree and domain overview of the p53 family of transcription factors from Homo sapiens and the orthologous proteins from Ciona intestinalis. For mammalian p53, p63, and p73 only the most important isoforms are shown, for the p53‐like proteins from C.int. all isoforms are presented. Isoform 5 of C.int. p53/p73‐a lacks the TD and parts of the DBD. TAD: transactivation domain, DBD: DNA‐binding domain, TD: tetramerization domain, SAM: sterile alpha motif, TID transactivation inhibitory domain. The phylogenetic tree on the left depicts the homology relationship. All proteins derived from a common ancestor. Following speciation into two distinct evolutionary lines (marked with a gray circle) independent duplication events (marked with yellow circles) resulted in the different genomic copies.
For the p53‐like protein from C. elegans, the closer relationship to p63/p73 than to p53 due to the presence of a SAM domain was not identified by sequence comparison but only detected after structure determination.14 Some of the splice forms of C.int. p53/p73‐a contain large C‐terminal extensions that might harbor folded domains as well. In addition to the presence of a SAM domain, the observation of a second helix as part of the tetramerization domain in vertebrates also distinguishes p63/p73 from p53. To determine if the C‐terminus of C.int. p53/p73‐a contains a SAM or any other folded domain and if a second helix as characteristic for p63/p73‐like proteins is formed, we investigated the tetramerization domains of C.int. p53/p73‐a and C.int. p53/p73‐b by nuclear magnetic resonance (NMR) spectroscopy.
Results
The extended C‐terminus of C.int. p53/p73‐a Isoform 2 does not contain a folded domain
To obtain information about the domain architecture of the p53/p73‐like proteins of the two tunicate species C.int. and C.sav. we aligned their sequences against the sequences of the p53, p63, and p73 proteins of five representative vertebrate species (Homo sapiens, Mus musculus, Xenopus tropicalis, Danio rerio, and Callorhynchus milii). Protein sequences from three other species C. elegans (nematode), D. melanogaster (arthropod), and Branchiostoma floridae (cephalochordate, lancelet), which are taxonomically more distant to vertebrates, were also included. The DBD is the only domain conserved in all included species, showing sequence identities between the tunicate proteins and vertebrate proteins of 38–51% and 19–32% between the tunicate proteins and Dmp53 or Cep‐1. In contrast, the sequence of the OD is only conserved among deuterostome species. The sequences of the ODs were aligned in a second calculation with the NMR solution structure of the TD of human p73 used as a reference. Dmp53 and Cep‐1 were excluded from this alignment since the structures of their ODs show architectures that differ from those of vertebrate p53 family proteins (Fig. 3). For p53/p73‐a, a splice site located directly adjacent to the OD contributes to the formation of three different C‐terminally isoforms with the C‐terminus of Isoform 2 being considerably extended. Structural investigations of the ODs of the p53 homologs from Drosophila and C. elegans had revealed that in both cases the C‐terminus contains secondary structure elements (Drosophila) or entire domains (C. elegans) that interact with the core OD.14 To investigate if such interactions occur in C.int. p53/p73‐a Isoform 2 as well, we expressed a construct spanning only the core p53‐like OD (415–460) and a construct spanning a significant part of the extended C‐terminus (415–511) in Escherichia coli. A construct containing the OD and the entire C‐terminus could unfortunately not be expressed under all conditions screened. The [15N, 1H]‐TROSY spectrum of the core OD consisting of the predicted β‐strand and the α‐helix shows a chemical shift dispersion reminiscent of a well folded domain, indicating that the core domain alone is sufficient to fold into a stable structure. Comparison with the [15N, 1H]‐TROSY spectrum of the construct spanning amino acids 415–511 reveals that virtually all additional signals resonate in the 1H dimension between 7.7 and 8.6 ppm, indicating that they do not form a stable structure. In addition, the amide peaks of the core OD show virtually the same chemical shifts as in the isolated case, demonstrating that the core domain does not interact with amino acids located in this C‐terminal extension (Supporting Information Fig. S1). These observations also predict that the OD does not possess a second α‐helix as present in the TDs of p63, p73 and the p53 species from zebra fish.22 This interpretation was supported by the analysis of the backbone chemical shifts of amino acids 415–489 of Isoform 1 which indicated the existence of one β‐strand and one helix within the core of the domain (418–446) and no stable additional C‐terminal secondary structure elements (Supporting Information Figs. S2 and S3). Analogous, the presence of additional N‐terminal structural elements as present in the OD of Dmp53 was excluded by the observation of only additional random coil chemical shift values in an N‐terminally extended construct (405–489) of Isoform 1 and no changes in the chemical shifts of the residues in the core domain (Supporting Information Fig. S4).
Figure 3.
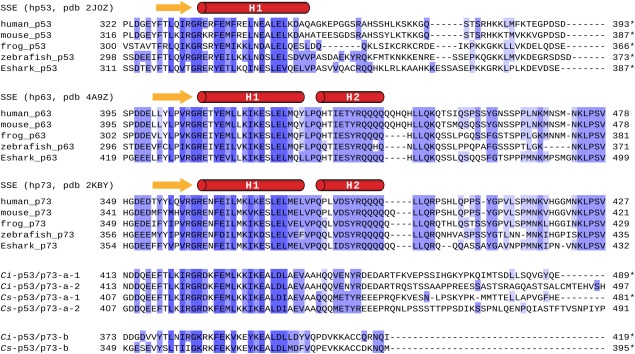
Alignment of protein sequences corresponding to the tetramerization domain of p53/p63/p73 proteins from vertebrate species and p53/p73‐like proteins from C.int. and C.sav. Representative vertebrate species Xenopus tropicalis (frog), Danio rerio (zebrafish), and Callorhynchus milii (elephant shark; Eshark) were selected in addition to human and mouse. Protein sequences are grouped by homology. An asterisk indicates the C‐terminal end of the natural protein sequence. Secondary structure elements are depicted above the sequences. Residues in dark blue fields show the highest, amino acids in light blue the lowest degree of homology.
The core tetramerization domain of C.int. p53/p73‐a Isoform 1 and 2 is capable to support a second α‐helix
The observation that no second α‐helix exists within the TD of C.int. p53/p73‐a was surprising based on sequence alignments with the TDs of p63 and p73 which both contain a second helix. Residues within the first helix that interact with the second helix in p63 and p73 are well conserved in C.int. p53/p73‐a (Fig. 3). In addition, the Tyr‐Arg dipeptide within the second helix of p63/p73 that is important to anchor this helix on the core domain,17, 18 is conserved in C.int. and in C.sav. p53/p73‐a. We wanted to investigate whether the C.int. p53/p73‐a core domain is in principle capable of supporting the formation of a second helix and if so which residues are crucial for stabilizing such a potential helix. We therefore created a chimeric sequence consisting of the C.int. p53/p73‐a core domain (415–444) and the second helix of human p73 (381–404). The corresponding [15N, 1H]‐TROSY spectrum is presented in Figure 4(a) as an overlay with the spectrum of the wild type reference. The overlay shows significant chemical shift differences between both spectra for almost all residues, including those from the β‐sheet. This result revealed that the core OD interacts in the chimeric construct with the sequence of the second helix from human p73. For further analysis, the backbone chemical shifts (CA, CB, HN, and N) of the chimeric construct were assigned and used as input for a secondary structure prediction with the program TALOS+.23 The section that corresponds to the second helix in the TD of human p73 was indeed predicted to adopt an α‐helical conformation [Fig. 4(b,c)]. Compared to the wild type protein the first helix of the C.int. p53/p73‐a core domain was predicted to be three residues shorter at its C‐terminus. This result suggests that the first helix has to adopt to the same length as in human p73 to allow for a proper positioning of the interacting residues within the core and the second helix.
Figure 4.
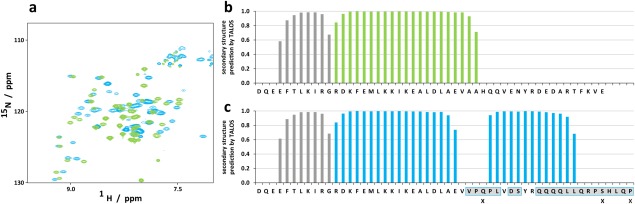
The chimeric protein consisting of the core domain of C.int. p53/p73‐a Isoform 1 and the second helix of human p73 forms a second helix. (a) Comparison of [15N, 1H]‐TROSY spectra of wild type C.int. p53/p73‐a Isoform 1 aa 415–489 (green) and the chimeric construct encompassing aa 415–444 of the Ciona protein and aa 381–404 from human TAp73α (blue). (b) Prediction of secondary structure by TALOS+ for the wild type construct and (c) for the chimeric construct. Bars colored in gray represent predictions for β‐strands, those depicted in the color designated to the respective construct represent α‐helical conformation. Residues for which no reliable prediction was obtained are labeled with an X.
Since the C.int. p53/p73‐a core domain is capable of supporting the formation of a second helix, we wanted to identify the crucial differences between p73 and C.int. p53/p73‐a. In all structure determinations of tetramerization domains that contain this second helix so far, a Tyr‐Arg dipeptide was found to form important contacts between the second and the first helix of two monomers. As mentioned above, this dipeptide is conserved in C.int. p53/p73‐a and in p73. To investigate its importance, we mutated the dipeptide to Ala‐Ala in our chimeric construct. The resonances of the core domain of this mutated chimeric form shifted back toward their corresponding positions in the wild type C.int. p53/p73‐a OD, indicating that the interaction between the core domain and the region of the second helix is diminished (Supporting Information Figs. S5 and S6).
The linker region between Helix 1 and Helix 2 is crucial for stabilizing the second helix
The combination of these results suggests that the Tyr‐Arg motif is crucial for anchoring the second helix on the core domain, but not sufficient to form a stable helix. We reasoned that the transition from the first to the second helix might be crucial as well. p73 contains in this transition segment the sequence Val‐Pro‐Gln and p63 the sequence Leu‐Pro‐Gln. In both proteins Pro is the last residue with nonhelical Φ, Ψ angles immediately preceding the N‐terminus of the second helix. Pro residues in the N‐cap position can form a stabilizing capping motif when accompanied by three hydrophobic residues in position N′, N3, and N4.24 The sequence fingerprint hPXXhh of this so called N‐Pro‐box motif is conserved in p73.
In C.int. p53/p73‐a the sequence corresponding to the transition sequence is Ala‐Ala‐His. To investigate the importance of this transition segment, we exchanged the dipeptide Val‐Pro in the chimera to the C.int. p53/p73‐a sequence Ala‐Ala. NMR analysis indicated again a destabilization of the second helix and a shift toward the wild type chemical shifts, thus confirming the importance of the transition segment (Supporting Information Figs. S7 and S8).
Using the structure of the tetramerization domain of p73 as a reference, we analyzed which other amino acids in this transition segment contribute to the folding of the second helix. The formation of the interface results in a gain in buried surface area for five residues, which are located within a seven residues long section. Of these five residues, the last one (Val 386) is conserved in C.int. p53/p73‐a. This leads to the prediction that the OD of C.int. p53/p73‐a Isoform 1 does not form a second helix due to deviations from the motif L‐V‐P‐X‐X‐L (V‐A‐A‐H‐Q‐Q in C.int. p53/p73‐a) within the hinge region. To investigate the importance of these four amino acids, we introduced them singly or in combination into the C.int. p53/p73‐a wild type sequence (aa 415–464) and determined the extent of helical folding using NMR chemical shifts. From a total of 16 mutants 10 including the wild type construct could be expressed in E. coli while the remaining ones all showed severe aggregation (Supporting Information Fig. S9).
Only residues that are separated by more than six amino acids in the primary sequence from the mutation site were included in the chemical shift analysis to exclude perturbations caused by the mutation itself to be considered. The two combinations, [LAPHQL], [LVPHQQ] and the combination of all four amino acids [LVPHQL] showed the largest chemical shift differences. For these three constructs the chemical shifts for the CA, CB, HN and N nuclei were assigned and the secondary structure was predicted using the programs δ2D25 and TALOS+23 and compared to the secondary structure contents of the wild type protein as well as the C.int. p53/p73‐a / human p73 chimera (Fig. 5 and Supporting Information Fig. S10). Compared to the wild type protein the first helix is three residues shorter for all mutants, ending with Glu 443, the last residue before the hinge region. The two mutants LVPHQL and LVPHQQ showed the most and the second most intense chemical shift changes, respectively. For both mutants TALOS+ and δ2D25 predict a second helix, which extends to Ala 458, however, without reaching the predicted helical propensities of the C.int. p53/p73‐a / human p73 chimera. Like in human p63 and human p73 the proline probably forms an N‐terminal cap of the second helix. For the mutant showing the third strongest chemical shift differences, LAPHQL, the prediction of a second helix is weak. All mutants showing even smaller chemical shift differences were, therefore, not further analyzed.
Figure 5.
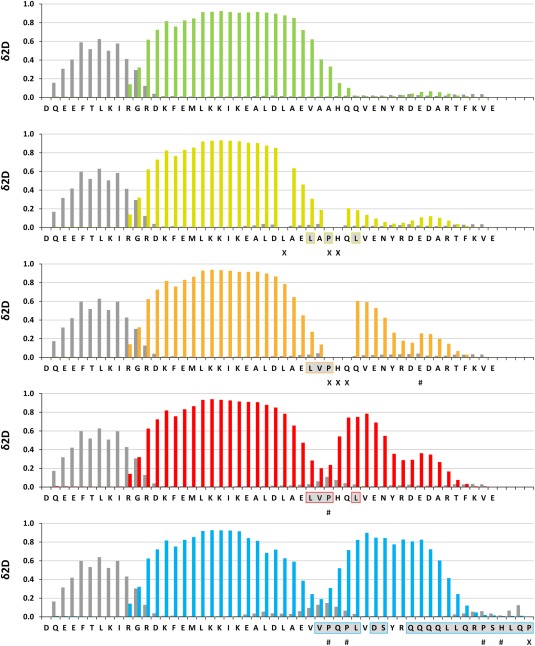
Propensities for β‐strand and α‐helical conformation determined for wild type C.int. p53/p73‐a Isoform 1 and several constructs with mutations in the hinge region. The secondary structure propensity was calculated using the δ2D method based on the chemical shifts of the CA, CB, HN, and N nuclei. Residues for which no reliable prediction was possible are labeled with # and unassigned residues are labeled with an X. Bars depicted in dark gray represent the propensity of the respective residue to adopt β‐strand conformation. The bars representing the propensity for an α‐helical conformation have the color that was assigned to the respective construct. Residues that differ from the C.int. wild type sequence are underlined in light gray and boxed in the color assigned to the respective mutant. The first bar diagram shows the results for the wild type sequence and the last one the results for the chimeric protein consisting of the C.int. p53/p73‐a Isoform 1 core domain and the second helix of human p73.
To evaluate if the second helix that forms has a similar orientation as in p73, we mutated again the Tyr‐Arg motif within the (LVPHQL) mutant that showed the strongest indication for the formation of a second helix. As expected, the chemical shifts became far more similar to the chemical shifts in the wild type protein, suggesting that the interaction of the core domain with the C‐terminus gets significantly destabilized (Supporting Information Fig. S11).
Determination of the gain in stability of the second helix by measurements of heteronuclear NOEs
As an independent measurement to investigate the formation of secondary structure elements, we measured 15N‐{1H}‐heteronuclear NOEs (hetNOEs) for the wild type and the three hinge region mutants LAPHQL, LVPHQQ and LVPHQL [Fig. 6(a)]. For the section, spanning the β‐strand and the first helix, hetNOEs between 0.6 and 0.8 were determined for all constructs, indicative of stable secondary structure elements. The hetNOE values for residues residing within the section corresponding to the second helix were on the contrary highly affected by substitutions within the hinge region. The analysis revealed a gain in rigidity for all three mutants with respect to the wild type, following the trend seen in the helical propensity prediction from chemical shifts with the LVPHQL mutant showing the highest rigidity.
Figure 6.
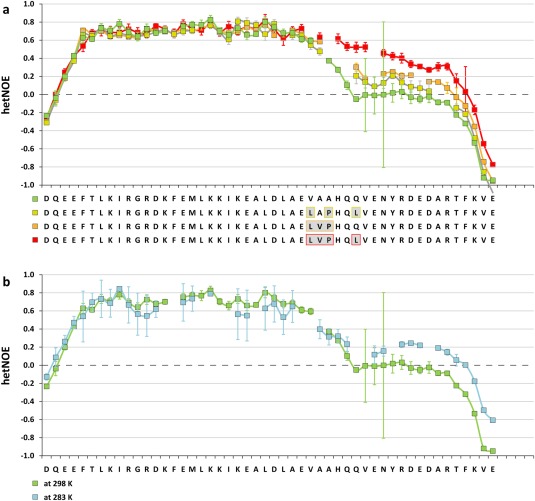
Measurement of heteronuclear NOEs confirm the stabilization of a second helix. (a) 15N‐{1H}‐heteronuclear nuclear Overhauser effects (hetNOEs) determined at 25°C for wild type and selected hinge region mutants of C.int. p53/p73‐a Isoform 1 aa 415–464. Residues that differ from the C.int. wild type sequence, are underlined in light gray and boxed in the color assigned to the respective mutant. (b) hetNOEs determined for wild type protein at 25°C and 10°C.
A second α‐helix can be stabilized in wild type C.int. p53/p73‐a at lower temperature
The mutational analysis described above indicated that some elements that can stabilize the second helix such as the Tyr‐Arg dipeptide are present but other crucial residues in the hinge region are missing. This observation suggests that lowering the temperature might stabilize this helix. To investigate this hypothesis, we determined 15N‐{1H}‐heteronuclear NOE values at 10°C. Figure 6(b) compares the hetNOE values measured at 10 and at 25°C. The data show an increase in rigidity within the C‐terminus that is consistent with a partial formation of a second helix.
Structure determination of the tetramerization domain of C.int. p53/p73‐b
As mentioned above, C.int. expresses a second p53/p73‐like protein. The protein p53/p73‐b is the result of a relatively recent gene duplication event, which happened independently of the gene duplications during the evolution of p53‐like proteins in vertebrates. This duplication is specific to the Ciona lineage and occurred after the divergence from the last common ancestor shared by Ciona species and vertebrates. Throughout the full length protein sequences p53/p73‐a and p53/p73‐b share 50% amino acid identity with maxima within the DBD (65% identity) and the OD (75% identity).
Analysis of the sequence revealed that p53/p73‐b lacks the important Tyr‐Arg dipeptide in the sequence stretch that would correspond to a second helix, however, contains a Pro residue in the segment between the first and the potential second helix. Analysis of the backbone chemical shifts of C.int. p53/p73‐b suggested that a second helix indeed exists despite the lack of the Tyr‐Arg motif. Since the entire sequence of this second helix lacks a significant degree of conservation relative to the vertebrate sequences, we decided to determine the structure of the TD (amino acids 374–419); the structure statistics are listed in Supporting Information Table 1 and the intermonomer NOEs that restrain the structure are depicted in Supporting Information Figure S12.
Despite the lack of sequence conservation in the second helix, the structure of the TD of C.int. p53/p73‐b shows great similarity to the TDs of human p63 and p73. Relative to the members of the mammalian p53 protein family, the conformation of the TD of C.int. p53/p73‐b is situated between p53 on the one hand and p63/p73 on the other hand. The angle at which the two dimers within the tetramer pack against each other is 82° in p53, 64° in p73 (or p63) and 74° in C.int. p53/p73‐b (Supporting Information Fig. S13). In all structures of members of the deuterostome p53 family that contain a second helix (p63, p73, Danio rerio p53) the Tyr‐Arg motif plays an essential role to anchor the second helix on the core OD. The only exception known so far is C.int. p53/p73‐b.
In mammalian p73 (and p63) the Tyr residue, from the characteristic Tyr‐Arg motif, is centered in a local hydrophobic pocket and contacts the aliphatic parts of the side chains of two glutamic acid residues, Glu 373 and Glu 376 (Glu 419 and Glu 422 in p63), as well as the residues Leu 377 and Leu 380 (Leu 423 and Tyr 426 in p63), while the guanidinium group of the Arg side chain forms a salt bridge with the carboxylate of Glu 373 (Glu 419 in p63) of the first helix.17, 26 The overlay of the TDs of human p73 and C.int. p53/p73‐b reveals that in the Ciona protein the second helix is positioned closer to the first helix and shifted by about 41° with respect to the arrangement in p73 (Fig. 7). In combination with the difference in the angle at which the primary dimers pack onto each other this causes the second helix in C.int. p53/p73‐b to pack onto its interacting first helix at a close to orthogonal angle of 86°, which is 35° less than in p73. In Danio rerio p53 the respective angle measures 73° giving C.int. p53/p73‐b an intermediate position. The closer proximity of the interacting helices in C.int. p53/p73‐b is caused by amino acids with smaller side chains on the second helix. The bulky side chains of Tyr and Arg are replaced by two Cys residues (Cys 413 and Cys 414), of which Cys 414, however, is not involved in binding the first helix (Supporting Information Fig. S14). Important contacts between both helices include also a Tyr residue which in C.int. p53/p73‐b, however, is provided by the first helix (Tyr 404). This side chain interacts with a hydrophobic pocket created by Val 409, Ala 412, Cys 413, and the aliphatic part of the side chain of Arg 416 of the second helix (Supporting Information Fig. S15). Interestingly this Tyr residue of the first helix is not only conserved in the respective protein from C.sav. (Phe instead of Tyr) but in most vertebrate p63 proteins as well where it is located two amino acids N‐terminal to the Pro residue of the hinge region.
Figure 7.
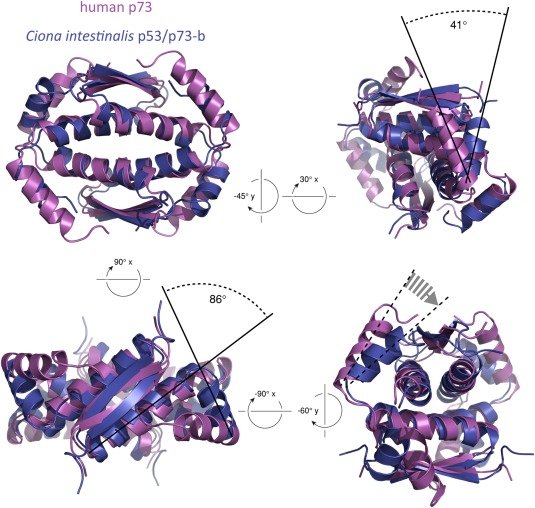
Overlay of the tetramerization domains of human p73 (pdb entry 2KBY) and C.int. p53/p73‐b (pdb entry 2MW4). The structures were aligned with respect to the β‐sheets and the first helices.
The second helix is further anchored on the core tetramerization domain by a salt bridge between Lys 410 and Glu 397. Interestingly, the position of this glutamic acid side chain corresponds to the Glu residue in p63 and p73 that is involved in the salt bridge with the important Arg residue of the Tyr‐Arg dipeptide. The Lys residue, however, precedes the position that would correspond to the Arg residue in p63 and p73 by four residues or one turn of the second helix. The N‐terminus of the second helix in the C.int. p53/p73‐b TD is not stabilized by a capping motif as the N‐cap position is occupied by Gln 406 while Pro 407 is already the first residue with alpha helical backbone conformation. Additional stabilization of the N‐terminus of the second helix might be provided by an intra‐chain salt bridge between Asp 408 and Lys 411.
The tetramerization domains of C.int. p53/p73‐a and p53/p73‐b do not form hetero oligomers
For human p63 and p73 it was shown that the TDs of the two proteins in vitro preferentially form hetero‐tetramers from homodimers,17, 18 and the dissociation of the kinetically more stable p73 homotetramer was identified as the rate‐limiting step.26 The formation of such mixed tetramers suggests functional cross talk, and therefore it was an important finding that human p53 does not form hetero‐tetramers with its family members. To investigate if C.int. p53/p73‐a and C.int. p53/p73‐b can form hetero‐oligomers, we mixed a 15N labeled sample of the TD of C.int. p53/p73‐b (aa 374–419) with a 10‐fold molar excess of unlabeled C.int. p53/p73‐a (aa 415–464). After incubation at 25°C for 2 days a [15N, 1H]‐TROSY experiment was recorded which showed no chemical shift changes with respect to a pure sample of the C.int. p53/p73‐b TD. To ensure that exchange is not inhibited by a high kinetic barrier and that thermodynamically stable hetero‐oligomers could form, the proteins were denatured with 6 M guanidium hydrochloride and subsequently refolded by a 50‐fold dilution with buffer. After restoring the original protein concentration the [15N, 1H]‐TROSY spectrum of the mixture still showed no chemical shift differences with respect to the reference sample, indicating that both TDs do not form hetero‐oligomers.
Many residues within the β‐strand and the first α‐helix are conserved between C.int. p53/p73‐a and ‐b (Supporting Information Fig. S16). To investigate if the second helix in C.int. p53/p73‐b prevents the formation of hetero‐oligomers, we performed the mixing experiments with the core ODs of C.int. p53/p73‐a (aa 415–450) and p53/p73‐b (aa 374–410), which lack the second helix. After denaturation and refolding the [15N, 1H]‐TROSY spectrum of C.int. p53/p73‐b again resembled that of the reference sample of pure C.int. p53/p73‐b aa 374–410, indicating that the core ODs do not form hetero‐tetramers, thus excluding functional cross talk via these domains. This result can be rationalized using the structure of C.int. p53/p73‐b. The side chains of Lys 391 and Glu 394 form a salt bridge across the tetramerization interface. In C.int. p53/p73‐a the corresponding residues are Met and Lys. Hetero‐tetramer formation based on homodimers would therefore result in the loss of four salt bridges and in addition the two directly opposing Lys side chains would result in charge repulsion.
Discussion
Knock out mouse studies of the vertebrate p53 family members have revealed that p63 and p73 have important functions in embryonic development.8, 9, 27, 28 Inactivating the p63 gene results in severe limb truncations, lack of a multilayered skin and other epithelial structures while p73 knock out mice suffer from hippocampal dysgenesis, hydrocephalus, chronic infections and inflammation, as well as abnormalities in pheromone sensory pathways. For p63 a second function was identified in oocytes where it serves as a quality control factor.5, 6 Detection of DNA double strand breaks results in the activation of p63 by phosphorylation and the induction of cell death.29 Most likely this monitoring function of the genetic quality in germ cells is the evolutionary oldest function of the entire family and their involvement in development as well as the tumor suppressor function arose later in evolution.30
So far only few studies have addressed the function of C.int. p53/p73‐a and C.int. p53/p73‐b. In agreement with the current hypothesis of the evolution of the p53 protein family, both proteins are expressed from maternal mRNA in eggs and are degraded during development.21 This expression profile suggests a function in monitoring the genetic quality in unfertilized eggs, similar to reported functions in vertebrates and in C. elegans.31 However, no detailed investigations of the effect of DNA damage on the activation state of both proteins has been conducted. In addition to the proposed quality control function, morpholino knock down studies have shown that both C.int. p53/p73‐a and C.int. p53/p73‐b are involved in developmental processes.21 Knock down led in the gastrula stage to inhibition of gastrulation movement and of closing of the blastopore. In addition, during the neurula and tailbud stages, neurulation and elongation of the tail was impaired.
Interestingly, mice knock out studies targeting only the TA isoform of p73 showed significant impairment of the development of the blastocyst.28, 32 Detailed analysis suggested that this effect is due to problems in proper formation of the spindle assembly complex resulting in genomic instability associated with enhanced aneuploidy. While some similarity between the developmental functions of C.int. p53/p73‐a and C.int. p53/p73‐b and p73 seem to exist, the underlying molecular processes have not been investigated in great detail so far. However, it seems likely, that C.int. p53/p73‐a and C.int. p53/p73‐b combine functions of both, mammalian p63 and p73.
So far the classification of a family member to be closer related to p63/p73 than to p53 was mainly based on the existence of a SAM domain.12 In principle, Isoform 2 of C.int. p53/p73‐a contains a C‐terminal extension that is long enough to harbor a folded domain such as a SAM domain. Our investigations, however, could not find any evidence for the existence of a folded domain. A second structural criterion that distinguishes p63/p73 from p53 is the existence of a second helix in the TD.17, 18, 26 A characteristic sequence that so far was used as an indicator for the existence of this second helix is the Tyr‐Arg motif. The structural investigation of the C.int. p53/p73‐a OD has, however, revealed that the presence of this dipeptide is not always correlated with the formation of a second helix. Equally surprising, we found a second helix in the TD of C.int. p53/p73‐b that lacks the Tyr‐Arg dipeptide. Our detailed mutational analysis has suggested that the most important determinant for the formation of a second helix is the hinge region between both helices. Of particular importance is a Pro residue in the N‐cap position of the second helix that is directly preceded by two hydrophobic residues. These results are in agreement with a bioinformatics study of the evolution of the p63/p73‐like TDs.22
We detected a more rigid behavior of the sequence C‐terminal to the OD of C.int. p53/p73‐a by lowering the temperature from 25 to 10°C which is consistent with a higher helical propensity. However, if the formation of a second helix at lower temperature would have any physiological relevance is currently not known. In addition to lower temperatures, the formation of a second helix might also be promoted by posttranslational modifications such as phosphorylation and acetylation.30
In conclusion, given the fact that C.int. p53/p73‐b likely is the product of a retro‐transposition event, it can be predicted that the common precursor of the tunicates and vertebrates contained a second helix which was lost in C.int. p53/p73‐a during evolution.
Materials and Methods
Site‐directed mutagenesis
Site‐directed mutagenesis was carried out using either PfuTurbo or PfuUltra high fidelity DNA polymerase (Stratagene). After the PCR reaction the methylated template DNA was specifically digested by DpnI during incubation for at least 3 h at 37°C. Afterward, the reaction mixture was directly used to transform E. coli DH5α cells. Mutations were verified by DNA sequencing.
Heterologous expression in E. coli
All constructs used in this study were cloned into the vector pBH4 (gift from Wendell Lim Laboratory, San Francisco, CA) encoding for an N‐terminal tobacco etch virus protease (TEV)‐cleavable His6 tag. Protein expression was carried out in E. coli BL21 DE3 cells harboring a co‐plasmid for expression of tRNAs for rare codons. Bacteria grown in unlabeled media were induced at an OD600 of 1.0–1.2, bacteria grown in standard M9 labeled media at an OD600 of 0.7–0.8 and the temperature was lowered to 20–25°C. After overnight expression cells were harvested by centrifugation and resuspended in buffer containing 400 mM NaCl, 10 mM β‐mercaptoethanol, and 25 mM Tris pH 7.8. Cells were disrupted in the presence of DNAse I, RNAse A, and EDTA‐free protease inhibitor cocktail (Roche) by the addition of lysozyme and by sonication. Protein purification was achieved by immobilized metal ion chromatography (IMAC) using columns with Ni Sepharose 6 Fast Flow resin on an ÄKTApurifier FPLC system at 4°C. After washing with buffer containing 100 mM imidazole the protein was eluted with 500 mM imidazole. 3 mL fractions were collected, inspected by SDS PAGE and those containing the protein of interest were pooled and further purified by reverse IMAC after cleaving off the His‐tag with TEV protease (molar ratio of 1:25 and overnight cleavage during dialysis against buffer at 4°C). The flow through was collected, concentrated and applied to a Superdex75™ size exclusion chromatography column equilibrated and run in RE buffer (50 mM L‐arginine, 50 mM L‐glutamate, 1 mM DTT, pH 7.0) on an ÄKTApurifier FPLC system at 4°C.
NMR spectroscopy
NMR experiments were performed on Bruker Avance spectrometers equipped with cryogenic z‐axis gradient triple resonance probes at proton Larmor frequencies of 950, 900, 800, 700, or 600 MHz, and on a Bruker Avance 500 MHz spectrometer using a room‐temperature x‐, y‐, z‐gradient, triple resonance probe. Unless otherwise stated, all NMR spectra were recorded at 298 K. The backbone assignment was obtained from TROSY‐based33, 34 HNCACB and HNCO experiments. Side chain resonances were assigned from an H(C)CH‐TOCSY as well as 3D‐[15N, 1H]‐TROSY‐H(CCCO)NH‐TOCSY, 3D‐[15N, 1H]‐TROSY‐(H)C(CCO)NH‐TOCSY, and 3D‐13C‐separated NOESY experiments using UCSF Sparky35 3.114 as the assignment software package.
Steady‐state 15N‐{1H}‐nuclear Overhauser effects were determined using a TROSY‐based 2D pulse sequence36 where spectra with and without proton saturation (5s saturation) were recorded in an interleaved manner. The residue specific hetNOE was calculated based on the intensities of the respective peaks in both spectra. The peak heights were determined with Sparky. The errors result from the varying ratios of the peak heights and the S/N of the spectra.
Calculation of chemical shift perturbation
To compare chemical shifts of assigned backbone amide (N and HN) resonances of wild type sequences with those from truncated or mutated sequences the chemical shift perturbations were calculated from the values of the chemical shift differences according to
where R N = 0.2, and ΔHN and ΔN are the chemical shift differences for a residue specific assigned backbone resonance in the two respective HSQC spectra to be compared.
Assignment of intermonomer NOEs in the C.int. p53/p73‐b tetramerization domain
Two samples of C.int. p53/p73‐b tetramerization domain either 13C or 15N uniformly labeled were separately expressed and purified. After mixing of equimolar amounts the proteins were denatured with 6 M guanidinium hydrochloride and subsequently refolded by dilution with a 50‐fold volume of reducing RE buffer. The protein solution was reconcentrated and applied onto preparative gel filtration using a Superdex75™ 16/60 column, equilibrated and run in reducing RE buffer. Intersubunit distance constraints were obtained with 4D‐CT‐J‐Resolved 13C‐separated NOESY37 (in D2O, τ M = 100 ms), with 3D 15N‐edited/13C‐separated NOESY (in H2O, τ M = 110 ms) and with 3D 15N/13C‐separated NOESY (in H2O, τ M = 110 ms) experiments.
Calculation of the solution structure of the C.int. p53/p73‐b tetramerization domain
The structure of the C.int. p53/p73‐b tetramerization domain was calculated using the software package CYANA38 (CYANA 3.9 development version) and refined with ARIA/CNS. The chemical shifts and 3D NOESY peak lists from 3D NOESY‐[15N, 1H]‐HSQC (in H2O, τ M = 60 ms), 3D NOESY‐[13C, 1H]‐HSQC (aliphatic and aromatic, in H2O, τ M = 60 ms and τ M = 50 ms, respectively), as well as intermolecular 3D and 4D NOESYs of the mixed 13C and 15N labeled samples were used as input for automated NOESY cross peak assignment and calibration with CYANA. The chemical shift tolerances were set to 0.035 and 0.40 ppm for the protons and heavy atoms respectively. In addition to NOE data, hydrogen bond distances and dihedral angle restraints (based on TALOS+ predictions) and confirmed with NOESY assignments and initial structure calculations as well as symmetry (distance difference) restraints were included in the standard structure calculation with CYANA 3.9 (100 structures per iteration, 20,000 refinement steps). The final bundle of 20 best structures was used as input for optimization with CNS 1.1 using adapted ARIA 1.2 setup and protocols for refinement in explicit water.39, 40 The restraints were converted to ARIA/CNS format and included in this refinement stage. The standard settings and allhdg5.3 force field were used with OPLS nonbonded parameters. The final structure bundle was analyzed with Procheck 3.5.4.41
Supporting information
Supporting Information
The authors declare no conflict of interest.
References
- 1. Belyi VA, Ak P, Markert E, Wang H, Hu W, Puzio‐Kuter A, Levine AJ (2010) The origins and evolution of the p53 family of genes. Cold Spring Harb Perspect Biol 2:a001198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lane DP, Madhumalar A, Lee AP, Tay BH, Verma C, Brenner S, Venkatesh B (2011) Conservation of all three p53 family members and Mdm2 and Mdm4 in the cartilaginous fish. Cell Cycle 10:4272–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Botchkarev VA, Flores ER (2014) p53/p63/p73 in the epidermis in health and disease. Cold Spring Harb Perspect Med 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G (2011) The p53 family: guardians of maternal reproduction. Nat Rev 12:259–265. [DOI] [PubMed] [Google Scholar]
- 5. Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F (2006) p63 protects the female germ line during meiotic arrest. Nature 444:624–628. [DOI] [PubMed] [Google Scholar]
- 6. Deutsch GB, Zielonka EM, Coutandin D, Weber TA, Schafer B, Hannewald J, Luh LM, Durst FG, Ibrahim M, Hoffmann J, Niesen FH, Senturk A, Kunkel H, Brutschy B, Schleiff E, Knapp S, Acker‐Palmer A, Grez M, McKeon F, Dotsch V (2011) DNA damage in oocytes induces a switch of the quality control factor TAp63alpha from dimer to tetramer. Cell 144:566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F (1998) p63, a p53 homolog at 3q27‐29, encodes multiple products with transactivating, death‐inducing, and dominant‐negative activities. Mol Cell 2:305–316. [DOI] [PubMed] [Google Scholar]
- 8. Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F (1999) p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398:714–718. [DOI] [PubMed] [Google Scholar]
- 9. Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A (1999) p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398:708–713. [DOI] [PubMed] [Google Scholar]
- 10. Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias JM, Dumont X, Ferrara P, McKeon F, Caput D (1997) Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 90:809–819. [DOI] [PubMed] [Google Scholar]
- 11. Lane DP (1992) Cancer. p53, guardian of the genome. Nature 358:15–16. [DOI] [PubMed] [Google Scholar]
- 12. Chi SW, Ayed A, Arrowsmith CH (1999) Solution structure of a conserved C‐terminal domain of p73 with structural homology to the SAM domain. Embo J 18:4438–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Serber Z, Lai HC, Yang A, Ou HD, Sigal MS, Kelly AE, Darimont BD, Duijf PH, Van Bokhoven H, McKeon F, Dotsch V (2002) A C‐terminal inhibitory domain controls the activity of p63 by an intramolecular mechanism. Mol Cell Biol 22:8601–8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ou HD, Löhr F, Vogel V, Mäntele W, Dötsch V (2007) Structural evolution of C‐terminal domains in the p53 family. EMBO J 26:3463–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeffrey PD, Gorina S, Pavletich NP (1995) Crystal structure of the tetramerization domain of the p53 tumor suppressor at 1.7 angstroms. Science 267:1498–1502. [DOI] [PubMed] [Google Scholar]
- 16. Lee W, Harvey TS, Yin Y, Yau P, Litchfield D, Arrowsmith CH (1994) Solution structure of the tetrameric minimum transforming domain of p53. Nat Struct Biol 1:877–890. [DOI] [PubMed] [Google Scholar]
- 17. Coutandin D, Löhr F, Niesen F, Ikeya T, Weber T, Schäfer B, Bullock A, Yang A, Güntert P, Knapp S, McKeon F, Ou H, Dötsch V (2009) Conformational stability and activity of p73 require a second helix in the tetramerization domain. Cell Death Differ 16:1582–1589. [DOI] [PubMed] [Google Scholar]
- 18. Joerger AC, Rajagopalan S, Natan E, Veprintsev DB, Robinson CV, Fersht AR (2009) Structural evolution of p53, p63, and p73: implication for heterotetramer formation. Proc Natl Acad Sci U S A 106:17705–17710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein DM, Harafuji N, Hastings KE, Ho I, Hotta K, Huang W, Kawashima T, Lemaire P, Martinez D, Meinertzhagen IA, Necula S, Nonaka M, Putnam N, Rash S, Saiga H, Satake M, Terry A, Yamada L, Wang HG, Awazu S, Azumi K, Boore J, Branno M, Chin‐Bow S, DeSantis R, Doyle S, Francino P, Keys DN, Haga S, Hayashi H, Hino K, Imai KS, Inaba K, Kano S, Kobayashi K, Kobayashi M, Lee BI, Makabe KW, Manohar C, Matassi G, Medina M, Mochizuki Y, Mount S, Morishita T, Miura S, Nakayama A, Nishizaka S, Nomoto H, Ohta F, Oishi K, Rigoutsos I, Sano M, Sasaki A, Sasakura Y, Shoguchi E, Shin‐i T, Spagnuolo A, Stainier D, Suzuki MM, Tassy O, Takatori N, Tokuoka M, Yagi K, Yoshizaki F, Wada S, Zhang C, Hyatt PD, Larimer F, Detter C, Doggett N, Glavina T, Hawkins T, Richardson P, Lucas S, Kohara Y, Levine M, Satoh N, Rokhsar DS (2002) The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science 298:2157–2167. [DOI] [PubMed] [Google Scholar]
- 20. Nedelcu AM, Tan C (2007) Early diversification and complex evolutionary history of the p53 tumor suppressor gene family. Dev Genes Evol 217:801–806. [DOI] [PubMed] [Google Scholar]
- 21. Noda T (2011) The maternal genes Ci‐p53/p73‐a and Ci‐p53/p73‐b regulate zygotic ZicL expression and notochord differentiation in Ciona intestinalis embryos. Dev Biol 360:216–229. [DOI] [PubMed] [Google Scholar]
- 22. Joerger AC, Wilcken R, Andreeva A (2014) Tracing the evolution of the p53 tetramerization domain. Structure 22:1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viguera AR, Serrano L (1999) Stable proline box motif at the N‐terminal end of alpha‐helices. Protein Sci 8:1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camilloni C, De Simone A, Vranken WF, Vendruscolo M (2012) Determination of secondary structure populations in disordered states of proteins using nuclear magnetic resonance chemical shifts. Biochemistry 51:2224–2231. [DOI] [PubMed] [Google Scholar]
- 26. Natan E, Joerger AC (2012) Structure and kinetic stability of the p63 tetramerization domain. J Mol Biol 415:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, Vagner C, Bonnet H, Dikkes P, Sharpe A, McKeon F, Caput D (2000) p73‐deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404:99–103. [DOI] [PubMed] [Google Scholar]
- 28. Tomasini R, Tsuchihara K, Wilhelm M, Fujitani M, Rufini A, Cheung CC, Khan F, Itie‐Youten A, Wakeham A, Tsao MS, Iovanna JL, Squire J, Jurisica I, Kaplan D, Melino G, Jurisicova A, Mak TW (2008) TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev 22:2677–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bolcun‐Filas E, Rinaldi VD, White ME, Schimenti JC (2014) Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science 343:533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coutandin D, Der Ou H, Lohr F, Dotsch V (2010) Tracing the protectors path from the germ line to the genome. Proc Natl Acad Sci U S A 107:15318–15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Derry WB, Putzke AP, Rothman JH (2001) Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294:591–595. [DOI] [PubMed] [Google Scholar]
- 32. Tomasini R, Tsuchihara K, Tsuda C, Lau SK, Wilhelm M, Ruffini A, Tsao MS, Iovanna JL, Jurisicova A, Melino G, Mak TW (2009) TAp73 regulates the spindle assembly checkpoint by modulating BubR1 activity. Proc Natl Acad Sci U S A 106:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salzmann M, Wider G, Pervushin K, Senn H, Wuthrich K (1999) TROSY‐type triple‐resonance experiments for sequential NMR assignments of large proteins. J Am Chem Soc 121:844–848. [Google Scholar]
- 34. Pervushin K, Riek R, Wider G, Wuthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole‐dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci U S A 94:12366–12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goddard TD, Kneller DG (2008) SPARKY 3. San Francisco: University of California. [Google Scholar]
- 36. Ferrage F, Piserchio A, Cowburn DGhose R (2008) On the measurement of 15N‐{1H} nuclear overhauser effects. J Magn Reson 192:302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Melacini G (2000) Separation of intra‐ and intermolecular NOEs through simultaneous editing and J‐compensated filtering: a 4D quadrature‐free constant‐time J‐resolved approach. J Am Chem Soc 122:9735–9738. [Google Scholar]
- 38. Guntert P (2004) Automated NMR structure calculation with CYANA. Methods Mol Biol 278:353–378. [DOI] [PubMed] [Google Scholar]
- 39. Linge JP, Nilges M (1999) Influence of non‐bonded parameters on the quality of NMR structures: a new force field for NMR structure calculation. J Biomol NMR 13:51–59. [DOI] [PubMed] [Google Scholar]
- 40. Linge JP, Williams MA, Spronk CA, Bonvin AM, Nilges M (2003) Refinement of protein structures in explicit solvent. Proteins 50:496–506. [DOI] [PubMed] [Google Scholar]
- 41. Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM (1996) AQUA and PROCHECK‐NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR 8:477–486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


