Abstract
Background:
Formation of biofilm and bacterial colonization within the endotracheal tube (ETT) are significant sources of airway contamination and play a role in the development of ventilator-associated pneumonia (VAP). This study was conducted to examine the effect of nebulized eucalyptus (NE) on bacterial colonization of ETT biofilm.
Materials and Methods:
We performed a randomized clinical trial in three intensive care units (ICUs) of an educational hospital. Seventy intubated patients were selected and randomly divided into intervention (n = 35) and control (n = 35) groups. The intervention group received 4 ml (5%) of eucalyptus in 6 ml normal saline every 8 h. The placebo group received only 10 ml of normal saline in the same way. On extubation, the interior of the tube was immediately sampled using a sterile swab for standard microbiological analysis. Chi-square and Fisher's exact tests were used for statistical analysis in SPSS. P values less than 0.05 were considered statistically significant.
Results:
In both samples, Klebsiella pneumoniae and Acinetobacter baumannii were the most frequently isolated bacteria. In the control group, heavy colonization was greater than in the intervention group (P = 0.002). The frequency of isolation of K. pneumoniae in the intervention group was lower than in the control group (P < 0.001). However, there was no difference between the two groups in other isolated bacteria.
Conclusions:
NE can reduce microbial contamination of the endotracheal tube biofilm in ventilated patients. Moreover, K. pneumoniae was the most sensitive to NE.
Keywords: Biofilms, endotracheal intubation, endotracheal tube, eucalyptus, Iran, nebulizers
INTRODUCTION
Endotracheal intubation is one of the standard and safe methods to protect the airway against aspiration. It facilitates secretion and improves pulmonary ventilation.[1] Despite the extensive use and many benefits of intubation, it has side effects and risks. In the study by Craven and Hjalmarson on intubation in patients hospitalized at intensive care units (ICUs), the incidence of these side effects has been reported at 28-54%.[2] One of the main disadvantages of the endotracheal tube (ETT) is the increased risk of infection and the entry of microorganisms into the inferior airways, which places the patients at risk of ventilator-associated pneumonia (VAP). Colonization of microorganisms and biofilm or microbial plaque formation inside the tracheal tube have been described as permanent sources of infection for the inferior airway. Following the damage caused by intubation and due to the host's immune system disorders, within 12 h after intubation, bacteria enter into the ETT and cause bacterial colonization and biofilm formation in the ETT.[3] In addition, biofilms can increase airway resistance, decrease tidal volume, increase respiratory activity, delay detachment from the ventilator, and even cause airway obstruction.[4,5]
Biofilm is a collection of bacterial cells that is considered as a permanent source of infection. It has been claimed that more than 60% of human infections are caused by biofilms.[6] Biofilm acts as a physical barrier which prevents the absorption of antibiotics and elimination of microbes. It is difficult to destroy biofilms using antimicrobial substances, and in laboratory tests, biofilms show high resistance to antibiotics.[7] In order to prevent the formation of biofilms in the ETT, several solutions have been proposed. Use of methylene blue solution as spray,[8] mucus wipes (mucus shaver),[4] or silver-coated, chlorhexidine-covered, and silver carbonate-covered ETTs has significantly reduced colonization in the ETT.[9] In 1994, the Center for Disease Control (CDC) emphasized the use of nebulizers for medication administration in the form of vapor inhaled into the lungs.[10] Inhalation is an effective method of drug delivery, which localizes the drug in the target organ and, thus, reduces the dosage and side effects.[11] ICU nurses are responsible for the care of patients using nebulizers.[12]
Many studies have been conducted on the use of inhaled antibiotics in the prevention and treatment of VAP.[13,14,15,16] The results of the study by Badia et al. in this respect show that the use of antibiotics through inhalation causes a significant reduction in the incidence of pneumonia.[17] The use of a nebulizer is one of the recommended methods for the delivery of antibiotics and other antimicrobial extracts to the inferior airways of ventilated patients.[18] A medication used in complementary medicine in the form of an inhaler is eucalyptus extract. Eucalyptus extract has antibacterial, antiviral, and antifungal properties.[19] It is effective against colds, influenza, and other respiratory infections, and runny nose, and sinus. Inhaling several drops of eucalyptus extract through a humidifier is safe and has been used since antiquity.[20] Most gram-positive and gram-negative bacteria have a tendency to form biofilms. These bacteria include Staphylococcus aureus, Staphylococcus epidermidis, viridans streptococci, Escherichia coli, Enterococcus, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa.[21] The antibacterial and antiviral effects of eucalyptus extract on the causes of respiratory infections, such as Streptococcus pneumoniae and Staphylococcus haemophilus, have been approved.[22] In compliance with the administration guidelines of this medication, incidence of serious side effects has not been reported.[23]
The increased prevalence and complexity of multidrug-resistant organisms in hospital-acquired infections has created incentives for the use of new therapies such as inhaled antibiotics, which are considered for the prevention and treatment of pneumonia. Eucalyptus is commonly used in many countries and is supplied in different forms. Due to the antimicrobial properties of eucalyptus, its safety,[23] and its topical use through a nebulizer, this clinical trial was conducted to assess the impact of eucalyptus incense on microbial plaque of ETT in patients undergoing mechanical ventilation.
MATERIALS AND METHODS
This study was a single-blind randomized clinical trial. The aim of the study was to investigate the effect of eucalyptus incense on ETT biofilms in 70 patients under mechanical ventilation. All patients who were hospitalized in the ICUs of a training hospital from August until November 2014 and met the inclusion criteria were selected via purposive sampling and assigned to two groups. Written informed consents were obtained from the patients’ legal guardians. They had the right to withdraw from the study at any time without any change in their treatment. The hospital has three ICUs (trauma, internal, and surgical) with 63 beds, and is located in Central Iran as a referral center. This project was approved by the Research Council and the Ethics Committee of Arak University of Medical Sciences, Iran, and was recorded in the clinical trial center in Iran with the code IRCT2014060217955N1.
The inclusion criteria were the following: Being in the age range of 18-65 years; having a tracheal tube through the mouth; being under ventilators for minimum of 3 days and maximum of 14 days; and lack of pneumonia, sepsis, pulmonary embolism, atelectasis, and inflammatory disease of the gastrointestinal tract, liver, and bile ducts, and lack of sensitivity to herbal compounds. If for any reason the patients were transferred, had sudden changes in hemodynamic status, or showed complications such as hives, itching, and rash that might be due to sensitivity to eucalyptus, they were excluded from the study. After selection, the patients were randomly assigned to intervention (35 patients) or control (35 patients) group. All patients received standard care and treatment based on their illness and doctor's prescription. According to the anesthesiologist's prescription, the patients in the intervention group received 4 ml of eucalyptus solution 5% and 6 ml of normal saline 0.9% using a standard nebulizer for 20-30 min every 8 h until ETT removal. To prevent rebreathing of exhaled air, filters were placed in the expiratory circuit. Patients in the control group received 10 ml of normal saline incense through the same method. Due to the number of wards and nurses who provided care for the patients, a training program was conducted for all nurses and a written protocol were used to equalize the study method. Considering the differences between patients and ICUs, the same ratio of patients was selected from each unit.
In both groups, if the intervention lasted more than 1 week, the nebulizer tank and its fittings were cleaned using 70% alcohol. After washing, all the pieces were dried and placed in sterile bags to prevent contamination. The intervention was completed by the removal of ETT. Immediately after the removal of ETT, it was placed in a sterile cover to prevent contamination before sampling. From the ETT of patients who were intubated for a minimum of 72 h and a maximum of 14 days, a culture was taken from 5 cm into the inner and inferior part of the ETT using a sterile swab. All samples were prepared by one person (nurse) through one method. The samples were tested in the microbiology laboratory of the hospital using transitional environments to determine the type and number of aerobic microorganisms. This study was a single-blind trial; thus, the laboratory technician was unaware of the patients’ placement in the groups.
Demographic information (age and gender) and basic information (cause of admission, invasive interventions, history of chronic disease, the cause of tracheal tube removal, the number of antibiotics, and intubation time) were recorded in a checklist at the beginning of the study. Type and number of microbes in the tracheal tube were the main criterion of the study and were compared between the two groups. Information collected using the questionnaire and the microbial cultures of the ETT biofilm was entered into SPSS software (version 20; SPSS Inc., Chicago, IL, USA) after coding. Descriptive statistics and calculation of central and dispersion indices were used. Therefore, chi-square test or Fisher's exact test (for qualitative variables) and independent t-test (for quantitative variables) were used. A significance level of P < 0.05 was considered for all the tests.
RESULTS
A total of 90 patients were enrolled in the study and 20 patients were excluded. Of the 47 patients who were in the intervention group, 12 patients were excluded for various reasons. Thus, five patients were excluded due to lack of observance of the protocol, one patient due to the prolonged duration of intubation for more than 14 days, two patients due to contamination of the ETT because of incorrect transference to the sterile cover, two patients due to positive blood culture, and two patients were excluded due to the risk of side effects. Of the 43 patients in the control group, 8 patients were excluded for various reasons. Of these, four patients were excluded due to lack of observance of the protocol, two patients due to the prolonged duration of intubation for more than 14 days, one patient due to contamination of the ETT because of incorrect transference to the sterile cover, and one patient was excluded due to positive blood culture. Therefore, 70 patients (35 in each group) completed the study. Since the allocation of the subjects to groups was performed randomly, the two groups were compared in terms of demographic and baseline characteristics. The results showed that there was no significant difference between the two groups in terms of gender (P = 0.78), age (P = 0.16), and the cause of hospitalization (P = 0.31). Moreover, no significant differences were observed between the groups in terms of invasive interventions [central venous catheter (P = 0.30), chest tube (P = 0.23), abdominal drainage (P = 0.19), brain drainage (P = 0.31), and catheter for hemodialysis (P > 0.99)]. There was also no significant difference between the two groups in terms of cause of tracheal tube removal (P = 0.47), duration of intubation (P = 0.50), and antibiotics use (P = 0.4). The two groups differed only in terms of some underlying diseases. In the intervention group, hypertension (P = 0.04) and in the control group, cancer (P = 0.04) were more common. However, there were no differences between the groups in terms of diabetes (P > 0.99) and chronic obstructive pulmonary disease (P > 0.99). In each group, 11.4% of the patients had diabetes and 5.7% of the patients were suffering from chronic obstructive pulmonary disease. To investigate the difference between the two groups in terms of qualitative variables, such as gender, cause of hospitalization in ICU, invasive interventions, cause of ETT removal, and type of underlying disease, chi-square and Fisher's exact tests (considering that in the chi-square test, more than 20% of the tables had a frequency of less than 5) were used. To investigate the difference between the two groups regarding quantitative variables, such as age, duration of intubation, and the amount of antibiotics, independent t-test was used.
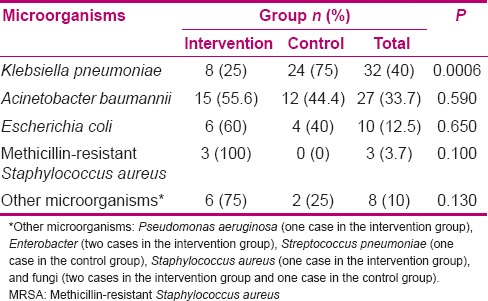
A total of 66 cases (94.3%) of contamination were found in 70 cultures. In the intervention group, four (5.7%) of the samples were negative [Table 1]. On the contrary, all the samples were positive in the control group. Most positive cultures were infected with a single microorganism (78.8%) and only in 14 positive cultures (21.2%), two types of microorganisms were found (7 in each group). The frequency of contaminating microorganisms in patients in the intervention group was less than in the control group, but this difference was not statistically significant (P = 0.11) [Table 2]. The most common microorganisms in both groups were K. pneumoniae (40%) and Acinetobacter baumannii (33.7%) [Table 2]. The frequency of K. pneumoniae was significantly lower in the intervention group than in the control group (P < 0.0001). Nevertheless, there was no significant difference between the groups in terms of other microorganisms. There was no significant relationship between the duration of intubation and severity of microbial contamination in the intervention group (P = 0.56) and the control group (P = 0.33). An effective factor in the formation of bacterial biofilms is the type and number of intravenous antibiotics which could affect the study results. Moreover, the type of underlying disease, days of intubation, and the number and the method of chips suction may affect the formation of the microbial biofilms. Therefore, with the random assignment of patients in both groups and comparison of the groups, it was attempted to control this affect.
Table 1.
Comparison of density of endotracheal tube colonization of the intervention and control groups
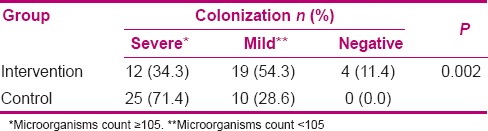
Table 2.
Comparison of frequency of contaminant microorganisms in the intervention and control groups
DISCUSSION
This study aimed to determine the effect of eucalyptus incense on the ETT biofilm microbes in patients under mechanical ventilation. The results showed that all the samples taken from the ETT biofilm of the control group patients had microbial contamination. However, in four cases of the intervention group, no pathogen was observed. In other words, eucalyptus incense can reduce microbial contamination of the ETT biofilms. Severe contamination was far less in the intervention group than in the control group.
To the knowledge of the researchers, only one study was conducted on the effects of eucalyptus as an aerosol in patients undergoing mechanical ventilation and hospitalized in ICUs. In a study conducted without a control group on only 12 patients, it was concluded that eucalyptus incense can reduce colonization of common nosocomial pathogens and the incidence of pneumonia related to mechanical ventilation.[24] The antimicrobial effects of different eucalyptus species on influenza virus,[25] local infections, and various species of bacteria, including K. pneumoniae, Pseudomonas, Proteus, E. coli, St. aureus, and methicillin-resistant St. aureus, in in vitro and in vivo conditions have been approved.[26] The antimicrobial effects of eucalyptus are attributed to the 1 and 8 cineol.[20] The essential oil used in this study consisted of 80% of 1 and 8 cineole.
In several studies, inhaled antibiotics were used to combat biofilms and microbial contamination of the ETT, and showed positive results like in the present study.[13,14,15,16] In a meta-analysis performed by Falagas et al., results of eight studies on 1877 patients showed that the use of antimicrobial drugs through inhalation or its administration into the ETT can reduce VAP.[16] This is the effect of increasing the drug concentration in the airways. Therefore, given that eucalyptus has antibacterial properties, its application through inhalation can increase its concentration in the airways of patients undergoing mechanical ventilation, and hence, it is effective in reducing the microbial contamination of ETT biofilms. Several studies have shown that clearing the airways and ventilation routes from microbial contamination can be effective in the prevention of VAP.[7,8,9] In the meta-analysis performed by Ioannidou et al., results of five clinical trials showed that using topical antibiotics (as inhalation or their administration into the tracheal tube), compared with intravenous antibiotics as placebo, can clinically reduce VAP, but the difference between the two groups in terms of biological improvement was not significant.[27]
Airways may be colonized by different microorganisms. In the present study, as well as a large number of other studies, the most common microorganisms colonizing the airways were K. pneumoniae and A. baumannii.[21,24,28,29] However, in a number of other studies, Acinetobacter and Pseudomonas were more common.[30,31,32] Difference in culture source,[33] intubation techniques, and demographic and clinical characteristics of patients[30] may be effective in this regard.
Another result of this study was that in addition to reducing the microbial load of ETT, eucalyptus significantly reduced K. pneumoniae contamination in the intervention group. In the study by Tabari et al., the antimicrobial properties of eucalyptus were evaluated on Klebsiella, E. coli, Proteus, Pseudomonas, and St. aureus.[34] They found that K. pneumoniae and E. coli had the highest sensitivity and Pseudomonas and Proteus had the least sensitivity to eucalyptus.[34] K. pneumoniae, as an opportunistic pathogen, is the leading cause of nosocomial infections.[35] Contamination and infections caused by this bacterium are highly destructive and can increase the rate of mortality by about 25-60%.[36] Given that these microorganisms can produce dense biofilm and can quickly lead to multidrug resistance to antibiotics, they cause numerous medical problems in the world that have limited treatment options.[35] Therefore, reducing the frequency of infections of these microorganisms using eucalyptus incense can have a significant role in reduction of nosocomial infections, especially pneumonia. According to their properties and other biological effects, essential oils are considered as an appropriate complementary treatment with antibiotics.[37]
In this study, 14 cases (21.2%) out of 66 positive cultures of ETT were contaminated with two microorganisms (7 cases in each group). In most studies, such as the present study, polymicrobial infection was less than contamination with a single microbe.[29,30,33] Friedland et al. stated that the existence of a microorganism alone in a biofilm matrix can prevent the growth of other bacteria.[33] Nevertheless, in some studies, the number of cases contaminated with multiple microbes was high. For example, in the study by Simoni and Wiatrak, all the samples showed polymicrobial contamination,[38] and in the study by Gil-Perotin et al., the protein of most of the samples (60%) was polymicrobial.[31] According to the present study, in which the number of two-microbial infections in the two groups was equal (in each group, seven cases were observed), it can be concluded that eucalyptus incense cannot be effective on polymicrobial contamination. This study had some limitations. There was no relationship between the duration of intubation, and severity and type of microbial contamination in this study, and it may be due to the small sample size. On the other hand, one of the contributing factors in bacterial biofilm formation was the type and number of intravenous antibiotics which could affect the study results.
CONCLUSION
Inhalation of eucalyptus incense in patients undergoing mechanical ventilation can reduce severe microbial contamination of the ETT biofilm. The prevalence of contaminating microorganisms in patients in the intervention group was less than in the control group, but this difference was not statistically significant. However, this method significantly reduced infection by K. pneumoniae. Therefore, reduction of microbial biofilms of the ETT may cause a reduction in VAP, which requires further research.
Financial support and sponsorship
This article was derived from an MSc thesis in Arak University of Medical Sciences, No. 1118.
Conflicts of interest
There are no conflicts of interest
Acknowledgments
The authors wish to thank the Research Deputy of Arak University of Medical Sciences, which approved and financially supported this project, the Clinical Research Development, and all the staffs of Al-zahra University Hospital for their help.
This article was derived from the Master's thesis of Nazanin Amini with project number 1118, Arak University of Medical Sciences, Arak, Iran.
REFERENCES
- 1.Plummer AL, Gracey DR. Consensus conference on artificial airways in patients receiving mechanical ventilation. Chest. 1989;96:178–80. doi: 10.1378/chest.96.1.178. [DOI] [PubMed] [Google Scholar]
- 2.Craven DE, Hjalmarson KI. Ventilator-associated tracheobronchitis and pneumonia: Thinking outside the box. Clin Infect Dis. 2010;51(Suppl 1):S59–66. doi: 10.1086/653051. [DOI] [PubMed] [Google Scholar]
- 3.Adair CG, Gorman SP, Feron BM, Byers LM, Jones DS, Goldsmith CE, et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999;25:1072–6. doi: 10.1007/s001340051014. [DOI] [PubMed] [Google Scholar]
- 4.Berra L, Coppadoro A, Bittner EA, Kolobow T, Laquerriere P, Pohlmann JR, et al. A clinical assessment of the Mucus Shaver: A device to keep the endotracheal tube free from secretions. Crit Care Med. 2012;40:119–24. doi: 10.1097/CCM.0b013e31822e9fe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park YS, Kee YW, Park KS, Lee J, Lee SM, Yim JJ, et al. Sound analysis in an in vitro endotracheal tube model. Korean J Intern Med. 2011;26:421–6. doi: 10.3904/kjim.2011.26.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsek MR, Greenberg EP. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 7.El-Azizi M, Rao S, Kanchanapoom T, Khardori N. In vitro activity of vancomycin, quinupristin/dalfopristin, and linezolid against intact and disrupted biofilms of staphylococci. Ann Clin Microbiol Antimicrob. 2005;4:2. doi: 10.1186/1476-0711-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biel MA, Sievert C, Usacheva M, Teichert M, Wedell E, Loebel N, et al. Reduction of endotracheal tube biofilms using antimicrobial photodynamic therapy. Lasers Surg Med. 2011;43:586–90. doi: 10.1002/lsm.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacheco-Fowler V, Gaonkar T, Wyer PC, Modak S. Antiseptic impregnated endotracheal tubes for the prevention of bacterial colonization. J Hosp Infect. 2004;57:170–4. doi: 10.1016/j.jhin.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Craven DE, Lichtenberg DA, Goularte TA, Make BJ, McCabe WR. Contaminated medication nebulizers in mechanical ventilator circuits. Source of bacterial aerosols. Am J Med. 1984;77:834–8. doi: 10.1016/0002-9343(84)90520-5. [DOI] [PubMed] [Google Scholar]
- 11.Rau JL. The inhalation of drugs: Advantages and problems. Respir Care. 2005;50:367–82. [PubMed] [Google Scholar]
- 12.Fares AA, Khodish B, Mona A, Mohammed D. Knowledge and performance of critical care nurses toward nebulizer therapy in the intensive care unit at Assiut University Hospital. Med J Cairo Univ. 2013;81:81–94. [Google Scholar]
- 13.Adair CG, Gorman SP, Byers LM, Jones DS, Feron B, Crowe M, et al. Eradication of endotracheal tube biofilm by nebulised gentamicin. Intensive Care Med. 2002;28:426–31. doi: 10.1007/s00134-002-1223-8. [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, Yang J, Liu Z, Gutierrez C, Aymard G, Rouby JJ. Nebulized Antibiotics Study Group. Nebulized ceftazidime and amikacin in ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2011;184:106–15. doi: 10.1164/rccm.201011-1894OC. [DOI] [PubMed] [Google Scholar]
- 15.Klick JM, du Moulin GC, Hedley-Whyte J, Teres D, Bushnell LS, Feingold DS. Prevention of gram-negative bacillary pneumonia using polymyxin aerosol as prophylaxis. II. Effect on the incidence of pneumonia in seriously ill patients. J Clin Invest. 1975;55:514–9. doi: 10.1172/JCI107957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falagas ME, Siempos II, Bliziotis IA, Michalopoulos A. Administration of antibiotics via the respiratory tract for the prevention of ICU-acquired pneumonia: A meta-analysis of comparative trials. Crit Care. 2006;10:R123. doi: 10.1186/cc5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badia JR, Soy D, Adrover M, Ferrer M, Sarasa M, Alarcón A, et al. Disposition of instilled versus nebulized tobramycin and imipenem in ventilated intensive care unit (ICU) patients. J Antimicrob Chemother. 2004;54:508–14. doi: 10.1093/jac/dkh326. [DOI] [PubMed] [Google Scholar]
- 18.O’Riordan TG, Greco MJ, Perry RJ, Smaldone GC. Nebulizer function during mechanical ventilation. Am Rev Respir Dis. 1992;145:1117–22. doi: 10.1164/ajrccm/145.5.1117. [DOI] [PubMed] [Google Scholar]
- 19.Bachir RG, Benali M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac J Trop Biomed. 2012;2:739–42. doi: 10.1016/S2221-1691(12)60220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadlon AE, Lamson DW. Immune-modifying and antimicrobial effects of Eucalyptus oil and simple inhalation devices. Altern Med Rev. 2010;15:33–47. [PubMed] [Google Scholar]
- 21.Vasanthi R, Karthikeyan D, Jeya M. Study of biofilm production and antimicrobial resistance pattern of the bacterial isolates from invasive devices. Int J Res Health Sci. 2014;31:274–81. [Google Scholar]
- 22.Elaissi A, Rouis Z, Salem NA, Mabrouk S, ben Salem Y, Salah KB, et al. Chemical composition of 8 eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement Altern Med. 2012;12:81. doi: 10.1186/1472-6882-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruenwald J, Brendler T, Jaenicke C. PDR for Herbal Medicines. 3rd ed. Montvale, NJ: Thomson Healthcare; 2004. pp. 293–7. [Google Scholar]
- 24.Corujo LO, Pére AP, Pérez NG, Oliva SP. Profilaxis de la neumonía asociada a ventilación con aerosoles de eucalipto [artículo en línea] [Last accessed on 2008 Oct 14];MEDISAN. 2008 12 Available from: http://bvs.sld.cu/revistas/san/vol12_1_08/san02108.htm . [Google Scholar]
- 25.Vratnica B, Djakov TA, Šukoviæ D, Damjanoviæ J. Antimicrobial effect of essential oil isolated from Eucalyptus globules Labill. from Montenegro. Czech J Food Sci. 2011;29:277–84. [Google Scholar]
- 26.Torabi B. Evaluation of components and antimicrobial effects of 10 species of eucalyptus on Escherichia coli and Micrococcus loteus. IJMAPR. 2011;27:440–49. [Google Scholar]
- 27.Ioannidou E, Siempos II, Falagas ME. Administration of antimicrobials via the respiratory tract for the treatment of patients with nosocomial pneumonia: A meta-analysis. J Antimicrob Chemother. 2007;60:1216–26. doi: 10.1093/jac/dkm385. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh B, Ghosh K, Roy A, Pal D, Ghosh A, Mandal K. Correlation between colonized bacteria of ET tube among suspected pneumonia patients of ICU. IJRTSAT. 2014;11:245–8. [Google Scholar]
- 29.Ghazvini K, Ghanaat J, Malek JM, Yazdan PM, Irani N. Incidence of nosocomial pneumonia and bacterial agents causing this infection in intensive care unit in Ghaem University Hospital in Mashhad. J Ilam Univ Med Sci. 2005;13:28–35. [Google Scholar]
- 30.Abdollahi A, Shoar S, Shoar N. Microorganisms’ colonization and their antibiotic resistance pattern in oro - tracheal tube. Iran J Microbiol. 2013;5:102–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Gil-Perotin S, Ramirez P, Marti V, Sahuquillo JM, Gonzalez E, Calleja I, et al. Implications of endotracheal tube biofilm in ventilator-associated pneumonia response: A state of concept. Crit Care. 2012;16:R93. doi: 10.1186/cc11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seyedalshohadaee M, Rafii F, Haghani H, Faridian Arani F. Evaluating the Effect of Mouth Washing with Chlorhexidine on the Ventilator Associated Pneumonia. IJN. 2012;25:34–44. [Google Scholar]
- 33.Friedland DR, Rothschild MA, Delgado M, Isenberg H, Holzman I. Bacterial colonization of endotracheal tubes in intubated neonates. Arch Otolaryngol Head Neck Surg. 2001;127:525–8. doi: 10.1001/archotol.127.5.525. [DOI] [PubMed] [Google Scholar]
- 34.Tabari MA, Youssefi MR, Ghasemi F, Tabari RG, Esmaili RH, Behzadi MY. Comparison of antibacterial effects of eucalyptus essence, mint essence and combination of them on Staphylococcus aureus and Escherichia coli isolates. Middle-East J Sci Res. 2012;11:536–40. [Google Scholar]
- 35.Hackstein H, Kranz S, Lippitsch A, Wachtendorf A, Kershaw O, Gruber AD, et al. Modulation of respiratory dendritic cells during Klebsiella pneumoniae infection. Respir Res. 2013;14:91. doi: 10.1186/1465-9921-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellifa S, Hassaine H, Balestrino D, Charbonnel N, M’hamedi I, Terki IK, et al. Evaluation of biofilm formation of Klebsiella pneumoniae isolated from medical devices at the University Hospital of Tlemcen, Algeria. Afr J Microbiol Res. 2013;7:5558–64. [Google Scholar]
- 37.Mahboubi M. Comparison of Respitol B® containing mint and eucalyptus essence with Menthophin®. Iran J Microbiol. 2007;1:39–44. [Google Scholar]
- 38.Simoni P, Wiatrak BJ. Microbiology of stents in laryngotracheal reconstruction. Laryngoscope. 2004;114:364–7. doi: 10.1097/00005537-200402000-00034. [DOI] [PubMed] [Google Scholar]


