Abstract
Autism is a neurodevelopmental disorder with multifactorial etiology and varied presentation, in which early diagnosis is crucial to the implementation of early treatment. A 6-year-old child clinically diagnosed with autism, and a normal magnetic resonance imaging underwent dedicated 18F-fluorodeoxyglucose brain positron emission tomography (PET) as an ancillary investigation. PET image showed diffuse bilateral temporal hypometabolism. Although PET imaging is currently not indicated in the evaluation of autism, characteristic imaging patterns on PET can provide corroborative information and increase the diagnostic confidence for the same.
Keywords: Autism, brain positron emission tomography, functional imaging
INTRODUCTION
Autism is a neurodevelopmental disorder with a multifactorial etiology and varied presentation. In this most severe form of autistic spectrum disorder, early diagnosis is crucial to the implementation of early treatment, which can lead to immense improvement in the behavioral as well the cognitive functions of a child. We present a case of a 6-year-old child, clinically diagnosed with autism, who underwent dedicated 18F-fluorodeoxyglucose (18F-FDG) brain positron emission tomography (PET) as an ancillary and baseline investigation. In today's era, when no imaging modality exists which can diagnose autism with complete certainty, PET/computed tomography (CT) can be a valuable tool to confirm the clinical suspicion as well as rule out any other cause.
CASE REPORT
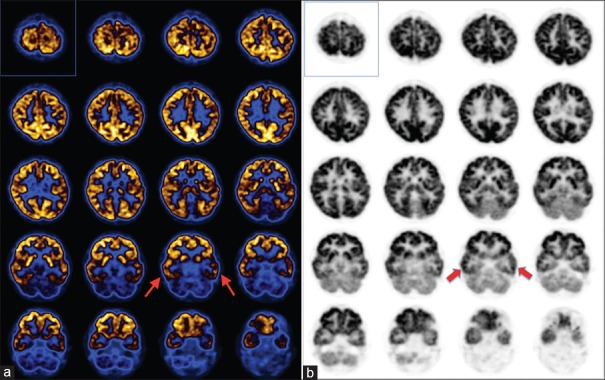
We present the case of a 6-year-old boy, born of a full term normal delivery, who had normal motor milestones as recalled by the parents. He presented with symptoms from the age of 3 years. There was delayed speech, with lack of social interactions and emotional development. He was hyperactive at school and home with occasional emotional outbursts. He had normal vision and hearing with slurred speech. Functionally, he was independent for most of the daily activities but needed assistance in fine motor activities. As per the Diagnostic and Statistical Manual of Mental Disorders-IV criteria, the child was clinically diagnosed as autistic. He was well oriented for his age and had no history of seizures or trauma. There was no history of intrauterine infection or any other illness. In spite of regular social and vocal rehabilitation since the age of 4 years, he showed minimal improvement with respect to behavior and social interaction. On examination, he had normal muscle tone and power in all the muscle groups. His Childhood Autism Rating Scale (CARS) score was 36, which is categorized as mildly-moderately autistic and Modified Checklist for Autism in Toddlers (M-CHAT) screening score indicated a high risk for autism. The patient was referred for brain PET as baseline study and also for imaging confirmation as magnetic resonance imaging (MRI) of the brain was normal. Axial PET image [Figure 1a and b] showed symmetrically decreased FDG uptake in bilateral temporal lobes, with rest of the brain parenchyma showing normal, physiological tracer distribution [Figures 2 and 3]. Diffuse temporal hypometabolism did not show any corresponding structural abnormality on the corresponding CT (acquired on dedicated PET/CT scanner). No focal hyper or hypometabolic lesions were noted elsewhere in the brain parenchyma. The patient is on treatment, and follow-up imaging is awaited.
Figure 1.
(a) Transaxial images of brain PET show diffuse hypometabolism in bilateral temporal lobes (marked by red arrows). Rest of the brain parenchyma shows physiological tracer distribution. (b) Transaxial image in grayscale showing bilateral temporal hypometabolism as marked
Figure 2.
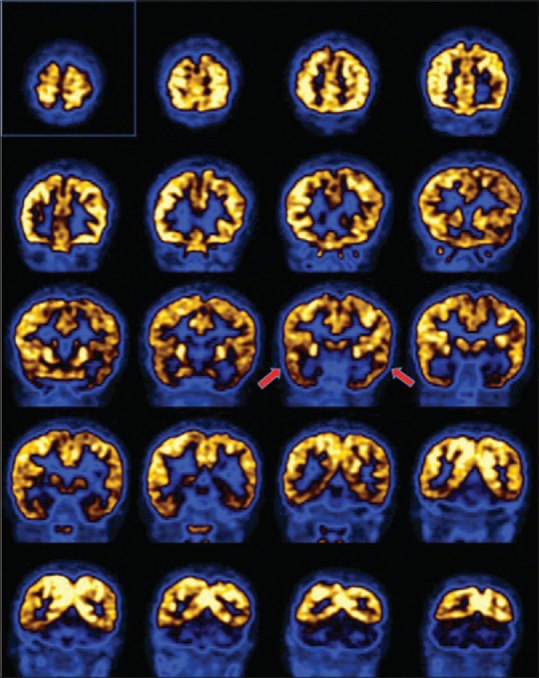
Coronal images of brain positron emission tomography from anterior to posterior direction showing low uptake in bilateral temporal lobes
Figure 3.
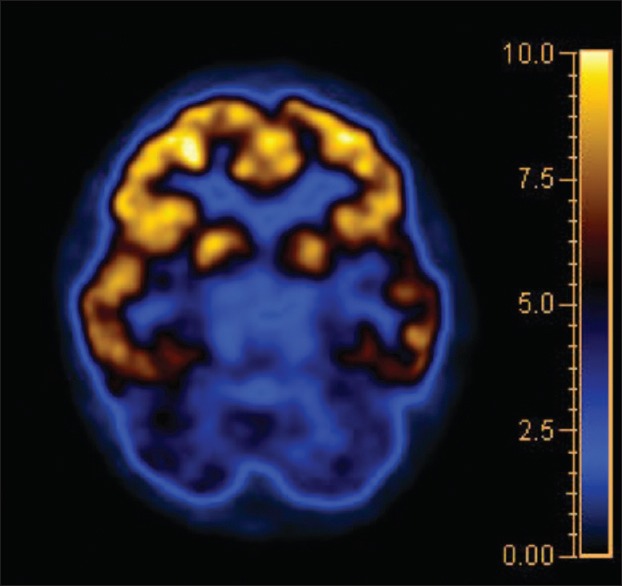
Transaxial section of brain positron emission tomography image with corresponding color scale, showing bilateral hypometabolism
DISCUSSION
Autism spectrum disorder (ASD) is a group of complex disorders of brain development. Usually noticed by parents at the age of 3 years, autism is characterized by the presence of markedly abnormal or impaired development in social interaction and communication and a markedly restricted repertoire of activity and interests.[1] In India, most children with ASD are diagnosed between 3 and 6 years of age.[2] The delay in diagnosis is most often due to limited knowledge among the clinicians, lack of awareness, inadequate screening, and the social stigma associated with the diagnosis.[3,4] Early diagnosis is crucial, as it is likely that this would facilitate the recognition of underlying genetic and neurobiological mechanisms, and provide an incentive to focus on specific behavioral or biological treatment. Moreover, it has been documented that early initiation of treatment in children with ASD leads to significant improvement in language, cognition, and behavior functioning. Primary goal of the diagnosis and treatment is to improve the quality of life of the patient by maximizing functional independence, with the help of various treatment options available. The current gold standard for ASD diagnosis is considered to be clinical judgment,[5,6] along with various screening tests and questionnaires such as CARS and M-CHAT.[7,8] MRI has been used by several investigators to determine the presence of structural changes if any, associated with this syndrome. However, no consistent abnormalities were found on MRI.[9,10] Dysfunction of temporal lobes has been often implicated in the etiopathogenesis of autism. This is because normal functioning of amygdala and hippocampus is essential for regulation of affect, memory, and learning. However, no structural changes have been demonstrated in these areas till today. Since the anatomic scans are normal, functional imaging may provide the best means for early diagnosis of this disorder. Though single-photon emission computed tomography (SPECT) perfusion studies are normal in many of the autism patients, it may show decreased temporal lobe perfusion. Up to 30% of autistic children eventually develop temporal lobe epilepsy.[11] PET imaging, performed with 18F-FDG, which is a radiolabeled glucose analog - the primary substrate for brain metabolism; provides improved resolution over SPECT. The neurotransmitter systems studied in ASD using PET or SPECT imaging include serotonin, dopamine, and gamma-aminobutyric acid. However, their limited availability makes their routine use challenging at present. Chivate et al. assessed 23 autistic patients and concluded that PET/CT can be a potential tool for the diagnosis of autism.[12] They demonstrated that majority of autistic patients showed low uptake in hippocampus and amygdala, followed by mesial temporal lobe and cerebellum, with high uptake in frontal lobes. The findings in our case report corroborated with what they have described. Currently, PET/SPECT imaging is not indicated in the evaluation and diagnosis of individuals who may have ASDs. However, characteristic imaging patterns on PET shall increase the diagnostic confidence of the neurophysicians in patients with strong clinical suspicion of ASD. Hence, based on the lines of this case, a larger pediatric patient population with clinical diagnosis of autism needs to be evaluated and followed up, to establish the role of PET imaging in the diagnostic algorithm of autism.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, D.C: American Psychiatric Association; 1994. American Psychiatric Association. [Google Scholar]
- 2.Malhi P, Singhi P. Recognition of autism in young children. Stud Psychol. 2003;45:75–80. [Google Scholar]
- 3.Daley TC. From symptom recognition to diagnosis: Children with autism in urban India. Soc Sci Med. 2004;58:1323–35. doi: 10.1016/S0277-9536(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 4.Singhi P, Malhi P. Clinical and neurodevelopmental profile of young children with autism. Indian Pediatr. 2001;38:384–90. [PubMed] [Google Scholar]
- 5.Klin A, Lang J, Cicchetti DV, Volkmar FR. Brief report: Interrater reliability of clinical diagnosis and DSM-IV criteria for autistic disorder: Results of the DSM-IV autism field trial. J Autism Dev Disord. 2000;30:163–7. doi: 10.1023/a:1005415823867. [DOI] [PubMed] [Google Scholar]
- 6.Volkmar F, Chawarska K, Klin A. Autism in infancy and early childhood. Annu Rev Psychol. 2005;56:315–36. doi: 10.1146/annurev.psych.56.091103.070159. [DOI] [PubMed] [Google Scholar]
- 7.Schopler E, Reichler R, Renner BR. Western Psychological Services. Los Angeles, CA: The Childhood Autism Rating Scale; 1988. [Google Scholar]
- 8.Robins DL, Fein D, Barton ML, Green JA. The modified checklist for autism in toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. 2001;31:131–44. doi: 10.1023/a:1010738829569. [DOI] [PubMed] [Google Scholar]
- 9.Ekman G, de Chateau P, Marions O, Sellden H, Wahlund LO, Wetterberg L. Low field magnetic resonance imaging of the central nervous system in 15 children with autistic disorder. Acta Paediatr Scand. 1991;80:243–7. doi: 10.1111/j.1651-2227.1991.tb11840.x. [DOI] [PubMed] [Google Scholar]
- 10.Garber HJ, Ritvo ER. Magnetic resonance imaging of the posterior fossa in autistic adults. Am J Psychiatry. 1992;149:245–7. doi: 10.1176/ajp.149.2.245. [DOI] [PubMed] [Google Scholar]
- 11.Lele RD. Principles and Practice of Nuclear Medicine and Correlative Medical Imaging. 1st ed. New Delhi: Jaypee Brothers Medical Publishers Pvt. Ltd.; 2009. p. 175. [Google Scholar]
- 12.Chivate R, Thakrar P, Narang J, Patkar D, Kumar S, Verma M. PET/CT in Autism – A Diagnostic Tool. Radiological Society of North America Scientific Assembly and Annual Meeting - Chicago IL. 2014 [Google Scholar]