Abstract
Introduction:
11C-methonine ([11C]-MET) positron emission tomography-computed tomography (PET-CT) is a well-established technique for evaluation of tumor for diagnosis and treatment planning in neurooncology. [11C]-MET reflects amino acid transport and has been shown to be more sensitive than magnetic resonance imaging (MRI) in stereotactic biopsy planning. This study compared fluorodeoxyglucose (FDG) PET-CT and MET PET-CT in the detection of various brain tumors.
Materials and Methods:
Sixty-four subjects of brain tumor treated by surgery, chemotherapy, and/or radiotherapy were subjected to [18F]-FDG, [11C]-MET, and MRI scan. The lesion was analyzed semiquantitatively using tumor to normal contralateral ratio. The diagnosis was confirmed by surgery, stereotactic biopsy, clinical follow-up, MRI, or CT scans.
Results:
Tumor recurrence was found in 5 out of 22 patients on [F-18] FDG scan while [11C]-MET was able to detect recurrence in 18 out of 22 patients in low-grade gliomas. Two of these patients were false positive for the presence of recurrence of tumor and later found to be harboring necrosis. Among oligodendroglioma, medulloblastoma and high-grade glioma out of 42 patients 39 were found to be concordant MET and FDG scans. On semiquantitative analysis, mean T/NT ratio was found to be 2.96 ± 0.94 for lesions positive for recurrence of tumors and 1.18 ± 0.74 for lesions negative for recurrence of tumor on [11C]-MET scan. While the ratio for FDG scan on semiquantitative analysis was found to be 2.05 ± 1.04 for lesions positive for recurrence of tumors and 0.52 ± 0.15 for lesions negative for recurrence of tumors.
Conclusion:
The study highlight that [11C]-MET is superior to [18F]-FDG PET scans to detect recurrence in low-grade glioma. A cut-off value of target to nontarget value of 1.47 is a useful parameter to distinguish benign from malignant lesion on an [11C]-MET Scan. Both [18F]-FDG and [11C]-MET scans were found to be useful in high-grade astrocytoma, oligodendroglioma, and medulloblastoma.
Keywords: [11C]-methonine, [18F]-fluorodeoxyglucose, gliomas, tumor recurrence
INTRODUCTION
In the era of multimodality imaging, modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound have been found to be very useful in early detection of malignant tumors as they give very precise information regarding the morphology of the lesion. In general, contrast enhanced magnetic and CT serves to define the tumor margins. Contrast enhancement is not a very reliable modality for detection of a viable brain tumor. It is falsely negative in viable tumor and is falsely positive in necrotic and inflamed areas.[1,2] MRI spectrometry data also suggests that standard T2-weighted contrast enhanced MRI may underestimate the volume of metabolically active tumors.[3] Advanced treatment techniques like neuronavigation in surgery, brachytherapy with radioisotopes, or radiotherapy needs an accurate definition of tumor extent for target volume planning than provided by standard MRI or CT techniques.[3]
Imbalance in DNA transmethylation is one of the earliest events associated with in vitro transformation thus inducing upregulation of the amino acid transporters expression, especially large amino acid transporter 1 (LAT1) to enhance the facilitated transport. The LAT1 is densely expressed in malignant tumors to support the proliferation of the tumors. The LAT1 and solute carrier family 1 (neutral amino acid transporter), member 5 (SLC7A5) are upregulated in a wide range of human cancers; this is positively correlated with the biological aggressiveness of tumors and are promising markers for prognostication.
L-[methyl-11C]-methionine ([11C]-MET) is the most popular amino acid imaging modality in positron emission tomography (PET) to image the size and spread of gliomas and have the advantage of showing selective uptake in the human brain tumor.[4,5,6] Low uptake of this tracer in normal brain and increased uptake in primary brain tumors makes this tracer highly useful in the field of neurooncology.[7,8,9,10] Increased uptake of methionine has shown to indicate increased cellular proliferation and microvessel density and proliferative cell nuclear antigen index in tumors.[11,12,13,14,15] Several studies have evaluated [11C]-MET for monitoring the efficacy of treatment and for differentiating recurrent tumors from radiation necrosis.[16,17,18,19,20] Uptake of [11C]-MET is facilitated by a small amino acid transporter which is upregulated in tumors.[21] Increased amino acid uptake in tumors appears to be due to increased transport mediated by type L-amino acid carriers.[18] The LAT1 expression in cancer relates to its malignant potential and prognostication which tend to increase from low-grade to high-grade neuroendocrine (NE) tumors. The previous study reported elsewhere suggests that LAT1 is involved in cellular proliferation, lymph node metastasis, and worse prognosis in patients with NE tumors of the lung.[22] [11C]-MET has the ability to detect most malignant regions and infiltrating areas in the tumors with high sensitivity and specificity.[13,14] Various studies have shown that the margins of tumors as assessed by PET using [11C]-MET are much wider than that found on MRI or CT.[23] Evidence in the literature suggests that amino acid-based tracers have better ability to delineate the margins of tumors as compared to [18F]-2-fluoro-2-deoxy-D-glucose ([18F]-FDG) in low-grade brain tumors. [11C]-MET is a sensitive tracer in tumor detection and it differentiates benign from malignant lesions with high sensitivity and specificity with low background activity in normal brain.[23,24] This study was undertaken with the aim of improving the prognostication in gliomas.
MATERIALS AND METHODS
In this retrospective study, 64 patients with age ranging from 5 to 56 years were investigated with [18F]-FDG and [11C]-MET-PET within last 4 years. There were 41 males and 23 females in the study group. Approval by Local Institutional Scientific Committee was taken for undertaking the study. Written informed consent was obtained from all the subjects.
Patients had been operated earlier for a brain tumor or undergone stereotactic biopsy and received a final histological diagnosis according to WHO grading system.[25] Individual patient data are given in Tables 1–5. They had undergone surgery, chemotherapy, and/or radiotherapy. They all underwent a follow-up PET-CT scan for detection of recurrent disease.
Table 1.
Patient and tumor characteristics in low-grade astrocytoma
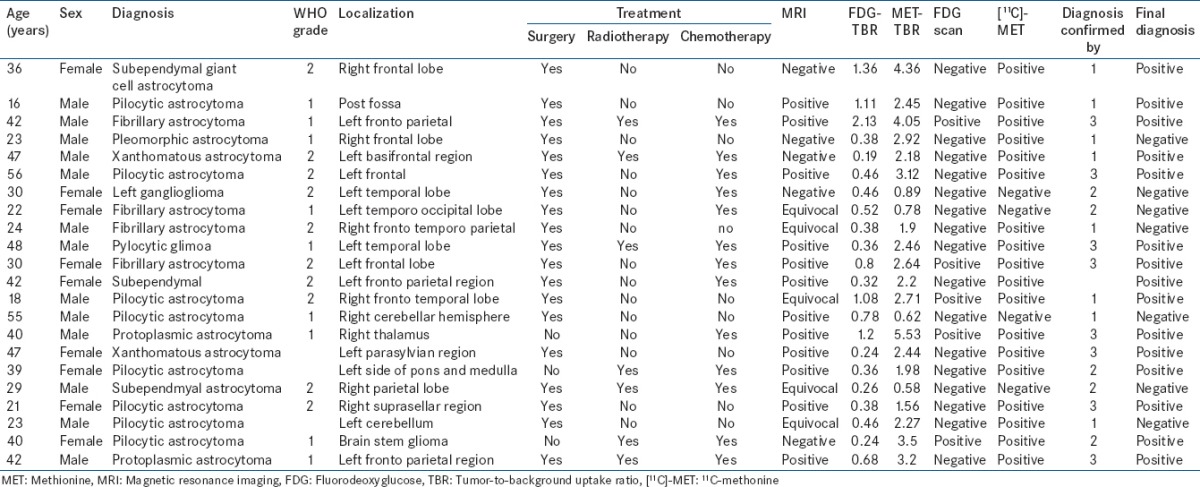
Table 5.
Patient and tumor characteristics in miscellaneous tumors
[18F]-FDG was prepared using automated module MX tracerlab (General Electric Medical System). [11CO2] was produced directly in the target chamber via the 14N (p, α) 11C nuclear reaction using a 0.5% oxygen balanced with nitrogen target. [11C]-methane was produced via [11C] carbon dioxide on a Nickel-Shimalite at high temperature (350°C. In order to enable sufficient conversion of [11C]-methane into [11C]-methyl iodide the gas phase iodination is performed as a circulation process. Thus, [11C]-MET was prepared by alkylation of 2-homocystiene hydrogen chloride with [11C]-CH3 I. This was purified by high-performance liquid chromatography and solid phase extraction on tC-18 cartridge to give 99% radiochemically pure [11C]-MET.
Patients fasted for 6 h before PET scan; however, liberal intake of water was encouraged. The patients were injected with 370 MBq (10 mCi) of [11C]-MET. A transmission scan was obtained from the level of the upper neck to the pelvis at 4 min/bed position. The [F-18] FDG scan was obtained a minimum of 2 h after completing the [11C]-MET scan. 60 min after injection of 370 MBq (10 mCi) [18F]-FDG, an emission scan was acquired from neck to the pelvis for 15-20 min/bed position Discovery STE 16 (GE) PET-CT Camera. All scans were reconstructed using vendor provided ordered-subsets expectation maximization.
Transverse, coronal, and sagittal images were evaluated visually by the three independent physicians. The [11C]-MET PET and [18F] FDG-PET scans were read independently. A PET scan was considered abnormal when ratio tracer uptake on [18F]-FDG or [11C]-MET uptake was more than that of normal background.
Regional [18F]-FDG and [11C]-MET uptake in tumor was quantified using a target [T] to nontarget [NT] ratio. Each lesion was identified on transaxial image and T/NT ratio was generated. A 96 mm2 region of interest was used to determine the maximum standardized uptake value (SUVmax) of the lesion, this region of interest was mirrored on the contralateral cortex to get the SUVmax of the normal area. The lesion was analyzed semiquantitatively using tumor to normal contralateral cortex ratio (T/N). The diagnosis was confirmed by histopathology after surgery, stereotactic biopsy, clinical follow-up, or follow-up MRI or CT scans.
RESULTS
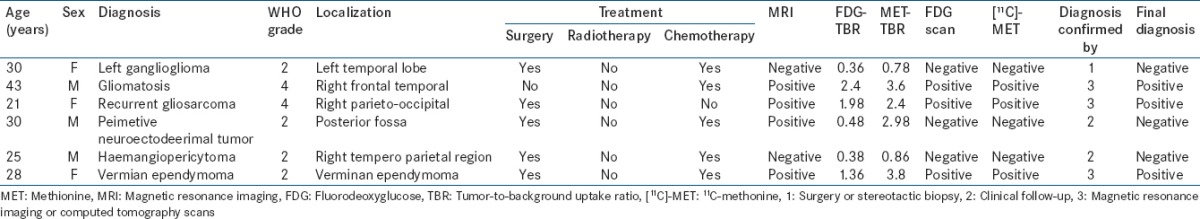
A total of 64 cases were included in the study. Of these, 22 were low-grade astrocytoma [Table 1], 16 had high-grade astrocytoma [Table 2], ten oligodendrogliomas [Table 3], ten had medulloblastoma [Table 4], and 6 had other miscellaneous brain tumors [Table 5]. Tumor recurrence was noted in 5 out of 22 patients of low-grade astrocytoma, 11 out of 16 patients of high-grade astrocytoma, 4 out of 10 oligodendrogliomas, 6 out of 10 patients in medulloblastoma, and 3 out of 6 miscellaneous brain tumor scans. On [18F]-FDG brain scans, it was found to be difficult to detect recurrence more so in patients of low-grade astrocytomas and oligodendrogliomas. High-grade astrocytomas and medulloblastomas were better visualized on FDG scan. In general, most of the low-grade gliomas did not show FDG tracer uptake in the recurrent glial tissue. In contrast, [11C]-MET PET-CT had the capacity to detect recurrent tumor tissue in low-grade glioma very well. Tumor recurrence was noted in 18 out of 22 patients of low-grade astrocytoma [Figures 1–4], 11 out of 16 patients of high-grade astrocytoma [Figure 5], 6 out of 10 oligodendroglioma [Figure 6], 6 out of 10 patients of medulloblastoma [Figure 7], and 3 out of 6 miscellaneous brain tumors in [11C]-MET PET-CT scan. The recurrent lesions were better appreciated with a good lesion to surrounding contrast on MET PET study compared to FDG-PET study especially in low-grade astrocytoma and oligodendrogliomas. However, in patients harboring high-grade astrocytoma and medulloblastomas both MET and FDG were able to detect the recurrence. Only one patient with anaplastic Grade III astrocytoma could not be diagnosed for recurrence on FDG scan while the MET scan and final diagnosis were positive for recurrence of a brain tumor. Two patients with low-grade astrocytoma showing MET uptake later turned out to be false positive on the final diagnosis. They were later found to have necrotic tissue in stereotactic brain biopsy. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of these two modalities were found to be 50%, 85.7%, 81.8%, and 60% for the FDG scans and 93.3%, 90%, 93.3%, and 90% for [11C]-MET scans for detection of low-grade gliomas. On semi-quantitative analysis, mean T/NT ratio was found to be 2.96 ± 0.94 for lesions positive for recurrence of tumors and 1.18 ± 0.74 for lesions negative for recurrence of tumor on [11C]-MET scan. In our study, 45 out of 47 (95.7%) patients who had a target to NT ratio of more than 1.47 were found to be positive for recurrence of the tumor. Two patients who had a ratio more than 1.47, however, were found to false positive for recurrent malignant disease. However, no cases were found to be false negative. Fifteen patients with low-grade glioma who had a positive MET scan did not show abnormal uptake on FDG scan. Of these 15 patients, 13 were later confirmed to have a recurrent tumor.
Table 2.
Patient and tumor characteristics in high-grade astrocytoma
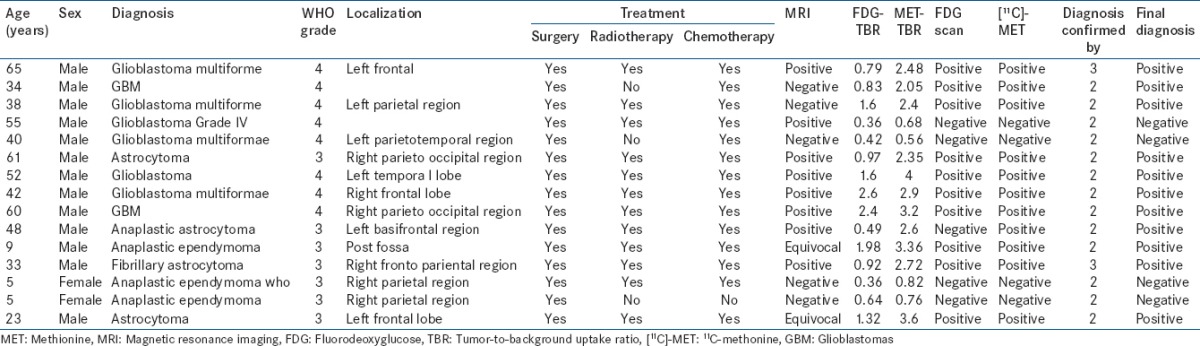
Table 3.
Patient and tumor characteristics in oligodendroglioma
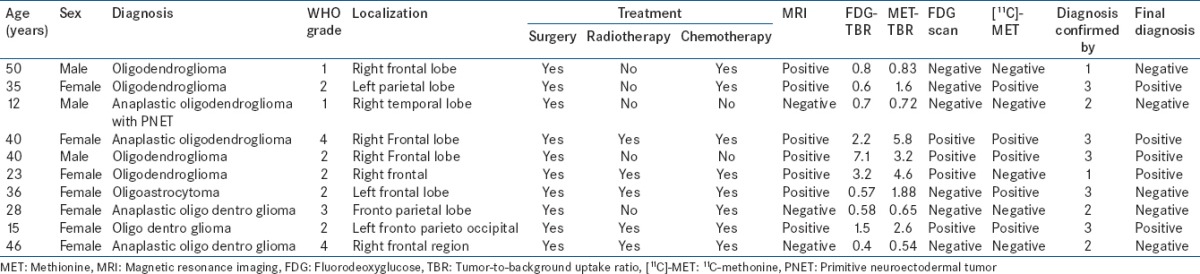
Table 4.
Patient and tumor characteristics in medulloblastoma
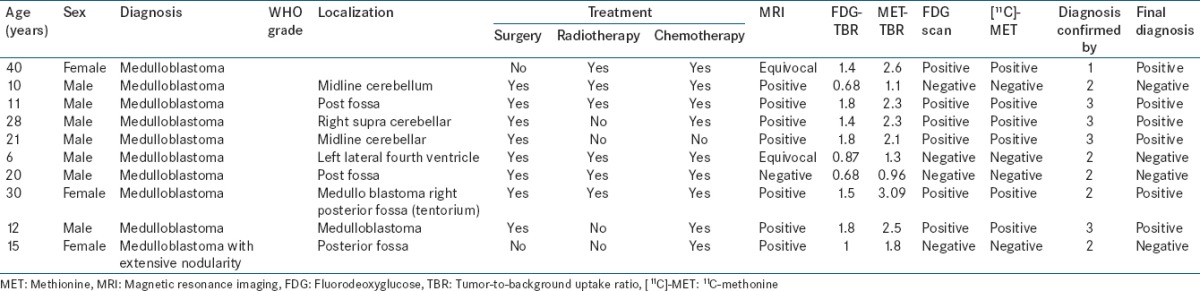
Figure 1.
Postoperative case of posterior fossa Grade I pilocytic astrocytoma. (a) Fluorodeoxyglucose scan negative for recurrence. (b) C-11 methionine scan showing residual tumor in the region of midbrain
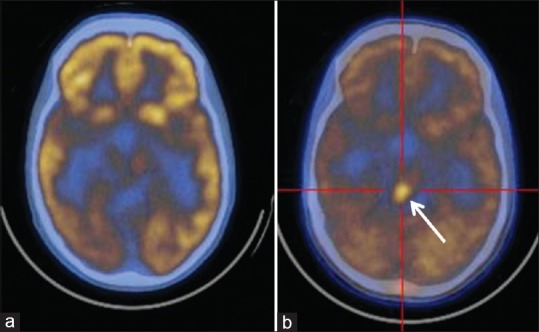
Figure 4.
Grade I pilocytic glioma in left temporal lobe after surgery, radiotherapy, and chemotherapy. (a) Fluorodeoxyglucose scan was negative. (b) C-11 methionine scan shows residual/recurrent lesion in left temporal region
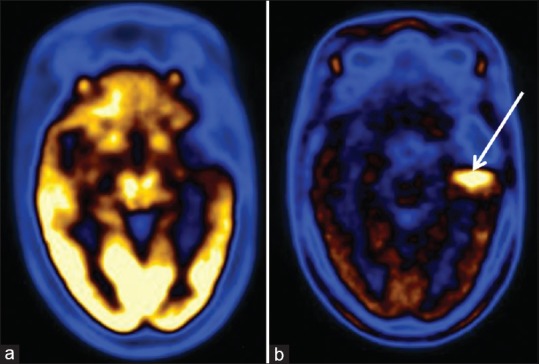
Figure 5.
Postoperative case of glioblastoma mutiformae undergone radio and chemotherapy. Both fluorodeoxyglucose (a) and methionine (b) scans show recurrent tumor mass in right temporal lobe. Note the extent of tumor noted is more on methionine scan as compared to fluorodeoxyglucose scan
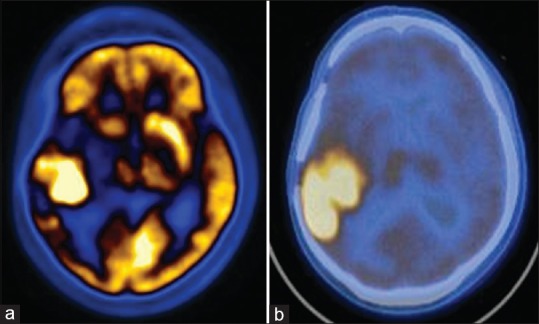
Figure 6.
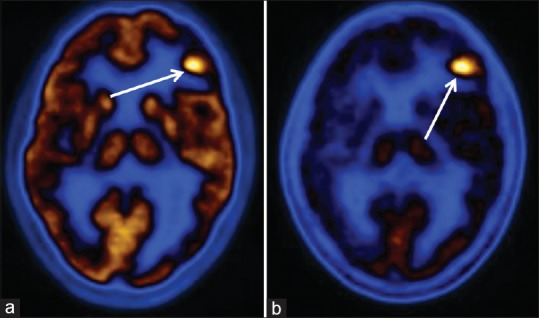
Anaplastic oligodendroglioma undergone surgery, runtime and computed tomography. Both fluorodeoxyglucose (a) and methionine (b) scans show recurrence of tumor in left frontal lobe
Figure 7.
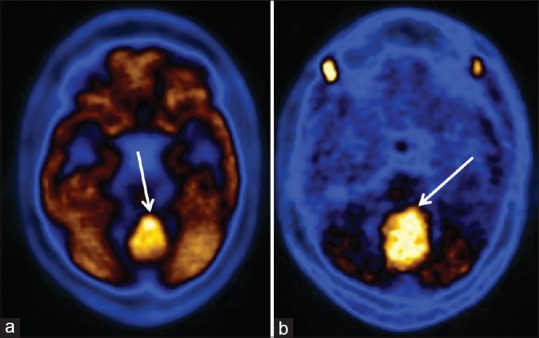
Midline cerebellar medulloblastoma on follow-up positron emission tomography scan after surgery. The recurrent/residual tumor is well-visualized in both fluorodeoxyglucose (a) and C-11 methionine (b) scan in the same region
Figure 2.
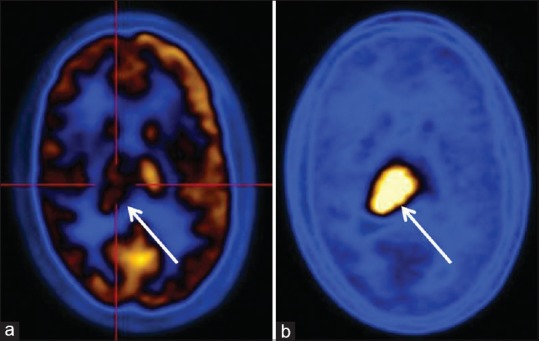
Postradiotherapy case of protoplasmic astrocytoma Grade I. (a) Fluorodeoxyglucose shows hypometabolism in the region of right thalamus. (b) C-11 methionine scan shows viable tumor in right thalamus region
Figure 3.
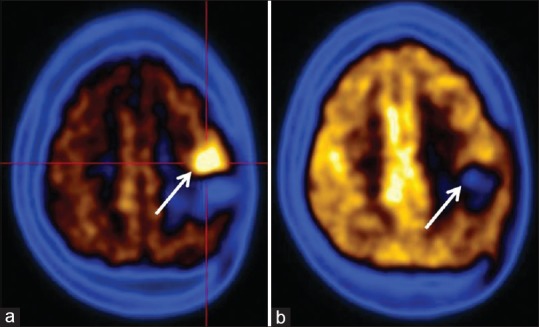
Postoperative case of fibrillary astrocytoma. (a) Fluorodeoxyglucose scan is negative for recurrence of tumor while (b) C-11 methionine scan shows definitive evidence of residual/recurrent tumor in left frontal lobe
Among high-grade astrocytoma, oligodendroglioma, medulloblastoma, and miscellaneous tumor out of 42 patients, 39 had concordant MET and FDG scans. Two patients with oligodendroglioma and 1 with high-grade astrocytoma did not show tracer uptake on FDG scan while MET scan was positive. All the three were later confirmed to harbor malignancy. On semi-quantitative analysis, the mean T/NT ratio was found to be 2.85 ± 0.98 for low-grade glioma and 2.9 ± 0.62 for high-grade gliomas.
DISCUSSION
In spite of great promise shown by [18F]-FDG as an oncology imaging agent, it is well-known now that [18F]-FDG-PET-CT scan has certain limitations when used for tumor detection especially in the brain.[24,25,26,27,28,29,30,31] A comparative study of 19 subjects showed the role of both [F-18] FDG-PET and 201Tl-single photon emission computed tomography (SPECT) in the detection of malignant disease. The sensitivity and specificity of these two modalities were found to be 69% and 40% and 81% and 40% for these two modalities, respectively. Though [F-18] FDG-PET had high sensitivity but specificity of this agent was found to be quite low when compared to the SPECT 201Tl examination.[28] Earlier Wong et al. have also reported the limitation of [F-18] FDG-PET in differentiating current high-grade tumors and radiation necrosis with high accuracy.[32]
Thus, [18F]-FDG-PET is not an ideal modality for imaging of cerebral gliomas. There are several limitation of [18F]-FDG. The sensitivity of detection by [18F]-FDG-PET especially for low-grade glioma is low as tumor to normal brain ratio is low.[33] Moreover, increase in tracer uptake is nonspecific as it is known to concentrate in inflammatory lesions also.[34] Even in tumor cells, 25% of [18F]-FDG concentrations is actually in macrophages.[35] The boundaries of the tumor cannot be easily localized due to high uptake of FDG in the normal brain tissue. The combination of MRI and MR spectroscopy techniques also have these limitations. Therefore, scientists have been working on non-FDG based agents to overcome the above-mentioned difficulties in case of FDG.
Interestingly, increased glucose uptake is associated with increased amino acid transport in cancer cells.[36] PET-CT using [11C]-MET is the most commonly used amino acid used for imaging brain tumors. Earlier clinical trials have suggested that MET-PET is more effective as compared to FDG-PET in delineating the tumor extent especially in the low-grade gliomas, and sensitivity has been reported the sensitivity to vary in the range of 75-95% in various studies.[14,37,38,39]
In a study by Chung et al. showed that 89% of 35 brain tumor patients with hypo- or isometabolic lesions on FDG-PET scan showed a high uptake on MET-PET scan.[39] In our study, 72% of 18 brain tumors with hypometabolic lesions on FDG-PET depicted high uptake on MET-PET scan. Detection rate is better with MET PET-CT in case of low-grade gliomas as compared to CECT and MRI. The study by Ribom et al. showed 30 out of 32 (94%) with low-grade glioma had an increased MET uptake, while only 12 out of 32 (38%) showed contrast enhancement.[40,41] In our study, 18 out of 22 (81.8%) with low-grade glioma had an increased MET uptake. While on FDG scan only 5 out of 22 (22.7%) low-grade gliomas showed recurrence. Hence, the study clearly highlights that MET-PET is better than FDG-PET scans to detect recurrence in low-grade gliomas. Of 18, only 2 cases of tumor came out to be false positive for a tumor on the final diagnosis.
Herholz et al. determined a specificity of 87% using tumor/normal tissue ratio of 1.47 as the cut-off value in a sample of 28 nontumoral cases.[37] None of the ten nontumorous lesions hypometabolic on FDG shared high uptake on MET-PET.[37] In our study, 45 out of 47 (95.7%) patients who had a target to NT ratio of more than 1.47 were found to be positive for recurrence of tumor. Two patients who had a ratio more than 1.47, however, were found to false positive for recurrent malignant disease. On semi-quantitative analysis, mean T/NT ratio was found to be 2.96 ± 0.94 for lesions positive for recurrence of tumor and 1.18 ± 0.74 for lesions negative for recurrence of tumor with [C-11] MET scan. Thus, we suggested that a cut-off value of 1.47 could be quite a useful parameter to distinguish malignant and benign lesion on an MET-PET scan. The reported cases of false positive on MET-PET brain scan include demyelination, necrosis, ischemia, leukoencephalitis, brain abscess, and hematoma.[14,37,38,39,42,43,44,45] In our study, two cases that were found to have a false positive accumulation of methionine were found to have necrotic tissue on the final diagnosis.
Ishii et al. have reported that the positive predictive value of MET-PET is high, but the NPV is expected to be low.[45] Padma et al. demonstrated that 86% (143/166) of patients with low FDG uptake had low-grade, whereas 94% (154/165) with high FDG uptake had high-grade glioma.[46] MET-PET, on the other hand, has not revealed such clear predictive values.[47] On semiquantitative analysis, although a significant difference in the MET uptake of low- and high-grade tumors has been demonstrated.[48] Sasaki et al. have reported a significant difference in the mean SUV of 1.94 ± 0.44 (mean ± standard deviation) for Garde II glioma and 3.20 ± 0.92 for Grade IV glioma.[49] In our study, the recurrent low- and high-grade astrocytoma, however, did not show a significant difference on MET study mean MET for recurrent low-grade astrocytoma being and that for recurrent high-grade astrocytoma being 2.85 ± 0.98 and 2.9 ± 0.62. However, FDG accumulated more avidly in high-grade glioma compared to low-grade glioma.
Several studies have demonstrated that conventional MRI underestimates the tumor extent in glioma.[50,51,52] Improved resolution and slice thickness of up to 3.6 and 3.125 mm, respectively, with the modern PET scanners, have resulted in a better delineation of tumor margins using MET-PET.[14] The extent of tumor delineated by MET-PET was found to be larger than contrast enhanced MRI or CT scan in 67% of brain tumor patients.[53] MET-PET has been found to delineate a larger area of increased uptake than FDG-PET.[54,55] MET-PET and CT-MR have been found to better delineate the residual glioma than CT-MRI alone.[56]
Noninvasive differentiation of recurrent tumor from radiation injury in a symptomatic patient on posttherapy follow-up can be difficult. Contrast enhancement patterns on both CT and MRI can occur in both the conditions caused by breakdown in Blood Brain Barrier (BBB). MET-PET has been reported to be useful by earlier workers in identifying recurrent tumor after surgery or radiotherapy.[57]
In our study, 18 patients out of 22 were found to show recurrence on an MET scan. However, two patients who had radiation necrosis were found to be positive on MET study.
CONCLUSION
The study clearly highlights that [11C]-MET is superior to [18F]-FDG PET scans to detect recurrence in low-grade glioma. The sensitivity, specificity, PPV, and NPV of these two modalities were found to be 50%, 85.7%, 81.8%, and 60% for the FDG scans and 93.3%, 90%, 93.3%, and 90% for [11C]-MET scans for detection of low-grade gliomas. A cut-off value of target to NT value of 1.47 is a useful parameter to distinguish benign from a malignant lesion on an [11C]-MET scan. While [11C]-MET scan was superior to [F-18] FDG scan in low-grade glioma, both [18F]-FDG and [11C]-MET scans were found to be useful in high-grade astrocytoma, oligodendroglioma, and medulloblastoma. [11C]-MET study was not able to distinguish recurrent low- and high-grade glioma as a target to nontarget ratio in both the tumor was not significantly different.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Burger PC, Heinz ER, Shibata T, Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg. 1988;68:698–704. doi: 10.3171/jns.1988.68.5.0698. [DOI] [PubMed] [Google Scholar]
- 2.Kelly PJ, Daumas-Duport C, Scheithauer BW, Kall BA, Kispert DB. Stereotactic histologic correlations of computed tomography-and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62:450–9. doi: 10.1016/s0025-6196(12)65470-6. [DOI] [PubMed] [Google Scholar]
- 3.Pirzkall A, McKnight TR, Graves EE, Carol MP, Sneed PK, Wara WW, et al. MR-spectroscopy guided target delineation for high-grade gliomas. Int J Radiat Oncol Biol Phys. 2001;50:915–28. doi: 10.1016/s0360-3016(01)01548-6. [DOI] [PubMed] [Google Scholar]
- 4.Kubota K, Matsuzawa T, Ito M, Ito K, Fujiwara T, Abe Y, et al. Lung tumor imaging by positron emission tomography using C-11 L-methionine. J Nucl Med. 1985;26:37–42. [PubMed] [Google Scholar]
- 5.Fujiwara T, Matsuzawa T, Kubota K, Abe Y, Itoh M, Fukuda H, et al. Relationship between histologic type of primary lung cancer and carbon-11-L-methionine uptake with positron emission tomography. J Nucl Med. 1989;30:33–7. [PubMed] [Google Scholar]
- 6.Kubota K, Matsuzawa T, Fujiwara T, Abe Y, Ito M, Hatazawa J, et al. Differential diagnosis of solitary pulmonary nodules with positron emission tomography using [11C] L-methionine. J Comput Assist Tomogr. 1988;12:794–6. doi: 10.1097/00004728-198809010-00015. [DOI] [PubMed] [Google Scholar]
- 7.Garnick MB, Fair WR. Combating prostate cancer. Sci Am. 1998;279:74–83. doi: 10.1038/scientificamerican1298-74. [DOI] [PubMed] [Google Scholar]
- 8.Mettlin C, Jones GW, Murphy GP. Trends in prostate cancer care in the United States, 1974-1990: Observations from the patient care evaluation studies of the American College of Surgeons Commission on Cancer. CA Cancer J Clin. 1993;43:83–91. doi: 10.3322/canjclin.43.2.83. [DOI] [PubMed] [Google Scholar]
- 9.Scher HI, Chung LW. Bone metastases: Improving the therapeutic index. Semin Oncol. 1994;21:630–56. [PubMed] [Google Scholar]
- 10.Sagalowsky AI, Wilson JD. Hyperplasia and carcinoma of the prostate. In: Fauci AS, Braunwald E, Iselbacher K, Wilson D, editors. Harrison's Principles of Internal Medicine. 14th ed. New York: McGraw-Hill; 1998. pp. 596–602. [Google Scholar]
- 11.Castelino RA, De La Paz RL, Larson SM. Imaging techniques in cancer. In: De Vita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 4th ed. Philadelphia, PA: Lippincott-Raven; 1993. [Google Scholar]
- 12.Fischer G, Ruschenburg I, Eigenbrodt E, Katz N. Decrease in glucokinase and glucose-6-phosphatase and increase in hexokinase in putative preneoplastic lesions of rat liver. J Cancer Res Clin Oncol. 1987;113:430–6. doi: 10.1007/BF00390036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kracht LW, Friese M, Herholz K, Schroeder R, Bauer B, Jacobs A, et al. Methyl-[11C]-l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging. 2003;30:868–73. doi: 10.1007/s00259-003-1148-7. [DOI] [PubMed] [Google Scholar]
- 14.Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, et al. Delineation of brain tumor extent with [11C] L-methionine positron emission tomography: Local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10:7163–70. doi: 10.1158/1078-0432.CCR-04-0262. [DOI] [PubMed] [Google Scholar]
- 15.Sato N, Suzuki M, Kuwata N, Kuroda K, Wada T, Beppu T, et al. Evaluation of the malignancy of glioma using 11C-methionine positron emission tomography and proliferating cell nuclear antigen staining. Neurosurg Rev. 1999;22:210–4. doi: 10.1007/s101430050018. [DOI] [PubMed] [Google Scholar]
- 16.Tsuyuguchi N, Takami T, Sunada I, Iwai Y, Yamanaka K, Tanaka K, et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery - In malignant glioma. Ann Nucl Med. 2004;18:291–6. doi: 10.1007/BF02984466. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs AH, Kracht LW, Gossmann A, Rüger MA, Thomas AV, Thiel A, et al. Imaging in neurooncology. NeuroRx. 2005;2:333–47. doi: 10.1602/neurorx.2.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs AH, Li H, Winkeler A, Hilker R, Knoess C, Rüger A, et al. PET-based molecular imaging in neuroscience. Eur J Nucl Med Mol Imaging. 2003;30:1051–65. doi: 10.1007/s00259-003-1202-5. [DOI] [PubMed] [Google Scholar]
- 19.Thiel A, Pietrzyk U, Sturm V, Herholz K, Hövels M, Schröder R. Enhanced accuracy in differential diagnosis of radiation necrosis by positron emission tomography-magnetic resonance imaging coregistration: Technical case report. Neurosurgery. 2000;46:232–4. [PubMed] [Google Scholar]
- 20.Van Laere K, Ceyssens S, Van Calenbergh F, de Groot T, Menten J, Flamen P, et al. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: Sensitivity, inter-observer variability and prognostic value. Eur J Nucl Med Mol Imaging. 2005;32:39–51. doi: 10.1007/s00259-004-1564-3. [DOI] [PubMed] [Google Scholar]
- 21.Miyagawa T, Oku T, Uehara H, Desai R, Beattie B, Tjuvajev J, et al. “Facilitated” amino acid transport is upregulated in brain tumors. J Cereb Blood Flow Metab. 1998;18:500–9. doi: 10.1097/00004647-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, et al. Expression of L-type amino acid transporter 1 (LAT1) in neuroendocrine tumors of the lung. Pathol Res Pract. 2008;204:553–61. doi: 10.1016/j.prp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs AH, Winkler A, Dittmar C, Gossman A, Deckert M, Kracht L, et al. Molecular and functional imaging technology for the development of efficient treatment strategies for gliomas. Technol Cancer Res Treat. 2002;1:187–204. doi: 10.1177/153303460200100304. [DOI] [PubMed] [Google Scholar]
- 24.Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: Basic aspects and clinical applications in oncology. J Nucl Med. 2001;42:432–45. [PubMed] [Google Scholar]
- 25.Kleihues P, Cavenee WK. Pathology and Genetics of Tumors of the Nervous System (WHO) Lyon, France: International Agency for Research on Cancer (IARC Press); 2000. [Google Scholar]
- 26.Pirotte B, Goldman S, David P, Wikler D, Damhaut P, Vandesteene A, et al. Stereotactic brain biopsy guided by positron emission tomography (PET) with [F-18]fluorodeoxyglucose and [C-11]methionine. Acta Neurochir Suppl. 1997;68:133–8. doi: 10.1007/978-3-7091-6513-3_25. [DOI] [PubMed] [Google Scholar]
- 27.Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP. Differentiating recurrent tumor from radiation necrosis: Time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol. 1998;19:407–13. [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn D, Follett KA, Bushnell DL, Nathan MA, Piper JG, Madsen M, et al. Diagnosis of recurrent brain tumor: Value of 201Tl SPECT vs 18F-fluorodeoxyglucose PET. AJR Am J Roentgenol. 1994;163:1459–65. doi: 10.2214/ajr.163.6.7992747. [DOI] [PubMed] [Google Scholar]
- 29.Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001;96:191–7. doi: 10.1002/ijc.1016. [DOI] [PubMed] [Google Scholar]
- 30.Olivero WC, Dulebohn SC, Lister JR. The use of PET in evaluating patients with primary brain tumours: Is it useful? J Neurol Neurosurg Psychiatry. 1995;58:250–2. doi: 10.1136/jnnp.58.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belohlávek O, Simonová G, Kantorová I, Novotný J, Jr, Liscák R. Brain metastases after stereotactic radiosurgery using the Leksell gamma knife: Can FDG PET help to differentiate radionecrosis from tumour progression? Eur J Nucl Med Mol Imaging. 2003;30:96–100. doi: 10.1007/s00259-002-1011-2. [DOI] [PubMed] [Google Scholar]
- 32.Wong TZ, van der Westhuizen GJ, Coleman RE. Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am. 2002;12:615–26. doi: 10.1016/s1052-5149(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 33.Benard F, Romsa J, Hustinx R. Imaging gliomas with positron emission tomography and single-photon emission computed tomography. Semin Nucl Med. 2003;33:148–62. doi: 10.1053/snuc.2003.127304. [DOI] [PubMed] [Google Scholar]
- 34.Kubota K, Ishiwata K, Yamada S, Kubota R, Sato T, Takahashi J, et al. Dose-responsive effect of radiotherapy on the tumor uptake of L-[methyl-11C] methionine; feasibility for monitoring recurrence of tumor. Int J Rad Appl Instrum B. 1992;19:27–32. doi: 10.1016/0883-2897(92)90181-w. [DOI] [PubMed] [Google Scholar]
- 35.Kubota R, Kubota K, Yamada S, Tada M, Takahashi T, Iwata R, et al. Methionine uptake by tumor tissue: A microautoradiographic comparison with FDG. J Nucl Med. 1995;36:484–92. [PubMed] [Google Scholar]
- 36.Isselbacher KJ. Increased uptake of amino acids and 2-deoxy-D-glucose by virus-transformed cells in culture. Proc Natl Acad Sci U S A. 1972;69:585–9. doi: 10.1073/pnas.69.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herholz K, Hölzer T, Bauer B, Schröder R, Voges J, Ernestus RI, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology. 1998;50:1316–22. doi: 10.1212/wnl.50.5.1316. [DOI] [PubMed] [Google Scholar]
- 38.Braun V, Dempf S, Weller R, Reske SN, Schachenmayr W, Richter HP. Cranial neuronavigation with direct integration of (11) C methionine positron emission tomography (PET) data - Results of a pilot study in 32 surgical cases. Acta Neurochir (Wien) 2002;144:777–82. doi: 10.1007/s00701-002-0942-5. [DOI] [PubMed] [Google Scholar]
- 39.Chung JK, Kim YK, Kim SK, Lee YJ, Paek S, Yeo JS, et al. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:176–82. doi: 10.1007/s00259-001-0690-4. [DOI] [PubMed] [Google Scholar]
- 40.Viader F, Derlon JM, Petit-Taboué MC, Shishido F, Hubert P, Houtteville JP, et al. Recurrent oligodendroglioma diagnosed with 11C-L-methionine and PET: A case report. Eur Neurol. 1993;33:248–51. doi: 10.1159/000116947. [DOI] [PubMed] [Google Scholar]
- 41.Ribom D, Schoenmaekers M, Engler H, Smits A. Evaluation of 11C-methionine PET as a surrogate endpoint after treatment of grade 2 gliomas. J Neurooncol. 2005;71:325–32. doi: 10.1007/s11060-004-2031-5. [DOI] [PubMed] [Google Scholar]
- 42.Dethy S, Goldman S, Blecic S, Luxen A, Levivier M, Hildebrand J. Carbon-11-methionine and fluorine-18-FDG PET study in brain hematoma. J Nucl Med. 1994;35:1162–6. [PubMed] [Google Scholar]
- 43.Dethy S, Manto M, Kentos A, Konopnicki D, Pirotte B, Goldman S, et al. PET findings in a brain abscess associated with a silent atrial septal defect. Clin Neurol Neurosurg. 1995;97:349–53. doi: 10.1016/0303-8467(95)00067-t. [DOI] [PubMed] [Google Scholar]
- 44.Massager N, David P, Goldman S, Pirotte B, Wikler D, Salmon I, et al. Combined magnetic resonance imaging- and positron emission tomography-guided stereotactic biopsy in brainstem mass lesions: Diagnostic yield in a series of 30 patients. J Neurosurg. 2000;93:951–7. doi: 10.3171/jns.2000.93.6.0951. [DOI] [PubMed] [Google Scholar]
- 45.Ishii K, Ogawa T, Hatazawa J, Kanno I, Inugami A, Fujita H, et al. High L-methyl-[11C] methionine uptake in brain abscess: A PET study. J Comput Assist Tomogr. 1993;17:660–1. doi: 10.1097/00004728-199307000-00029. [DOI] [PubMed] [Google Scholar]
- 46.Padma MV, Said S, Jacobs M, Hwang DR, Dunigan K, Satter M, et al. Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol. 2003;64:227–37. doi: 10.1023/a:1025665820001. [DOI] [PubMed] [Google Scholar]
- 47.Utriainen M, Metsähonkala L, Salmi TT, Utriainen T, Kalimo H, Pihko H, et al. Metabolic characterization of childhood brain tumors: Comparison of 18F-fluorodeoxyglucose and 11C-methionine positron emission tomography. Cancer. 2002;95:1376–86. doi: 10.1002/cncr.10798. [DOI] [PubMed] [Google Scholar]
- 48.Kameyama M, Shirane R, Itoh J, Sato K, Katakura R, Yoshimoto T, et al. The accumulation of 11C-methionine in cerebral glioma patients studied with PET. Acta Neurochir (Wien) 1990;104:8–12. doi: 10.1007/BF01842885. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki M, Kuwabara Y, Yoshida T, Nakagawa M, Fukumura T, Mihara F, et al. A comparative study of thallium-201 SPET, carbon-11 methionine PET and fluorine-18 fluorodeoxyglucose PET for the differentiation of astrocytic tumours. Eur J Nucl Med. 1998;25:1261–9. doi: 10.1007/s002590050294. [DOI] [PubMed] [Google Scholar]
- 50.Tovi M. MR imaging in cerebral gliomas analysis of tumour tissue components. Acta Radiol Suppl. 1993;384:1–24. [PubMed] [Google Scholar]
- 51.Hawighorst H, Schreiber W, Knopp MV, Essig M, Engenhart-Cabilic R, Brix G, et al. Macroscopic tumor volume of malignant glioma determined by contrast-enhanced magnetic resonance imaging with and without magnetization transfer contrast. Magn Reson Imaging. 1996;14:1119–26. doi: 10.1016/s0730-725x(96)00241-x. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992;34:463–9. doi: 10.1007/BF00598951. [DOI] [PubMed] [Google Scholar]
- 53.Voges J, Herholz K, Hölzer T, Würker M, Bauer B, Pietrzyk U, et al. 11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: A tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact Funct Neurosurg. 1997;69:129–35. doi: 10.1159/000099864. [DOI] [PubMed] [Google Scholar]
- 54.Ogawa T, Shishido F, Kanno I, Inugami A, Fujita H, Murakami M, et al. Cerebral glioma: Evaluation with methionine PET. Radiology. 1993;186:45–53. doi: 10.1148/radiology.186.1.8380108. [DOI] [PubMed] [Google Scholar]
- 55.Ogawa T, Inugami A, Hatazawa J, Kanno I, Murakami M, Yasui N, et al. Clinical positron emission tomography for brain tumors: Comparison of fludeoxyglucose. AJNR Am J Neuroradiol. 1996;17:345–53. [PMC free article] [PubMed] [Google Scholar]
- 56.Singhal T, Narayanan TK, Jacobs MP, Bal C, Mantil JC. MET-PET in the clinical management of cerebral gliomas 17F 18 and L-methyl- 11C-methionine. J Nucl Med. 2012;53:1709–15. doi: 10.2967/jnumed.111.102533. [DOI] [PubMed] [Google Scholar]
- 57.Grosu AL, Lachner R, Wiedenmann N, Stärk S, Thamm R, Kneschaurek P, et al. Validation of a method for automatic image fusion (BrainLAB System) of CT data and 11C-methionine-PET data for stereotactic radiotherapy using a LINAC: First clinical experience. Int J Radiat Oncol Biol Phys. 2003;56:1450–63. doi: 10.1016/s0360-3016(03)00279-7. [DOI] [PubMed] [Google Scholar]


