Abstract
Background
Type 1 Diabetes TrialNet is an NIH-sponsored clinical trial network aimed at altering the disease course of type 1 diabetes. The purpose of this study is to evaluate age-dependent heterogeneity in clinical, metabolic, and immunologic characteristics of individuals with recent-onset type 1 diabetes (T1D), to identify cohorts of interest and to aid in planning of future studies.
Methods
883 individuals with recent onset T1D involved in five TrialNet studies were categorized by age as: ≥ 18, age 12-17, ages 8-12, and age <8. Data was compared with healthy age-matched subjects in the National Health and Nutrition Examination Survey.
Results
While only 2.0 % of individuals overall were excluded due to insufficient C-peptide values (<0.2 pmol/ml), 9.0% of those < age 8 did not meet this entry criteria. Leukopenia was present in 21.2% of individuals and lymphopenia in 11.6%; these frequencies were markedly different than age-matched healthy population. 24.5% of the cohort was overweight or obese. 31.1% of adults and 21.1% of children had neither HLA DR3 nor DR4.
Conclusions
The ability of recent onset T1D patients to meet key entry criteria for TrialNet studies, including C-peptide >0.2 pmol/ml, varies by age. Lower C-peptide level requirements for younger participants should be considered in the design of future trials. These data also highlight subgroups of type 1 diabetes patients, such as those with abnormal WBC or who are overweight, which allow for targeted studies of etiopathology and interventions.
Keywords: type 1 diabetes clinical trials; Type 1 Diabetes TrialNet, C-peptide
INTRODUCTION
Type 1 Diabetes TrialNet is an international consortium of clinical diabetologists and immunologists whose aim is to conduct multiple clinical trials to alter the natural history of the disease; specifically by delaying or stopping beta cell destruction. In these studies, Rituximab[1] and Abatacept[2] both demonstrated improvement in residual insulin secretion in drug as compared to placebo treated individuals, whereas GAD65-alum[3], MMF/DZB[4] and Canakinumab[5] did not. Within all studies and treatment arms however, heterogeneous responses were apparent. For example, we and others have highlighted age as an important variable accounting for some of this heterogeneity, finding significant differences in the disease course in children as compared with adults [6-8]. As a result, future studies may be restricted to narrower age ranges of participants or age category may be used as a stratification variable.
With the aim to further dissect heterogeneity in type 1 diabetes, we use combined TrialNet data to evaluate clinical, immunological, and metabolic characteristics of these subjects at study entry according to age. This evaluation should aid in the planning and design of future type 1 diabetes intervention trials.
MATERIALS AND METHODS
Clinical sites
Studies took place at 15 clinical centers in North America and one in Italy. Protocols and consent documents were approved by the institutional review board or independent ethics committee at each participating clinical center as previously reported and all subjects underwent informed consent and assent prior to participation in any study activities.
Study Interventions
The studies were designed to evaluate therapies with an array of mechanisms aimed at immunomodulation to preserve beta cells, including immunosuppressive agents (mycophenolate mofetil [MMF] and daclizumab), a therapy directed at B cells (anti-CD20 rituximab), a therapy directed at antigen-specific tolerance (GAD-alum vaccine), co-stimulation blockade (abatacept), and anti IL1B (canakinumab).
Eligibility Criteria
Study eligibility criteria were similar across studies with the exception of age and autoantibodies as described below. Inclusion criteria included Mixed Meal Tolerance Test (MMTT) stimulated peak C-peptide levels of at least 0.2 pmol/ml conducted within 3 weeks to 3 months after diagnosis, and randomization within 100 days of clinical diagnosis.
Patients were eligible to participate in the GAD-alum study if they had glutamic acid decarboxylase-65 antibodies (GAD65ab). Eligibility for all other studies required at least one diabetes-related autoantibody: microassayed insulin antibodies (mIAA) [if duration of insulin therapy was less than 7 days]; GAD65ab; insulinoma antigen 2 antibodies (IA-2ab) or islet-cell autoantibodies (ICA). ICA was often measured only when mIAA, GAD65ab, and IA-2ab were negative. In sum, a total of 754 subjects in the five studies underwent testing for all three antibodies (GADab, ICA, and IA-2ab). Znt8 antibodies were only measured in ten otherwise antibody negative subjects in the most recent study testing canakinumab. All trials had age 45 as the upper age limit for eligibility; the lower age limit for eligibility was 8 years for Rituximab and MMF/DZB studies, 6 years for canakinumab and abatacept studies and 3 years for the GAD-alum trial.
Exclusion criteria included complicating medical issues, active infection, positive PPD, serologic evidence of HIV, hepatitis B or hepatitis C infection, history of immunodeficiency or lymphopenia, or chronic use of steroids or other immunosuppressive agents. EBV and CMV serology was measured in all 5 studies along with EBV PCR to rule out active infection in all studies with the exception of the GAD-alum trial.
Study Assessments
Similar, but not identical information was obtained during all trials. For example, in some studies, HLA typing was performed on all screened subjects, while in others HLA was done only on randomized subjects.
Methods
Samples were sent to a central laboratory for measurements of HbA1c, C-peptide, glucose, autoantibodies, chemistries, viral serology and PCR as previously published. CBCs were determined at the center's local clinical laboratory. All local clinical laboratories at US sites were CLIA certified, Canadian sites were certified by Ontario Medical Association Laboratory Quality Management Program-Laboratory Services; similar certification was obtained at the Italian site. Values outside normal ranges for either the central laboratory or local laboratory measures were graded according to Common Terminology Criteria for Adverse Events (CTCAE) criteria.
While children were enrolled in all of these studies, the age of children eligible for trials may be limited in some studies due to regulatory or ethical considerations. Frequently, considerations for enrollment of children involve consideration of emotional and intellectual maturation stages as children vs adults (< or ≥ age 18); teenagers ages 12-17; children who are considered developmentally mature enough to understand study participation (age 8-12) and younger children (<age 8). These age categories were thus applied to the data presented.
For comparison of TrialNet type 1 diabetes participants with healthy subjects, CBC data from 2009-2010 National Health and Nutrition Examination Survey (NHANES) study participants, ages 3-45, was used (n=5172). Age and gender-specified cutoffs for normal values available in the 2009-2010 NHANES study documentation[9] were applied to WBC, PMN and lymphocyte counts.
Analysis
Categorical variables were compared among age groups by Pearson's chi-square test or Fisher Exact test when cell sizes were insufficient. Continuous variables were summarized either by mean and standard deviation or median and inter-quartile range. ANOVA F-tests were done to determine significant differences among age groups in mean values. The association of age with level of WBC, polymorphonuclear neutrophils (PMN) and lymphocyte counts was tested using the multivariable logistic regression model. Time from diagnosis, HbA1c, autoantibody status and c-peptide level were included in the model to adjust for possible confounding factors. Tests of significance were two-tailed. Probability (p) value < 0.05 was considered to be statistically significant. Statistical analyses were performed with SAS Version 9.2 (Cary, NC).
RESULTS
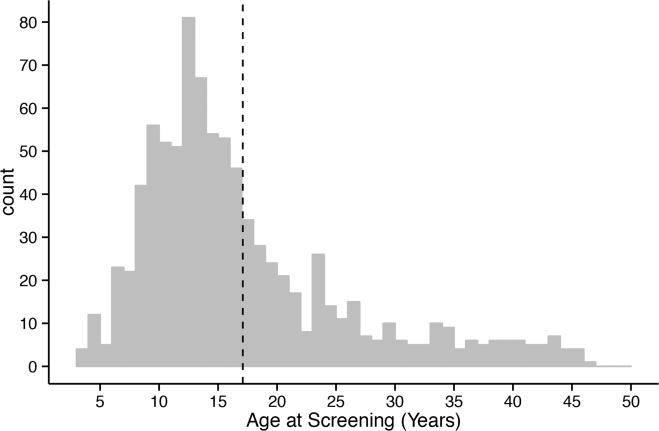
A total of 883 subjects were screened and 541 (61%) patients enrolled in one of the five intervention trials. The mean age of patients who presented for screening was 17.1 (±9.0) years, with a range from 3.5 to 46 years; the age distribution favored younger subjects (Figure 1). Descriptive characteristics of the patients screened for the intervention studies by age category are presented in Table 1. 90.5% of research participants were white and 8.8% were Hispanic or Latino. They were more often male (58.9%), particularly among older subjects, and were screened a mean of 54 days and a median of 56 days from type 1 diabetes diagnosis. While the information was not available on almost a third of participants, 24.7% of the participants reported having other family members with type 1 diabetes and 5.3% report other autoimmune disease themselves or within the family.
Figure 1.
Age distribution of participants screened for type 1 diabetes TrialNet studies
TABLE 1.
Descriptive characteristics by age category
| Age <8 (N=66) | Age 8-12 (N=203) | Age 12-17 (N=299) | Age>17 (N=315) | Total (N=883) | P Value* | |
|---|---|---|---|---|---|---|
| Race | 0.167 | |||||
| White | 55(83.34%) | 182(89.66%) | 278(92.98%) | 284(90.16%) | 799(90.49%) | |
| Black or African America | 3(4.54%) | 4(1.97%) | 3(1.00%) | 10(3.17%) | 20(2.26%) | |
| Other | 3(4.54%) | 10(4.93%) | 13(4.35%) | 10(3.17%) | 36(4.08%) | |
| Unknown | 5(7.58%) | 7(3.45%) | 5(1.67%) | 11(3.50%) | 28(3.17%) | |
| Ethnicity | 0.113 | |||||
| Not Hispanic or Latino | 56(84.85%) | 178(87.68%) | 268(89.63%) | 286(90.79%) | 788(89.24%) | |
| Hispanic or Latino | 7(10.61%) | 23(11.33%) | 28(9.36%) | 20(6.35%) | 78(8.83%) | |
| Unknown | 3(4.55%) | 2(0.99%) | 3(1.00%) | 9(2.86%) | 17(1.93%) | |
| Gender | 0.010 | |||||
| Male | 34(51.52%) | 104(51.23%) | 193(63.55%) | 189(60.00%) | 520(58.89%) | |
| Duration of T1D (days) | 52(36-71) | 55(41-72) | 58(39-73) | 57(37-72) | 56(38-72) | 0.717 |
| Family members with T1D? | 0.023 | |||||
| Yes | 23(34.85%) | 55(27.09%) | 68(22.74%) | 72(22.86%) | 218(24.69%) | |
| No | 23(34.85%) | 104(51.23%) | 146(48.83%) | 136(43.17%) | 409(46.32%) | |
| Unknown | 20(30.30%) | 44(21.67%) | 85(28.43%) | 107(33.97%) | 256(28.99%) | |
| Other Autoimmune Disease? | 0.232 | |||||
| Yes | 2(3.03%) | 13(6.40%) | 10(3.34%) | 22(6.98%) | 47(5.32%) | |
| No | 55(83.33%) | 174(85.71%) | 260(86.96%) | 254(80.63%) | 743(84.14%) | |
| Unknown | 9(13.64%) | 16(7.89%) | 29(9.70%) | 39(12.39%) | 93(10.54%) | |
| BMI Z Score; mean (± SD) | 0.37(± 0.90) | 0.44(±0.99) | 0.418(±0.88) | 0.24(±0.92) | 0.39(±0.92) | 0.294 |
| Overweight1 | 5(7.58%) | 33(16.26%) | 43(14.38%) | 63(20.00%) | 144(16.31%) | 0.045 |
| Obese2 | 5 (7.58%) | 21(10.34%) | 17(5.69%) | 29(9.21%) | 72(8.15%) | |
| HbA1c | 0.658 | |||||
| N Tested | 35 | 142 | 237 | 216 | 630 | |
| % (± SD) | 7.01(± 0.69) | 7.10(±1.27) | 7.16 ±1.27) | 7.01(±1.53) | 7.09 ±1.34) | |
| MMTT C-peptide; pmol/mL (± SD) | ||||||
| N Tested | 52 | 192 | 267 | 269 | 780 | |
| 2-hr AUC mean | 1.21 (±0.53) | 1.92 (±0.96) | 2.40 (±1.08) | 2.48 (±1.13) | 2.24 (±1.14) | <0.001 |
| 2-hr peak | 1.52 (±0.65) | 2.29 (±1.22) | 2.97 (±1.34) | 3.19 (±1.71) | 2.78 (±1.50) | <0.001 |
| Auto-antibodies; N positive/N tested (%) | ||||||
| GAD65 | 28/50(56%) | 100/165(61%) | 180/255(80%) | 203/293(69%) | 511/763(67%) | 0.047 |
| IA-2ab | 34/50 (68%) | 108/165(66%) | 175/255(69%) | 135/293(46%) | 452/763(59%) | <0.001 |
| ICA | 43/63 (68%) | 130/202(64%) | 188/287(66%) | 153/311(43%) | 514/863(60%) | <0.001 |
** For categorical data, Chi-sq or Fisher exact test was used; for continuous data, ANOVA was done to test the mean difference among the groups.
BMI>25 for age>18 or zBMI 85th-95th %ile for age<18.
BMI>30 for age>18 or zBMI >95th %ile for age<18.
The proportions of overweight and obese individuals were different by age category (p=0.045). While the mean BMI for adults was within the normal range, 24.5% of all enrolled subjects were classified as overweight or obese by BMI or BMIZ criteria including 43 obese children.
The mean HbA1c was 7.09% and this did not differ significantly by age category. Stimulated C-peptide values increased across age categories whether measured by AUC or peak value during MMTT. However, there was no significant relationship between age and HbA1c or age and C-peptide when considered as continuous variables (data not shown).
When considering only those subjects who were tested for GAD65, IA-2ab, and ICA, adults were less likely ICA or IA-2ab positive then the cohorts under age 18, yet little differences were found with prevalence of antibodies between the three younger cohorts. Too few subjects were assessed for ZnT8 antibodies (N=10) to evaluate the effect of age on rate of positivity.
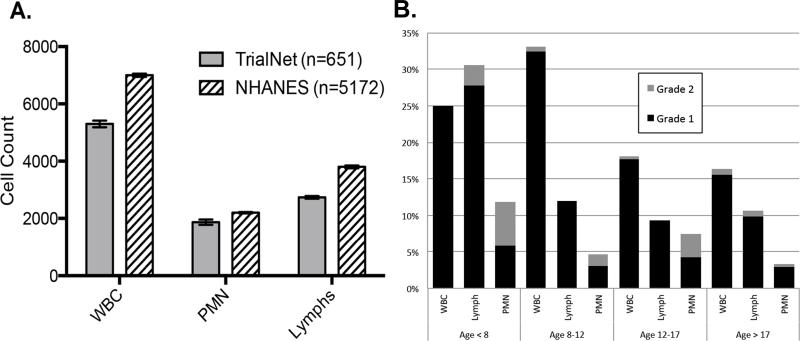
Figure 2 highlights results from laboratory tests obtained during eligibility assessment between 21-100 days from type 1 diabetes diagnosis. Median white blood cell, lymphocyte and PMN counts were significantly lower than healthy NHANES subjects of the same age (Figure 2A). In total, 21.2% of T1D subjects had a WBC below the lower limit of normal. Most of these low WBC were Grade 1 by CTCAE criteria (between 3000 and lower limit of normal). Abnormal WBC counts were more common in children <12 years (Figure 2B). Age at diagnosis, time from diagnosis, HbA1c, autoantibody status and C-peptide level (both 4h and 2h AUC) were not found to be significantly associated with a low total WBC using logistic regression analysis (data not shown). Lymphopenia was found in 11.6% TrialNet (vs. 1.4% of the NHANES population, p<0.001) with most abnormalities being Grade 1 (between 800 and lower limit of normal). The frequency of lymphopenia was also age dependent, being more commonly observed in the youngest subjects. This is in contrast to the proportion of those with abnormally low PMN counts (grade 1 - 3.6%, grade 2 -1.9%) that did not vary significantly by age.
Figure 2.
(A) Median cell counts by cell type for new-onset type 1 diabetes patients (TrialNet) and healthy controls [National Health and Nutrition Examination Survey (NHANES)]. Error bars represent 95% confidence intervals. (B) Frequency of cell count abnormalities by Common Terminology Criteria for Adverse Events criteria and age group in type 1 diabetes TrialNet participants. White blood cell (WBC), grade 1 = 3000–lower limit of normal (LLN), grade 2 = 2500–3000; Lymphocytes, grade 1 = 800–LLN, grade 2 = 500–800; polymorphonuclear neutrophils (PMN), grade 1 = 1500–LLN, grade 2 = 1000–1500
Risk for infectious viral disease was also assessed. A total of 29.4% of individuals were CMV IgG positive at screening and this was not different by age. In contrast 53.3% of individuals were EBV IgG positive with an increasing trend with age (p=0.001). While 29.1% of those < age 8 were EBV IgG positive, 75.8% of adults had prior exposure to EBV. Recent or active infection denoted by IgM antibodies was unusual for both CMV and EBV with only about 1% positive for each. About 4% of individuals in which EBV viral load was determined by PCR were deemed viral load positive; this did not differ by age category as shown in the Supplemental Table 1.
HLA information was available on 661 subjects, including all that actually enrolled in the study. Among these individuals, 75.5% carried at least one high risk DR3 or DR4 allele and only 1% had DQB1*0602. HLA did not differ significantly by age both overall (p=0.60), and with respect to HLA type although no subjects < age 8 had DQB1*0602 (Supplemental Table 1).
There were many reasons why patients did not enroll in a study and these varied by age category as detailed in Supplemental Table 2.
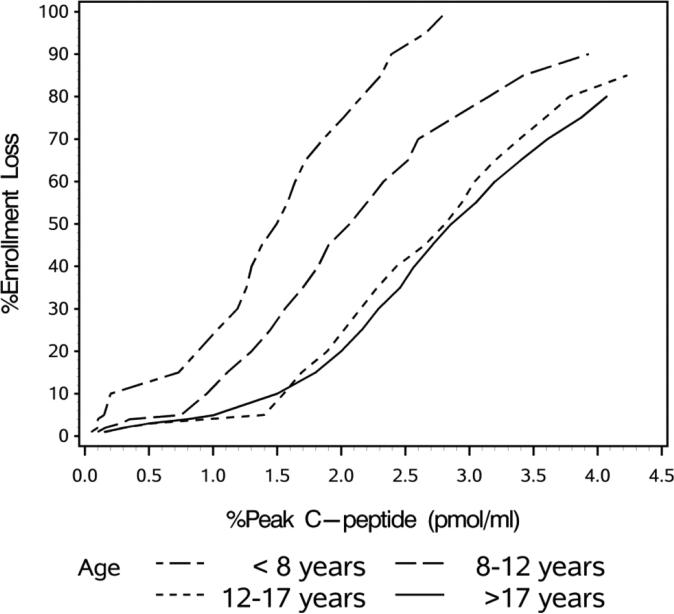
A stimulated C-peptide value less than <0.2 pmol/ml was the reason for exclusion in only 18 (2.0%) of the 883 patients screened. This was significantly more common in younger subjects, accounting for 9% of those under age 8. We evaluated how changing the minimum entry C-peptide values would have impacted enrollment (Figure 3). As illustrated, decreasing the minimum peak C-peptide value required to 0.1 pmol/ml would have decreased the number of children < age 8 who would have been excluded to 4%. No older subjects would have been excluded. Conversely, raising the entry criteria to 0.5 pmol/ml would have excluded more than 10% of the younger children as well as 4% of those age 8-12 and 3% of older subjects.
Figure 3.
Predicted enrollment loss according to C-peptide level required for study eligibility. Percent of individuals not eligible for enrollment according to age <8 years, 8–12 years, 12–17 years and >17 years
DISCUSSION
In this analysis of a large cohort of 883 new onset type 1 diabetes patients <100 days from diagnosis using combined data from five recently conducted studies with similar entry criteria, we evaluated the characteristics of subjects by age categories that reflect common “cut points” in clinical trials. The results reported here provide information about key elements of entry criteria and other characteristics by age categories which both suggest subgroup cohorts for directed studies and to facilitate design of future clinical trials.
Four key findings stand out from our analyses.
First, the requirement to have a stimulated C-peptide value ≥0.2 pmol/ml excluded only 2.0% of the 883 screened subjects. This criterion had been selected for TrialNet studies on the basis of the Diabetes Control and Complications Trial (DCCT) analysis suggesting this was a clinically significant value among DCCT subjects all of whom were over age 13[10]. We found that the impact of this C-peptide value on eligibility was strongly age dependent as 9% of those < age 8 failed to meet this entry criterion.
Recent re-analysis of DCCT data indicates that even lower C-peptide values are associated with reductions in hypoglycemia and retinopathy[11], thus suggesting that 0.2 pmol/ml should not necessarily be the level required for entry into clinical trials to preserve beta cell function. We therefore explored the impact of differing levels of C-peptide on enrollment by age group. We found that reducing the required stimulated C-peptide level by half (0.1 pmol/ml) would exclude 4% of children under age 8; less than half the amount excluded when using 0.2 pmol/ml. In other studies, as suggested by regulators, much higher stimulated C-peptide levels (0.4 pmol/ml) have been used as entry criteria with the aim to be able to reliably measure possible adverse effects of therapy (i.e. significant worsening rather than maintenance of beta cell function). This value would exclude more than 10% of the youngest cohort, and less than 5% of older individuals. While we and others have previously reported that younger children have lower C-peptide levels at the time of presentation, the data here for the first time provide needed quantification about the impact of C-peptide entry criteria according to age. Lower levels of C-peptide as entry criteria should be considered in trials of children less than age 8 so as not to exclude a significant proportion of otherwise eligible subjects. This is particularly important in light of the disproportionate increase in incidence of type 1 diabetes among the very young
Third, the mean WBC, lymphocyte and PMN counts for our type 1 diabetes subjects <100 days from diagnosis were all significantly lower than healthy, age-matched NHANES study volunteers. Further, in our TrialNet studies, just over 20% of individuals screened for our studies had leukopenia, as defined by their local laboratories age related normal values. This high frequency of slightly abnormal WBC is markedly different than that found in age-matched NHANES subjects. Similarly, about 4% of subjects had mild neutropenia and 2% of subjects presented with more severely abnormal values (PMN counts between 1000-1500). Findings regarding lymphocytes were similar to those observed with total leukocytes, with more than 10% of individuals having mild abnormalities; markedly more than seen in healthy NHANES individuals. Others have reported leukocyte abnormalities associated with onset of type 1 diabetes [12] and they may reflect the etiopathology of disease or serve as a clinical biomarker of a subgroup of individuals who develop type 1 diabetes..
Finally, around a quarter of our subjects were considered overweight or obese. This is both consistent with increasing body weight in the general population and with what has been reported in other studies [13]. While BMI had no impact on response to immunomodulatory therapies tested to date, these data raise the possibility that interventions targeting exercise and weight loss or insulin action in selected subsets might reduce the weight associated insulin resistance and improve glucose tolerance. Whether this, in turn, would reduce beta cell demand and prolong endogenous beta cell function is unknown.
Our data provide reassurance that enrollment of different age groups should have limited impact on most, but not all, other eligibility parameters. For example, there was little difference in the frequency of adults as compared with children with respect to the absence of DR3 or DR4 alleles. However, overall, these data highlight the recruitment challenge for a study that requires specific alleles; for example, a requirement for DR4 would exclude about 40% of subjects. Similarly, in considering a hypothetical trial requiring prior exposure to EBV (i.e. past infection indicated by IgG positivity), 70% of children under age 8 and about 60% of older children would not be eligible as compared with less than 25% of adults.
Designing entry criteria for phase 2 clinical trials requires careful consideration of potential efficacy (aiming to include those subjects in which efficacy of therapy could potentially be determined), safety (aiming to exclude those who may be potentially susceptible to increased risks), and future applicability of therapy (avoiding unnecessary exclusion of populations that may benefit). These considerations then must be judged in the context of feasibility. Too stringent criteria may make the trial impossible to conduct or may prolong the duration of the trial so long as to exhaust the resources of funders or make answering the question ethically and scientifically moot. Thus, decisions about entry criteria particularly when conducting trials of new therapeutic indications often involve considered judgments heavily influenced by the age of the subject for ethical and regulatory considerations. These decisions must be made in the context of the biology of the disease. We now know that there are differences in the rate of fall of C-peptide between children and adults after type 1 diabetes diagnosis and that preservation of C-peptide long after diagnosis depends upon the age at diagnosis. Therefore, age should be considered as part of entry criteria and analysis for all type 1 diabetes trials.
In summary, collective Type 1 Diabetes TrialNet data from subjects in five new onset trials allow for robust analysis of the clinical, immunological, and metabolic characteristics of these subjects at study entry according to age. In this way, we have identified cohorts with selected characteristics such as abnormal WBC or obesity that may allow for more targeted investigation or interventions to be undertaken. This analysis also provides key data important for planning future trials, particularly those that may have more specific requirements due to safety concerns or scientific rationale.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the patients and their families for their participation in Type 1 Diabetes TrialNet studies.
FUNDING
The sponsor of the trials was the Type 1 Diabetes TrialNet Study Group. Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061016, U01 DK061034, U01 DK061036, U01 DK061040, U01 DK061041, U01 DK061042, U01 DK061055, U01 DK061058, U01 DK084565, U01 DK085453, U01 DK085461, U01 DK085463, U01 DK085466, U01 DK085499, U01 DK085505, U01 DK085509, and a contract HHSN267200800019C; the National Center for Research Resources, through Clinical Translational Science Awards UL1 RR024131, UL1 RR024139, UL1 RR024153, UL1 RR024975, UL1 RR024982, UL1 RR025744, UL1 RR025761, UL1 RR025780, UL1 RR029890, UL1 RR031986, and General Clinical Research Center Award M01 RR00400; the Juvenile Diabetes Research Foundation International (JDRF); and the American Diabetes Association (ADA). The contents of this Article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, JDRF, or ADA. Members of the TrialNet Study Group are listed in the online appendix.
Abbreviations
- CMV
Cytomegalovirus
- EBV
Epstein Barr Virus
- GAD65ab
glutamic acid decarboxylase-65 antibodies
- ICA
Islet Cell Autoantibodies
- mIAA
microassayed insulin antibodies
- NHANES
National Health and Nutrition Examination Survey
- TrialNet
Type 1 diabetes TrialNet
Footnotes
CONTRIBUTION STATEMENT
All authors are members of the Type 1 Diabetes TrialNet study group and as such contributed to data used in this paper. JBB, PX, CJG and CAB wrote the manuscript. Drs. Greenbaum and Beam are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. DMW and AJB contributed to discussion and reviewed/edited the manuscript.
Clinical Trial Registration Numbers: NCT00100178, NCT00279305, NCT00529399, NCT00505375 and NCT00947427.
DUALITY OF INTEREST
The authors have no conflicts of interest to report pertinent to this manuscript.
REFERENCES
- 1.Pescovitz MD, Greenbaum CJ, Bundy B, et al. B-lymphocyte depletion with rituximab and beta-cell function: two-year results. Diabetes Care. 2014;37(2):453–9. doi: 10.2337/dc13-0626. doi: 10.2337/dc13-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–9. doi: 10.1016/S0140-6736(11)60886-6. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludvigsson J, Krisky D, Casas R, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366(5):433–42. doi: 10.1056/NEJMoa1107096. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb PA, Quinlan S, Skyler JS, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care. 2010;33(4):826–32. doi: 10.2337/dc09-1349. doi: 10.2337/dc09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran A, Bundy B, Becker DJ, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381(9881):1905–15. doi: 10.1016/S0140-6736(13)60023-9. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenbaum CJ, Beam CA, Boulware D, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61(8):2066–73. doi: 10.2337/db11-1538. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker A, Lauria A, Schloot N, et al. Age-dependent decline of beta-cell function in type 1 diabetes after diagnosis: a multi-centre longitudinal study. Diabetes Obes Metab. 2014;16(3):262–7. doi: 10.1111/dom.12216. doi: 10.1111/dom.12216. [DOI] [PubMed] [Google Scholar]
- 8.Ludvigsson J, Carlsson A, Deli A, et al. Decline of C-peptide during the first year after diagnosis of Type 1 diabetes in children and adolescents. Diabetes Res Clin Pract. 2013;100(2):203–9. doi: 10.1016/j.diabres.2013.03.003. doi: 10.1016/j.diabres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention/National Center for Health Statistics Plan and Operation of the Third National Health and Nutrition Examination Survey. 1994:1988–94. [Google Scholar]
- 10.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care. 2003;26(3):832–6. doi: 10.2337/diacare.26.3.832. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 11.Lachin JM, McGee P, Palmer JP, DCCT/EDIC Research Group Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes. 2014;63(2):739–48. doi: 10.2337/db13-0881. doi: 10.2337/db13-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valle A, Giamporcaro GM, Scavini M, et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes. 2013;62(6):2072–7. doi: 10.2337/db12-1345. doi: 10.2337/db12-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redondo MJ, Rodriguez LM, Escalante M, et al. Beta cell function and BMI in ethnically diverse children with newly diagnosed autoimmune type 1 diabetes. Pediatr Diabetes. 2012;13(7):564–71. doi: 10.1111/j.1399-5448.2012.00875.x. doi: 10.1111/j.1399-5448.2012.00875.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.