Abstract
Most woody plants are animal-pollinated, but the global problem of habitat fragmentation is changing the pollination dynamics. Consequently, the genetic diversity and fitness of the progeny of animal-pollinated woody plants sired in fragmented landscapes tend to decline due to shifts in plant-mating patterns (for example, reduced outcrossing rate, pollen diversity). However, the magnitude of this mating-pattern shift should theoretically be a function of pollinator mobility. We first test this hypothesis by exploring the mating patterns of three ecologically divergent eucalypts sampled across a habitat fragmentation gradient in southern Australia. We demonstrate increased selfing and decreased pollen diversity with increased fragmentation for two small-insect-pollinated eucalypts, but no such relationship for the mobile-bird-pollinated eucalypt. In a meta-analysis, we then show that fragmentation generally does increase selfing rates and decrease pollen diversity, and that more mobile pollinators tended to dampen these mating-pattern shifts. Together, our findings support the premise that variation in pollinator form contributes to the diversity of mating-pattern responses to habitat fragmentation.
Keywords: mating system, plant genetic resources, plant–pollinator mutualisms, pollen competition, pollen discounting
Introduction
Habitat fragmentation is a globally pervasive problem that continues to drive changes to woody plant ecosystems (FAO, 2012). As most species of woody plants are animal-pollinated (Ollerton et al., 2011), studying the impacts of fragmentation on woody plant–pollinator interactions seems particularly important, especially because significant amounts of biodiversity rely on these plant–pollinator interactions.
Fragmentation causes the spatial arrangement of plants to change (Young et al., 1996; Aguilar et al., 2008) and may change the abundance and foraging behaviour of pollinators (Schleuning et al., 2011; Hadley and Betts, 2012). However, populations of animal-pollinated woody plants do not appear to be as susceptible as many other taxa to the small population paradigm effects resulting from habitat fragmentation (for example, genetic drift), which dominate conservation genetics—the ‘paradox of forest fragmentation genetics' (Kramer et al., 2008; Vranckx et al., 2011).
The reasons for this population-level robustness are: (1) that woody plants tend to have many overlapping generations within populations; (2) are long-lived in relation to when most habitat fragmentation occurred and (3) undergo regular far-reaching gene flow, mediated by their pollinators, even in fragmented landscapes (Petit and Hampe, 2006; Kremer et al., 2012). These traits result in substantial genetic inertia within populations of animal-pollinated woody plants, which generally maintains genetic diversity within fragmented populations (Vranckx et al., 2011). This paradox of forest-fragmentation genetics also identifies that animal-pollinated woody plants are not sensitive to fitness effects resulting from post-fragmentation genetic drift.
However, recent work has shown that the progeny of animal-pollinated woody plants in fragmented forests is the life stage where fitness effects are expected to manifest (Yates et al., 2007; Vranckx et al., 2011; Breed et al., 2012a, 2012b). Reduced conspecific density following habitat fragmentation has been demonstrated to change the mating patterns of standing adult animal-pollinated woody plants (for example, increasing inbreeding, reducing pollen diversity; Eckert et al., 2010; Breed et al., 2012a). These changes in mating patterns drive an immediate gain or loss of genetic diversity in the progeny generation (Lowe et al., 2005). Furthermore, fitness of the progeny generation is expected to be directly influenced by mating pattern changes via increased inbreeding depression (Keller and Waller, 2002) and reduced pollen competition and/or reduced female choice (Skogsmyr and Lankinen, 2002; Breed et al., 2012b). Both these effects should increase the frequency of expression of low-fitness phenotypes resulting from high genetic load (Breed et al., 2012a).
Additionally, species with less mobile pollinators should theoretically be more sensitive to the drivers of these fitness effects (Heinrich and Raven, 1972; Charnov, 1976; Hadley and Betts, 2012). As plant densities decline, animal pollinators are less likely to shift from one plant to another because of the increased costs of doing so—the theory of optimal foraging (Heinrich and Raven, 1972; Charnov, 1976; Ottewell et al., 2009). A pollinator foraging on a plant that is a self-compatible hermaphrodite for longer periods of time increases the probability of selfing (increases either via autogamy or geitonogamy; that is, pollination from the same or a different flower on the same plant; Karron et al., 2009). As a consequence of increased self-pollen being received, an increase in pollen discounting is expected (Barrett, 1998). A recent review of outcrossing rates in undisturbed versus disturbed plant populations across 27 species confirmed the expectation of decreased outcrossing rate in disturbed plant populations (Eckert et al., 2010).
One factor that may mitigate against decreased outcrossing in fragmented systems is mobile pollinators (Lowe et al., 2005; Ottewell et al., 2009). Consequently, the generic expectation of decreased outcrossing with increased habitat fragmentation may need refining to be a function of pollinator mobility. In cases where pollinators have limited mobility (for example, small insects), these pollinators will tend to shift from plant to plant less freely than mobile pollinators, increasing the degree of pollen discounting and reducing the diversity of available pollen (Heinrich and Raven, 1972; Charnov, 1976).
Pollen diversity (often measured by correlated paternity, rp) is a parameter that also describes the mating patterns of plants, but is reported less often than the outcrossing rate. This is surprising, as it can be a strong predictor of offspring fitness (Breed et al., 2012a). Pollen diversity should decline with lower conspecific density because as density declines the number of different pollen sources received into a given canopy is also expected to decline (Bianchi and Cunningham, 2012; Breed et al., 2012b). This relationship is due to reduced numbers of pollen donors in the landscape and the fact that animal pollinators are less likely to shift from one plant to another because of the imposed costs of movement (Bianchi and Cunningham, 2012). As a consequence of reduced numbers of pollen sources, the correlated paternity within a given progeny array should increase. The effect of density on correlated paternity should again be a function of pollinator mobility, as more mobile pollinators should overcome greater distances between canopies more easily than less mobile pollinators, resulting in larger pollination neighbourhoods (Bianchi and Cunningham, 2012; Breed et al., in press).
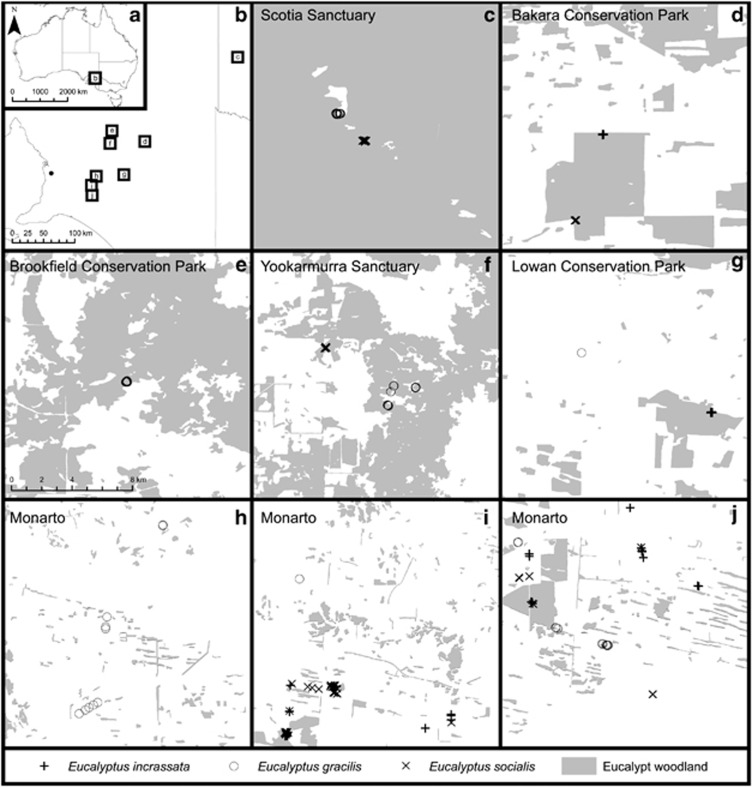
In this paper, we examine the relationships between habitat fragmentation and mating patterns in animal-pollinated woody plants. We present an in-depth study of mating patterns of three closely related eucalypt species that vary in the mobility of their pollinators. The effect of pollinator mobility on mating-pattern shifts in fragmented systems has been highlighted as a research gap (Ghazoul, 2005; Lowe et al., 2005; Eckert et al., 2010), but to the best of our knowledge no previous study has comprehensively explored this topic. We sampled 199 progeny arrays from 13 groups of maternal plants within a single landscape in southern Australia across a habitat fragmentation gradient (Figure 1).
Figure 1.
Map showing regional overview of the six sites where seeds were collected from maternal plants from three eucalypt species across a density gradient caused by habitat fragmentation (a, b). Inset maps (c–g) show intact woodland sites: Scotia Sanctuary (c), Bakara Conservation Park (d), Brookfield Conservation Park (e), Yookamurra Sanctuary (f), Lowan Conservation Park (g). Sampling from the more fragmented Monarto region is shown in inset maps (h–j).
We then investigate the generality of habitat fragmentation–mating-pattern relationships of animal-pollinated woody plants with a meta-analysis. No previous review has performed a quantitative assessment of habitat fragmentation or density effects on mating patterns of plants. Furthermore, previously published qualitative reviews have focussed on outcrossing rates of Neotropical trees, despite other mating-pattern data, taxa and regions being well represented (for example, pollen diversity, shrubs, southern Australia and east Asia).
We predict that for animal-pollinated woody plants: (1) outcrossing rate will negatively associate with habitat fragmentation and lower plant density; (2) biparental inbreeding will positively associate with habitat fragmentation and lower plant density, although this relationship should be weaker because of the variation in spatial genetic structure across populations; (3) correlated paternity will negatively associate with habitat fragmentation and lower plant density; and (4) these predicted relationships will be dampened by increased pollinator mobility, which should buffer the impacts of reduced plant density or losses of conspecifics due to habitat fragmentation.
Materials and methods
Mating patterns and density in mallee eucalypts
Study species
Eucalyptus gracilis F. Muell, E. incrassata Labill. and E. socialis F. Muell. ex. Miq. are multistemmed, sclerophyllous trees common throughout the sand and sand-over-limestone soils of the Murray–Darling Basin, southern Australia (Nicolle, 1997; Figure 1). They generally grow from 2–8 m high. E. socialis and E. gracilis have small white hermaphroditic flowers (diameter of mature flowers with reflexed stamens: <15 mm) and are pollinated primarily by small insects and, to a lesser degree, by birds and small marsupials (Slee et al., 2006). E. incrassata has larger reddish-white hermaphroditic flowers (diameter of mature flowers with reflexed stamens: <35 mm) and is pollinated primarily by birds (Family Meliphagidae) and to a lesser degree by insects (Bond and Brown, 1979).
Eucalypt flowers are protandrous (that is, male reproductive phase precedes female phase within flowers), and flower development within and between inflorescences is sequential and gradual. Therefore, flowers in male or female phase may be in close proximity, allowing geitonogamous selfing to occur (House, 1997). Data from closely related eucalypts suggest that the species investigated here probably have late-acting self-incompatibility mechanisms, resulting in mixed mating to preferential outcrossing (tm generally >0.60; Horsley and Johnson, 2007). Serotinous fruits (that is, seeds released in response to an environmental trigger) are held over numerous years, with drying triggering seed release. Seeds are small (<2 mm diameter) and gravity-dispersed. On the basis of the data for E. incrassata and our field observations, ants generally exhaust soil seed banks, except during particularly heavy seed release such as post fire (Wellington and Noble, 1985).
Seed collection
Open-pollinated seeds were collected from maternal plants (n=199) across six sites in the Murray–Darling Basin (Figure 1). At Scotia Sanctuary, Bakara Conservation Park, Brookfield Conservation Park, Yookamurra Sanctuary and Lowan Conservation Park sites, maternal plants were from generally higher-density woodlands with no history of known habitat fragmentation (Figures 1c–g inset maps respectively; see Supplementary Table S1 for sampling information). Monarto maternal plants (Figures 1h–j inset maps) were either located in higher-density small-remnant woodlands or were isolated pasture trees (Supplementary Table S1). Small remnant woodlands were natural habitats surrounded by agricultural land. Isolated pasture trees were in very small clusters of vegetation (often only a single tree) either in agricultural land or between public roads and agricultural land. In all cases, care was taken to avoid sampling near neighbours. We attempted to include each species from all sites and to sample populations of each species that had similar fragmentation histories and/or stand densities (Supplementary Table S1). A subset of the genotype data included in this study has been included in previous studies. This includes E. socialis data from Yookamurra Sanctuary and Monarto (n=47 maternal plants; Breed et al., 2012b) and E. incrassata data from Monarto (n=37 maternal plants; Breed et al., in press).
We used two methods to measure the conspecific density. For isolated pasture trees, we counted the number of conspecifics within a 30-m radius of each tree (that is, nearest-neighbours), then extrapolated this to trees per hectare. The 30-m radius was a manageable distance, given the reasonably evenly spaced distribution of the isolated pasture trees. To account for potential clumped distribution of conspecifics in large intact and small remnant woodlands, we estimated conspecific density by counting all conspecifics in eight replicates of 30 m × 10 m transects and extrapolated this to trees per hectare.
Genotyping
Leaf tissue and seeds were collected from all maternal plants. Seeds were germinated under semicontrolled glasshouse conditions and we sampled their leaf tissues. DNA was extracted from leaf tissue using Machery-Nagel Nucleospin Plant II Kit at the Australian Genome Research Facility (Adelaide, Australia). Nine direct-labelled microsatellite markers were selected from the set of EST-derived markers by Faria et al. (2010; EMBRA914; EMBRA1284; EMBRA1363; EMBRA1382; EMBRA1445; EMBRA1468; EMBRA1928; EMBRA1990; EMBRA2002). Polymerase chain reaction (PCR) was performed in a single 10-μl multiplex PCR following standard Qiagen Multiplex PCR conditions (Qiagen, Hilden, Germany; see Supplementary Appendix S1 for PCR conditions).
Analysis
Maternal genotypes were used to estimate genetic diversity of maternal plants, which were presumed to reflect preclearance dynamics because all trees sampled were estimated to be >80 years old and most land clearance occurred <80 years ago. Maternal genotypes were also used to screen for null alleles in MICRO-CHECKER (Oosterhout et al., 2004). GENEPOP (http://genepop.curtin.edu.au) was used to test for heterozygote deficit/excess and linkage disequilibrium, applying sequential Bonferroni correction for multiple testing where appropriate. Additionally, per-locus probability of paternity exclusion (Q) and combined probability of paternity exclusion (QC) were estimated in GENALEX (Peakall and Smouse, 2006).
We estimated the following genetic diversity parameters for maternal plants and progeny groups using GENALEX: number of alleles (A), Nei's (1973) unbiased expected and observed heterozygosity (HE and HO, respectively) and fixation index (F). To account for differences in sample size, we rarefied the mean number of alleles per locus (AR) using HP-RARE (Kalinowski, 2005).
We estimated the following mating system parameters for each group of maternal plants from progeny array genotypes in MLTR (Ritland, 2002): multilocus outcrossing rate (tm; where the selfing rate S=1−tm), biparental inbreeding (tm−ts) and multilocus-correlated paternity (rp). Families were bootstrapped 1000 times to calculate variance estimates for each parameter.
Meta-analysis
Data
We searched for relevant articles on the ISI Web of Knowledge database (http://apps.webofknowledge.com) on 12 December 2012 using the following search terms: (density OR habitat disturbance OR habitat frag* OR logging) AND (inbreed* OR mating system OR mating pattern OR outcross* OR selfing OR pollen diversity OR correlated paternity OR correlation of paternity). From this list, we reviewed articles and retained those that reported at least one mating system parameter for groups of trees or shrubs that differed in their history of habitat fragmentation or density. We recorded the mating system parameters observed in the intact forest or ‘best on offer' locations (for example, unlogged habitat) and the fragmented or disturbed habitat. If these data were presented in a figure, we extracted values using DATATHIEF (http://www.datatheif.org). We recorded the form of the species (tree or shrub), pollinator mobility (mobile versus less mobile) and all mating system parameters reported. Pollinator mobility was classified as ‘mobile' if the authors specified that pollination was conducted by mobile vertebrates (for example, birds, bats or large insects). Pollinator mobility was classified as ‘less mobile' if the authors specified that small insects did most of the pollination (for example, small moths, bees). We also included mating-pattern data presented in this study (see Supplementary Table S2 for data sets included in the meta-analysis).
Analysis
We conducted our meta-analysis following methods described by Borenstein et al. (2009). For each study, we calculated Hedges' g; the difference between forest or ‘best on offer' and the disturbed habitat mean mating system parameters standardised using the pooled standard deviation of the two groups (Borenstein et al., 2009). As Hedges' g is a biased estimator of effect size, we used a conversion factor to compute a bias-corrected metric, Hedges' g*. We then calculated the average effect size using the random-effect model, where effect sizes of individual comparisons are weighted by the inverse of within-study variance plus between-study variance. We calculated the effect size of the entire data set and for subgroups of data divided into pollinator mobility (mobile versus less mobile). We tested for publication bias by visually examining funnel plots of effect sizes plotted against standard errors to assess the symmetry of study precision around effect size. Relatively symmetrical funnel plots suggest that there were no relationships between effect size and study size, and that studies with small effect sizes do not have a lower probability of being published (see Supplementary Figure S1 for funnel plots).
Results
Mating patterns and density in mallee eucalypts
Marker quality
We genotyped 199 open-pollinated progeny arrays (=2867 progeny) from 13 groups of maternal plants from six sites across the Murray–Darling Basin (Supplementary Table S1). The combined probability of paternity exclusion if neither parent is known indicates good resolution for genetic markers used across all maternal plant groups (QC=1.00 in all cases; see Supplementary Table S3 for detailed microsatellite data). No significant excesses or deficits of heterozygotes were observed in the groups of maternal plants (Supplementary Table S1). We found no significant levels of null alleles at any loci within any maternal plant group. No significant linkage disequilibrium was observed between pairs of loci.
Genetic diversity
No significant differences in allelic richness and expected heterozygosity between progeny and maternal plants were observed across species (all t-test P-values >0.05; Supplementary Table S1). However, progenies were significantly more homozygous than maternal plants, particularly progenies from isolated pasture trees (isolated pasture trees: E. socialis t=3.24, df=16, P-value <0.01; E. gracilis t=2.42, df=16, P-value <0.05; E. incrassata t=5.25, df=14, P-value <0.01; Supplementary Table S1).
Mating patterns and density
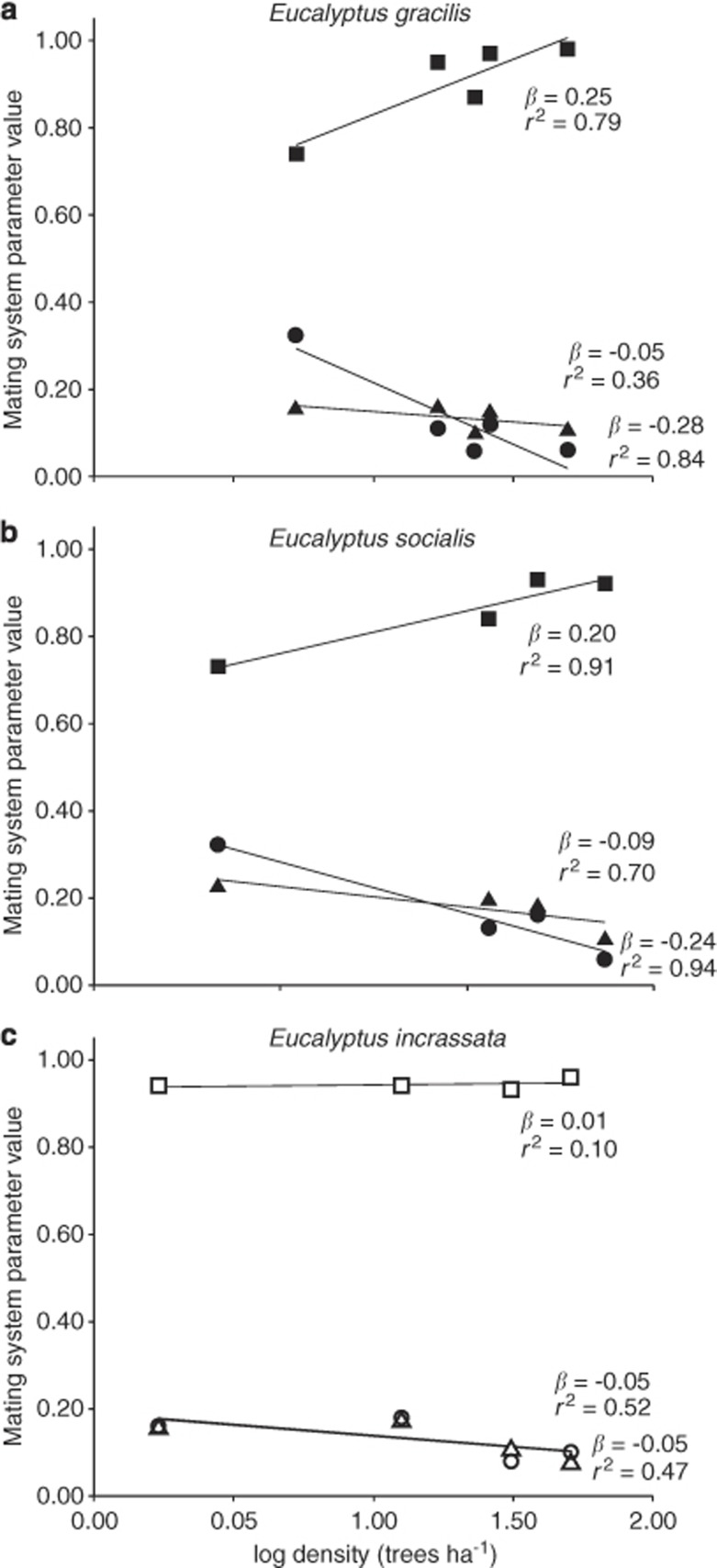
Each species experienced low levels of selfing (all groups s <0.30; see Supplementary Table S4 for mating-pattern results), but we observed significant shifts towards increased selfing, biparental inbreeding and correlated paternity in the insect-pollinated E. socialis and E. gracilis maternal plants sampled from lower-density stands (linear regression of density (log) effect: tm ß=0.21–0.25, r2=0.79–0.91, P-value <0.001; tm−ts ß=−0.05 to −0.09, r2=0.36–0.70, P-value <0.05; rp ß=−0.24 to −0.28, r2=0.84–0.94, P-value <0.001; Figure 2). The bird-pollinated E. incrassata showed no significant change in mating patterns across a 30-fold decrease in density (from 1.7 to 51 trees per hectare, linear regression of density(log) effect: tm ß=0.01, r2=0.10; tm−ts ß=−0.05, r2=0.52; rp ß=−0.05, r2=0.47; all P-values >0.05).
Figure 2.
Relationships between mating patterns and density of three mallee eucalypts. Insect-pollinated Eucalyptus gracilis (a) and E. socialis (b) outcrossing rate (tm, where the selfing rate s=1−tm) shown by filled squares, biparental inbreeding (tm−ts) by filled triangles and correlated paternity (rp) by filled circles. The bird-pollinated E. incrassata mating patterns are shown by corresponding open shapes (c). The trendlines show slopes (ß) and goodness of fit (r2) of linear regressions between log density and mating patterns. 95% CI are not shown as they fall within the outer edge of each symbol.
Meta-analysis
Our analysis involved 36 studies that presented 44 comparisons of selfing rate, 30 biparental inbreeding comparisons and 29 correlated paternity comparisons (Supplementary Table S2).
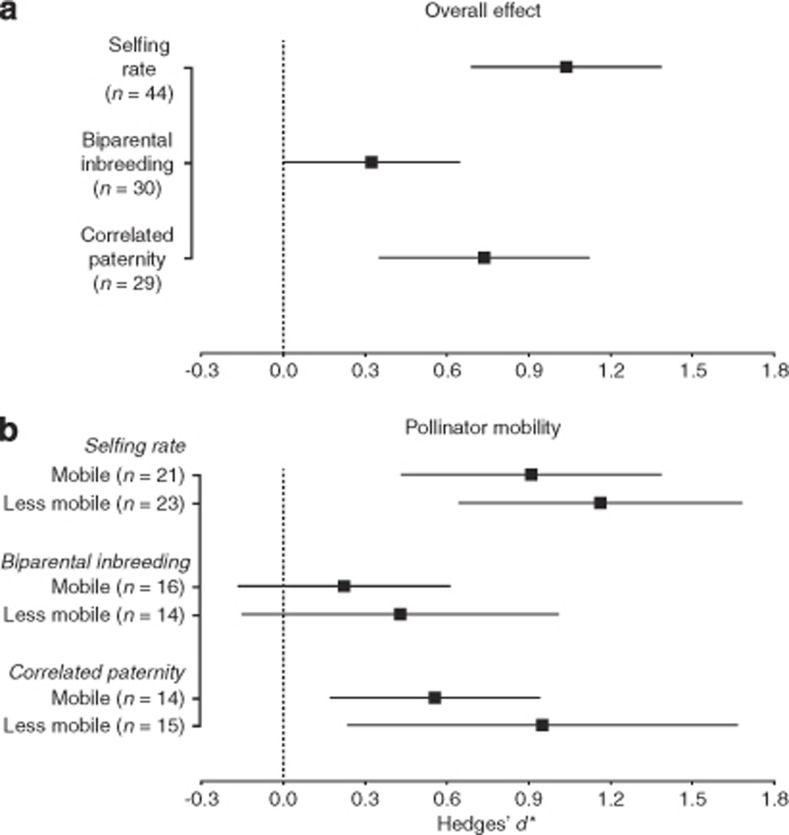
Habitat fragmentation had significant effects on increasing selfing rate (Hedges' g*=1.04; 95% CI, 1.39–0.69; z=5.33, P-value <0.0001) and correlated paternity (Hedges' g*=0.74; 95% CI, 0.35–1.13; z=3.73, P-value <0.001; Figure 3a). Habitat fragmentation did not significantly change biparental inbreeding, but a general increase was observed (Hedges' g*=0.32; 95% CI, 0.00–0.65; z=1.96, P-value=0.05).
Figure 3.
Mean effect sizes (Hedges' d*) of habitat fragmentation on animal-pollinated woody plant-mating patterns. Overall mean effect shown in (a) and effects separated by pollinator mobility in (b). Error bars show bias-corrected 95% bootstrap CIs. A mean effect size is significantly different from zero when its 95% CI does not overlap zero. Positive mean effect sizes indicate that the fragmented group of maternal plants had on average larger values for the given mating pattern. The number of studies is shown in parentheses.
When data were analysed separately for species with mobile versus less mobile pollinators, habitat fragmentation still had a significant positive effect on selfing rates and correlated paternity in both groups (mobile and less mobile pollinated species: selfing rate P-value <0.001 and <0.0001; correlated paternity P-value <0.05 and <0.01, respectively). In both cases, the effect was generally stronger for plants with less mobile pollinators, although the mean effects were not statistically different between groups (Figure 3b). No significant effect of habitat fragmentation on biparental inbreeding was apparent when the data were analysed separately by pollinator groups (both P-values >0.05).
Discussion
Our study aimed to test whether human-mediated stand density changes were correlated with changes in selfing rates, biparental inbreeding and correlated paternity. Additionally, we wanted to test whether greater pollinator mobility acted as a buffer against mating-pattern shifts as a consequence of reduced stand density. To the best of our knowledge, our eucalypt case study is the most in-depth study performed into these predictions conducted thus far (Supplementary Table S2) and our meta-analysis is the first quantitative review of this topic.
Generality of habitat fragmentation–mating pattern interactions
Our meta-analysis of the available literature (44 data sets) demonstrates that the negative effect of reduced stand density on mating patterns, which we observed in our eucalypt study, is a general one for animal-pollinated woody plants. We observed a general negative trend between habitat fragmentation (a surrogate for lower stand density; Ghazoul, 2005; Lowe et al., 2005) and less pollen diversity received into animal-pollinated woody plant canopies. This general trend has not been previously reported. In addition, we report that habitat fragmentation has a quantifiable effect on selfing rates, which is consistent with previous qualitative reviews (Ghazoul, 2005; Lowe et al., 2005; Eckert et al., 2010).
Our meta-analysis is the first review to investigate the general impact of habitat fragmentation on pollen diversity of plants. There are numerous studies that demonstrate how reduced pollen diversity received into plant canopies can associate with reduced offspring fitness in fragmented systems (Hoebee and Young, 2001; Cascante et al., 2002; Fuchs et al., 2003; González-Varo et al., 2010; Breed et al., 2012a); however, more work is required to investigate the mechanism of this fitness impact. Increased correlated paternity has been hypothesised to associate with less pollen competition to fertilise receptive ovules (Breed et al., 2012a). However, pollen grain size and tube growth rates were not measured in these studies, leaving the possibility that alternative hypotheses may explain the relationship between progeny vigour and pollen diversity (for example, heterosis effect from more genetically diverse pollen; Skogsmyr and Lankinen, 2002). Additionally, there is uncertainty about how pollen diversity received onto a stigma relates to correlated paternity after fertilisation, but we assume that these parameters are inversely proportional (that is, more pollen diversity received on stigma=lower correlated paternity). This relationship may breakdown in the presence of female choice for specific pollen grains or if certain males tend to provide more competitive pollen—‘supermales' (Skogsmyr and Lankinen, 2002).
There are numerous cases in the literature that demonstrate a link between the offspring fitness of animal-pollinated woody plants and increasing selfing caused by habitat fragmentation (Cascante et al., 2002; Fuchs et al., 2003; Quesada et al., 2004; Hirayama et al., 2007; González-Varo et al., 2010; Breed et al., 2012a). The mechanism whereby fitness is influenced because of increased selfing has been clearly articulated in the literature on inbreeding and inbreeding depression (for example, Keller and Waller (2002)). Animal-pollinated woody plants should be particularly susceptible to inbreeding depression, as most predominantly outcross, leading them to accumulate genetic load (Klekowski, 1988). Much work has been conducted on the evolutionary patterns of plant outcrossing (Barrett, 1998). However, more studies are needed to investigate what types of habitat fragmentation make plants most susceptible to induced increases in inbreeding (for example, fragmentation, logging and disruption to pollinator community). The future viability of populations that produce high levels of low-fitness offspring (that is, inbred or poor pollen–ovule combinations) also requires further investigation. Natural selection generally purges low-fitness offspring as a cohort ages; this has been documented in both non-fragmented (for example, Ueno et al. (2002)) and fragmented systems (Vranckx et al., 2011). However, the viability of fragmented populations that produce these high levels of low-fitness offspring remains unknown, but a tendency of demographic decline may be expected.
Pollinator mobility interactions with habitat disturbance
As predicted, we observed that the mating patterns of two insect-pollinated mallee eucalypts were negatively correlated with stand density. This is an important finding because a change in mating patterns is expected to result in progeny of lower fitness (Breed et al., 2012a). Furthermore, as predicted, there was no relationship between stand density and mating patterns for E. incrassata, which is primarily pollinated by highly mobile birds.
Our study highlights how pollinator mobility may affect a species' susceptibility to shifts in mating patterns in response to fragmentation. Pollination neighbourhoods of small-insect-pollinated woody plants appear to decline as the density declines. These reduced pollination neighbourhoods are expected to lead to greater pollen discounting (that is, selfing increases because of a reduction in outcrossed siring success; Barrett, 1998), provision of less diverse pollen (increasing correlated paternity; Bianchi and Cunningham, 2012) and proportionally more pollen from close relatives (increasing biparental inbreeding; Dubreuil et al., 2010). The bird-pollinated E. incrassata appeared not to be suffering from these pollen limitation and pollination-neighbourhood effects likely because its bird pollinators have the ability to sample larger and more diverse pollination neighbourhoods, even in fragmented landscapes (Paton, 2004).
The results from our meta-analysis generally support the findings of our eucalypt study in which mating patterns shifted in fragmented habitats as a function of pollinator mobility. More mobile pollinators (for example, birds and bats) should function within larger pollination neighbourhoods than less mobile pollinators (for example, small insects; Bianchi and Cunningham, 2012). Thus, the pollen provision of more mobile pollinators should be less affected by changes in stand density than less mobile pollinators. However, the pollinator mobility effect was not significant in our meta-analysis. Additionally, it is possible that the strong effect of pollinator mobility we observed in our eucalypt study might have been unusual because the bird pollinators of E. incrassata are particularly mobile (Family Meliphagidae; Paton, 2004).
The binary grouping of pollinators into mobility categories (mobile versus less mobile) seems to over-simplify reality, as despite many birds being mobile in fragmented landscapes (Paton, 2004; Byrne et al., 2007), some species are susceptible to fragmentation (population declines: Turner, 1996; foraging area reductions: Ibarra-Macias et al., 2011). Likewise, although many studies show that insect pollination declines in fragmented populations (Aizen and Feinsinger, 1994), there are cases showing long distance pollination by small insects in fragmented landscapes (>1 km; Dick et al., 2003; Byrne et al., 2008). Therefore, despite the expectation that selfing and pollen diversity should be a function of pollinator mobility, in line with the landscape ecology models (Charnov, 1976; Karron et al., 2009), more experimental studies into woody plant density, pollen movement and mating patterns are required to further understand these associations.
A central argument for the paradox of forest-fragmentation genetics is that average pollen flow distances may locally increase in fragmented animal-pollinated woody plant populations, genetically buffering these populations (Kramer et al., 2008). This may indeed be the case in some populations; however, there are now two well-supported general patterns that are inconsistent with this genetic buffering effect. Firstly, genetic diversity of progeny from animal-pollinated woody plant populations declines with fragmentation (Vranckx et al., 2011). Secondly, as we show here, animal-pollinated woody plant-mating patterns are influenced by habitat fragmentation. We suggest that when pollen neighbourhoods decline because of fragmentation, the mean gene flow will locally increase because of increased spacing between plants. However, as a consequence of this increased spacing, pollinators will spend more time in individual canopies (increasing selfing rates) and decrease the provision of different pollen sources (increasing correlated paternity). Together, these effects reduce progeny genetic diversity and show an impact on adult mating patterns, which may both have fitness consequences for the progeny generation.
Data archiving
Eucalypt genotype data are available through Dryad at doi:10.5061/dryad.g7j86.
Acknowledgments
This work was supported by ARC Linkage project (LP110200805) and SA Premier's Science and Research Fund awarded to AJL; Native Vegetation Council SA (grant 09/10/27), Nature Foundation SA Inc, Australian Geographic Society, Biological Society of SA, Field Naturalist Society of SA, Wildlife Preservation Society of Australia, NCCARF Travel Grants awarded to MFB. We thank Rob Murphy from Rural Solutions SA, Matt Hayward and Phil Scully from Australian Wildlife Conservancy and volunteers for fieldwork assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Heredity website (http://www.nature.com/hdy)
Supplementary Material
References
- Aguilar R, Quesada M, Ashworth L, Herrerias-Diego Y, Lobo J. (2008). Genetic consequences of habitat fragmentation in plant populations: susceptible signals in plant traits and methodological approaches. Mol Ecol 17: 5177–5188. [DOI] [PubMed] [Google Scholar]
- Aizen MA, Feinsinger P. (1994). Habitat fragmentation, native insect pollinators, and feral honey bees in Argentine 'Chaco Serrano'. Ecol Appl 4: 378–392. [Google Scholar]
- Barrett SCH. (1998). The evolution of mating strategies in flowering plants. Trends Plant Sci 3: 335–341. [Google Scholar]
- Bianchi FJJA, Cunningham SA. (2012). Unravelling the role of mate density and sex ratio in competition for pollen. Oikos 121: 219–227. [Google Scholar]
- Bond HW, Brown WL. (1979). The exploitation of floral nectar in Eucalyptus incrassata by honeyeaters and honeybees. Oecologia 44: 105–111. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. (2009) Introduction to Meta-Analysis. John Wiley and Sons, Ltd.: Hoboken, NJ, USA. [Google Scholar]
- Breed MF, Gardner MG, Ottewell K, Navarro C, Lowe A. (2012. a). Shifts in reproductive assurance strategies and inbreeding costs associated with habitat fragmentation in Central American mahogany. Ecol Lett 15: 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breed MF, Marklund MHK, Ottewell KM, Gardner MG, Harris JCB, Lowe AJ. (2012. b). Pollen diversity matters: revealing the neglected effect of pollen diversity on fitness in fragmented landscapes. Mol Ecol 21: 5955–5968. [DOI] [PubMed] [Google Scholar]
- Breed MF, Ottewell KM, Gardner MG, Marklund MHK, Stead MG, Harris JCB et al. (in press). Mating system and early viability resistance to habitat fragmentation in a bird-pollinated eucalypt. Heredity (e-pub ahead of print 28 November 2012; doi: 10.1038/hdy.2012.72). [DOI] [PMC free article] [PubMed]
- Byrne M, Elliott C, Yates C, Coates D. (2008). Maintenance of high pollen dispersal in Eucalyptus wandoo, a dominant tree of the fragmented agricultural region in Western Australia. Conserv Genet 9: 97–105. [Google Scholar]
- Byrne M, Elliott CP, Yates C, Coates DJ. (2007). Extensive pollen dispersal in a bird-pollinated shrub, Calothamnus quadrifidus, in a fragmented landscape. Mol Ecol 16: 1303–1314. [DOI] [PubMed] [Google Scholar]
- Cascante A, Quesada M, Lobo JJ, Fuchs EA. (2002). Effects of dry tropical forest fragmentation on the reproductive success and genetic structure of the tree Samanea saman. Conserv Biol 16: 137–147. [DOI] [PubMed] [Google Scholar]
- Charnov EL. (1976). Optimal foraging, the marginal value theorem. Theor Popul Biol 9: 129–136. [DOI] [PubMed] [Google Scholar]
- Dick CW, Etchelecu G, Austerlitz F. (2003). Pollen dispersal of tropical trees (Dinizia excelsa: Fabaceae) by native insects and African honeybees in pristine and fragmented Amazonian rainforest. Mol Ecol 12: 753–764. [DOI] [PubMed] [Google Scholar]
- Dubreuil M, Riba M, González-Martínez SC, Vendramin GG, Sebastiani F, Mayol M. (2010). Genetic effects of chronic habitat fragmentation revisited: strong genetic structure in a temperate tree, Taxus baccata (Taxaceae), with great dispersal capability. Am J Bot 97: 303–310. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Kalisz S, Geber MA, Sargent R, Elle E, Cheptou P-O et al. (2010). Plant mating systems in a changing world. Trends Ecol Evol 25: 35–43. [DOI] [PubMed] [Google Scholar]
- FAO. (2012) State of the World's Forests. Food and Agriculture Organization of the United Nations: Rome. [Google Scholar]
- Faria DA, Mamani EMC, Pappas MR, Pappas GJ Jr, Grattapaglia D. (2010). A selected set of EST-derived microsatellites, polymorphic and transferable across 6 species of Eucalyptus. J Hered 101: 512–520. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Lobo J, Quesada M. (2003). Effects of forest fragmentation and flowering phenology on the reproductive success and mating patterns of the tropical dry forest tree Pachira quinata. Conserv Biol 17: 149–157. [Google Scholar]
- Ghazoul J. (2005). Pollen and seed dispersal among dispersed plants. Biol Rev (Camb) 80: 413–443. [DOI] [PubMed] [Google Scholar]
- González-Varo JP, Albaladejo RG, Aparicio A, Arroyo J. (2010). Linking genetic diversity, mating patterns and progeny performance in fragmented populations of a Mediterranean shrub. J Appl Ecol 47: 1242–1252. [Google Scholar]
- Hadley AS, Betts MG. (2012). The effects of landscape fragmentation on pollination dynamics: absence of evidence not evidence of absence. Biol Rev (Camb) 87: 526–544. [DOI] [PubMed] [Google Scholar]
- Heinrich B, Raven PH. (1972). Energetics and pollination ecology. Science 176: 597–602. [DOI] [PubMed] [Google Scholar]
- Hirayama K, Ishida K, Setsuko S, Tomaru N. (2007). Reduced seed production, inbreeding, and pollen shortage in a small population of a threatened tree, Magnolia stellata. Biol Conserv 136: 315–323. [Google Scholar]
- Hoebee SE, Young AG. (2001). Low neighbourhood size and high interpopulation differentiation in the endangered shrub Grevillea iaspicula McGill (Proteaceae). Heredity 86: 489–496. [DOI] [PubMed] [Google Scholar]
- Horsley TN, Johnson SD. (2007). Is Eucalyptus cryptically self-incompatible? Ann Bot (Lond) 100: 1373–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House SM. (1997). Reproductive biology of eucalypts. In: Williams J, Woniarski J (eds) Eucalypt Ecology: Individuals to Ecosystems. Cambridge University Press, Cambridge. [Google Scholar]
- Ibarra-Macias A, Robinson WD, Gaines MS. (2011). Experimental evaluation of bird movements in a fragmented Neotropical landscape. Biol Conserv 144: 703–712. [Google Scholar]
- Kalinowski ST. (2005). HP-RARE 1.0: a computer program for performing rarefaction on measures of allelic richness. Mol Ecol Notes 5: 187–189. [Google Scholar]
- Karron JD, Holmquist KG, Flanagan RJ, Mitchell RJ. (2009). Pollinator visitation patterns strongly influence among-flower variation in selfing rate. Ann Bot (Lond) 103: 1379–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller LF, Waller DM. (2002). Inbreeding effects in wild populations. Trends Ecol Evol 17: 230–241. [Google Scholar]
- Klekowski EJ. (1988). Genetic load and its causes in long-lived plants. Trees-Struct Funct 2: 195–203. [Google Scholar]
- Kramer AT, Ison JL, Ashley MV, Howe HF. (2008). The paradox of forest fragmentation genetics. Conserv Biol 22: 878–885. [DOI] [PubMed] [Google Scholar]
- Kremer A, Ronce O, Robledo-Arnuncio JJ, Guillaume F, Bohrer G, Nathan R et al. (2012). Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol Lett 15: 378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AJ, Boshier D, Ward M, Bacles CFE, Navarro C. (2005). Genetic resource impacts of habitat loss and degradation; reconciling empirical evidence and predicted theory for neotropical trees. Heredity 95: 255–273. [DOI] [PubMed] [Google Scholar]
- Nei M. (1973). Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70: 3321–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle D. (1997) Eucalypts of South Australia. Lane Print Group: Adelaide, South Australia. [Google Scholar]
- Ollerton J, Winfree R, Tarrant S. (2011). How many flowering plants are pollinated by animals? Oikos 120: 321–326. [Google Scholar]
- Oosterhout CV, Hutchinson WF, Wills DPM, Shipley P. (2004). MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4: 535–538. [Google Scholar]
- Ottewell KM, Donnellan SC, Lowe AJ, Paton DC. (2009). Predicting reproductive success of insect- versus bird-pollinated scattered trees in agricultural landscapes. Biol Conserv 142: 888–898. [Google Scholar]
- Paton D. (2004). Birdscaping the environment: restoring the woodland systems of the Mt Lofty region, South Australia. In: Lunney D (ed) Conservation of Australia's Forest Fauna 2nd edn. Royal Zoological Society of New South Wales: Mosman, NSW, Australia. [Google Scholar]
- Peakall R, Smouse PE. (2006). GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit RJ, Hampe A. (2006). Some evolutionary consequences of being a tree. Annu Rev Ecol Evol Syst 37: 187–214. [Google Scholar]
- Quesada M, Stoner KE, Lobo JA, Herrerias-Diego Y, Palacios-Guevara C, Munguía-Rosas MA et al. (2004). Effects of forest fragmentation on pollinator activity and consequences for plant reproductive success and mating patterns in bat-pollinated Bombacaceous trees. Biotropica 36: 131–138. [Google Scholar]
- Ritland K. (2002). Extensions of models for the estimation of mating systems using n independent loci. Heredity 88: 221–228. [DOI] [PubMed] [Google Scholar]
- Schleuning M, Farwig N, Peters MK, Bergsdorf T, Bleher B, Brandl R et al. (2011). Forest Fragmentation and Selective Logging Have Inconsistent Effects on Multiple Animal-mediated ecosystem processes in a tropical forest. PLoS One 6: e27785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogsmyr IO, Lankinen Å. (2002). Sexual selection: an evolutionary force in plants? Biol Rev (Camb) 77: 537–562. [DOI] [PubMed] [Google Scholar]
- Slee A, Brooker M, Duffy S, West J. (2006) EUCLID: Eucalyptus of Australia 3rd edn Centre for Plant Biodiversity Research: Canberra. [Google Scholar]
- Turner IM. (1996). Species loss in fragments of tropical rain forest: a review of the evidence. J Appl Ecol 33: 200–209. [Google Scholar]
- Ueno S, Tomaru N, Yoshimaru H, Manabe T, Yamamoto S. (2002). Size-class differences in genetic structure and individual distribution of Camellia japonica L. in a Japanese old-growth evergreen forest. Heredity 89: 120–126. [DOI] [PubMed] [Google Scholar]
- Vranckx GUY, Jacquemyn H, Muys B, Honnay O. (2011). Meta-analysis of susceptibility of woody plants to loss of genetic diversity through habitat fragmentation. Conserv Biol 26: 228–237. [DOI] [PubMed] [Google Scholar]
- Wellington AB, Noble IR. (1985). Post-fire recruitment and mortality in a population of the mallee Eucalyptus incrassata in semi-arid, south-eastern Australia. J Ecol 73: 645–656. [Google Scholar]
- Yates CJ, Elliott C, Byrne M, Coates DJ, Fairman R. (2007). Seed production, germinability and seedling growth for a bird-pollinated shrub in fragments of kwongan in south-west Australia. Biol Conserv 136: 306–314. [Google Scholar]
- Young A, Boyle T, Brown T. (1996). The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11: 413–418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.