Abstract
Background:
Chronic stress has been found to suppress adult neurogenesis, but it remains unclear whether it may affect the maturation process of adult-born neurons. Here, we examined the influence of chronic social defeat stress on the morphological and electrophysiological properties of adult-born dentate granule cells at different developmental stages.
Methods:
Adult C57BL/6 mice were subjected to 10 days of chronic social defeat stress followed by a social interaction test 24 hours after the last defeat. Defeated mice were segregated into susceptible and unsusceptible subpopulations based on a measure of social interaction test. Combining electrophysiology with retrovirus-mediated birth-dating and labeling, we examined the impact of chronic social defeat stress on temporal regulation of synaptic plasticity of adult-born dentate granule cells along their maturation.
Results:
Chronic social defeat stress decreases the survival and dendritic complexity of adult-born dentate granule cells. While chronic social defeat stress doesn’t alter the intrinsic electrophysiological properties and synaptic transmission of surviving adult-born dentate granule cells, it promotes the developmental switch in synaptic N-methyl-D-aspartate receptors from predominant GluN2B- to GluN2A-containing receptors, which transform the immature synapse of adult-born dentate granule cells from one that exhibits enhanced long-term potentiation to one that has normal levels of long-term potentiation. Furthermore, chronic social defeat stress increases the level of endogenous repressor element-1 silencing transcription factor mRNA in adult-born dentate granule cells, and knockdown of the repressor element-1 silencing transcription factor in adult-born dentate granule cells rescues chronic social defeat stress-induced morphological deficits and accelerated developmental switch in synaptic N-methyl-D-aspartate receptor subunit composition.
Conclusions:
These results uncover a previously unsuspected role of chronic social defeat stress in regulating adult neurogenesis and suggest that chronic social defeat stress can affect synaptic maturation process of adult-born dentate granule cells.
Keywords: chronic social defeat stress, adult neurogenesis, NMDA receptor, REST, dentate granule cell
Introduction
In the adult mammalian brain, new neurons are continuously generated from neural stem/progenitor cells that reside predominantly in the subventricular zone of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) (Ming and Song, 2005). Adult neurogenesis is an extremely dynamic process that is regulated by various physiological, pathological, and pharmacological stimuli (Duman et al., 2001; Ming and Song, 2005). Stress is one of the most potent negative regulators of adult neurogenesis, as demonstrated in several mammalian species (Gould et al., 1997, 1998; Schoenfeld and Gould, 2012). Indeed, multiple acute stress paradigms, such as exposure to predator odor (Tanapat et al., 2001; Hill et al., 2006), restraint (Pham et al., 2003; Bain et al., 2004), electric footshock (Malberg and Duman, 2003), social defeat (Lagace et al., 2010), and cold swim (Heine et al., 2004), have been reported to suppress adult hippocampal neurogenesis by lowering the rate of cell proliferation. Likewise, chronic mild stress has also been shown to decrease the proliferation and survival of newly generated dentate granule cells (DGCs) in the adult hippocampus of different species (Pham et al., 2003; Simon et al., 2005; Czéh et al., 2007; Ferragud et al., 2010; Van Bokhoven et al., 2011). However, one study reported that the survival of adult-born DGCs is increased after chronic social defeat stress (CSDS) selectively in mice that display persistent social avoidance (Lagace et al., 2010), suggesting a more complicated relationship may exist between CSDS and cell survival. Although the molecular mechanisms by which stress regulates adult neurogenesis remain unclear, some evidence suggests that adrenal steroids may play an important role (Anacker et al., 2013; Lehmann et al., 2013). Consistently, exogenous administration of corticosterone to rats was found to reduce the proliferation and survival of adult-born DGCs (Brummelte and Galea, 2010).
The development of adult-born DGCs is a highly plastic process that recapitulates many aspects of early development. Adult-born DGCs extend their dendrites and receive functional inputs from the existing neural circuits as early as 2 weeks after birth (Schmidt-Hieber et al., 2004; Ge et al., 2006). We and others have previously shown that immature adult-born DGCs exhibit lower activation threshold and enhanced synaptic plasticity compared with mature DGCs (Schmidt-Hieber et al., 2004; Ge et al., 2007). Furthermore, such enhanced plasticity is associated with developmentally regulated synaptic expression of GluN2B-containing N-methyl-D-aspartate receptors (NMDARs). While stress has been shown to regulate adult neurogenesis at different stages, no previous studies have explored whether it may affect the synaptic maturation process of adult-born DGCs. Combining electrophysiology with retrovirus-mediated birth-dating and labeling, we examined the impact of CSDS on temporal regulation of synaptic plasticity of adult-born DGCs along their maturation. Here, we show that CSDS can affect the maturation of synaptic plasticity of surviving adult-born DGCs by accelerating the developmental switch in synaptic NMDAR subunit composition from predominantly GluN2B to GluN2A through increased expression of the repressor element-1 silencing transcription factor (REST).
Materials and Methods
A full detailed description of Materials and Methods is included in the supplementary Material.
Animals
Adult male C57BL/6 (8–12 weeks old) and CD1 retired breeder (6–10 months old) mice were used. C57BL/6 (4 per cage) and CD1 (1 per cage) mice were housed in a humidity- and temperature-controlled (25±1°C) room on a 12-hour -light/-dark cycle (lights on 6:00 am 6:00 pm) with access to food and water ad libitum and were acclimated in the animal research facility for at least 1 week prior to use in behavioral experiments. All behavioral procedures were carried out during the light cycle between 10:00 am and 3:00 pm. All animal procedures described were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of National Cheng Kung University.
Social Defeat Stress
C57BL/6 mice were subjected to CSDS for 10 consecutive days as previously described (Krishnan et al., 2007). During each defeat episode, a C57BL/6 mouse was introduced into the home cage of an unfamiliar and aggressive CD1 mouse for physical encounter. Following a 10-minute agonistic interaction, the aggressor and the test mouse were then housed in the same cage separated by a wire mesh partition for the next 24 hours, which allowed continuous sensory contact without physical interaction. Control mice were housed in equivalent cages with members of the same strain. The social interaction test was performed 24 hours after the last training day by measuring the time spent in the interaction zone (supplementary Figure 1A) during the first 5 minutes (target absent) and second 5 minutes (target present) trials. Social approach-avoidance behaviors were recorded using a digital video camera and analyzed with the Ethovision tracking system (Noldus). The interaction ratio was calculated as (time spent in the interaction zone in the presence of target)/(time spent in the interaction zone in the absence of target) × 100% (supplementary Methods).
BrdU Injection and Quantification of the Survival of Newborn Neurons
Mice were injected 6 times intraperitoneally with 5-bromo-2’-deoxyuridine (BrdU; 50mg/kg) at 12-hour intervals starting 24 hours after the social interaction test (SIT). Fluorescent immunolabeling and quantification of BrdU-labeled cells were performed as previously described (West et al., 1991) (supplementary Methods).
Retrovirus Production, Stereotaxic Injection, and Analysis
Engineered self-inactivating murine retroviruses expressing enhanced green fluorescent protein (GFP) were used to label proliferating cells and their progeny in the DG of adult mice as described previously (Ge et al., 2006). The purified retroviruses were stereotaxically injected into the DG as described previously (Ge et al., 2007) (supplementary Methods).
Electrophysiological Recordings
For whole-cell patch-clamp recordings, hippocampal slices were prepared using standard procedures as described previously (Ge et al., 2007) (supplementary Methods).
Statistical Analysis
Data are presented as mean±SEM. All statistical analyses were performed using the Prism 6 software package (GraphPad Software). For LTP experiments, statistical analysis was performed using the nonparametric Mann-Whitney U test. The significance of any difference between 2 groups was calculated using the unpaired Student’s t test. ANOVA tests were used for multiple groups’ comparison, and Bonferroni’s posthoc analyses were used to assess the significance between isolated groups. Number of animals or neurons examined is indicated by n. Probability values of P<.05 were considered to represent significant differences.
Results
Individual Differences in Responses to CSDS
To investigate the effects of stress on the morphological and functional maturation of adult-born DGCs, we utilized CSDS, a mouse model of chronic stress with psychotic features of major depressive disorder and posttraumatic stress disorder (Krishnan et al., 2007). Consistent with previous studies (Krishnan et al., 2007), C57BL/6 mice subjected to CSDS displayed clear depressive-like behaviors, characterized by enduring deficits in social interaction, although individual responses were highly heterogeneous (supplementary Figure 1A-C). Based on a measure of SIT at 24 hours after the last defeat, mice subjected to CSDS can be separated into susceptible and unsusceptible subpopulations. Susceptible mice displayed significantly reduced social interaction compared with undefeated control and unsusceptible defeated mice (supplementary Figure 1C). Susceptible mice spent more time in the corner zone and less time in the interaction zone than control and unsusceptible mice in the presence of a social target (supplementary Figure 1D). To determine whether our CSDS paradigm has an effect on basal depressive-like state, 2 classical experimental models were used to evaluate behavioral despair and anhedonia, respectively. When examined 7 days after SIT, we found only susceptible mice displayed a significant increase in the immobility time in the tail suspension test of behavioral despair (supplementary Figure 1E). Furthermore, a significant negative correlation was found in individual mice between the time spent in the interaction zone obtained from the SIT and the immobility time in the tail suspension test (supplementary Figure 1F). Similarly, susceptible mice displayed a significant anhedonia in preference to a 1% sucrose solution (supplementary Figure 1G). These results suggest that the development of social avoidance in susceptible mice is concomitant with increased depressive-like behaviors. We therefore chose susceptible mice for all subsequent experiments designed to investigate the influence of CSDS on adult-born neurons.
CSDS Decreases the Survival of Adult-born DGCs
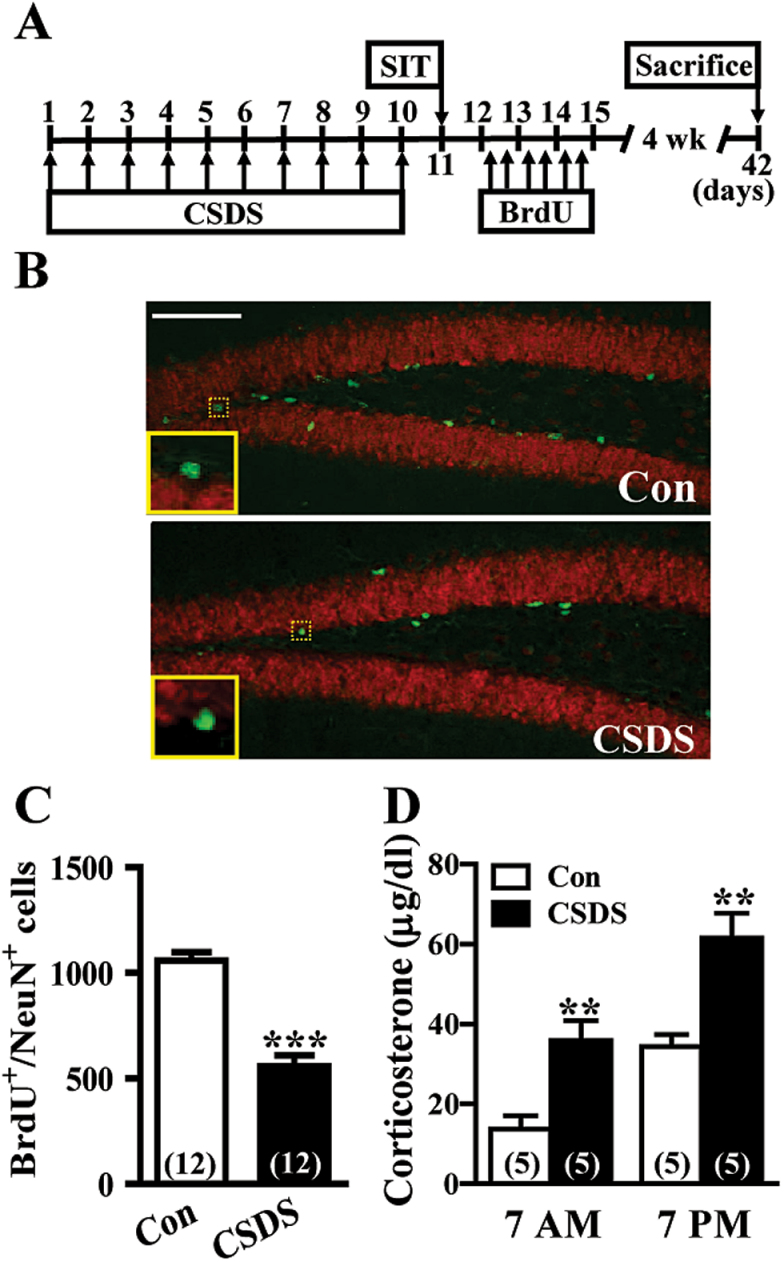
To determine whether chronic stress may affect the survival of adult-born DGCs, CSDS mice were subjected to multiple BrdU injections, and double fluorescent labeling for detection of BrdU-positive (BrdU+) neuronal cells (neuronal specific nuclear protein [NeuN+]) was performed on hippocampal sections 4 weeks later (Figure 1A). As illustrated in Figure 1B, BrdU+/NeuN+-labeled cells were found along the hilar border of the DGC layer. CSDS elicited a significant decrease in the number of BrdU+/NeuN+-labeled cells compared with control mice (Figure 1C). To assess the effect of CSDS on the circadian hypothalamic-pituitary-adrenal axis activity, blood samples were taken the next day after the last defeat at 2 different time points (7 am and 7 pm) for corticosterone analysis. CSDS mice showed elevated plasma levels of corticosterone compared with control mice, confirming successful stress induction (Figure 1D).
Figure 1.
Chronic social defeat stress (CSDS) results in a decrease in the number of 5-bromo-2’-deoxyuridine (BrdU)-labeled cells in the dentate gyrus (DG). (A) Schematic representation of the experimental designs for comparing individual differences in the effect of CSDS on adult-born dentate granule cell (DGC) survival. Following a 10-day CSDS, mice were separated into susceptible and unsusceptible subpopulations at day 11. Control (Con) and susceptible mice to CSDS were then injected 6 times intraperitoneally with BrdU (50mg/kg) at 12-hour intervals starting 24 hours after social interaction test (SIT). Mice were sacrificed 4 weeks after the last BrdU injection. (B) Representative images with immunofluorescent staining showing expression of BrdU and neuronal specific nuclear protein (NeuN) in the DG from Con and CSDS mice. Scale bar=100 µm. (C) Quantification of numbers of BrdU+/NeuN+ cells in the DG from Con and CSDS mice (t (22) = 7.6, P<.001; unpaired Student’s t test). (D) Summary bar graphs depicting plasma corticosterone levels in Con and CSDS mice next day after the last defeat at 7 am (t (8) = 3.7, P=.006; unpaired Student’s t test) and 7 pm (t (8) = 3.9, P=.005; unpaired Student’s t test). The total number of animals examined is indicated by n in parenthesis. Data are presented as mean ± SEM. **P<.01 and ***P<.001 vs Con.
CSDS Alters Dendritic Morphogenesis of Adult-born DGCs
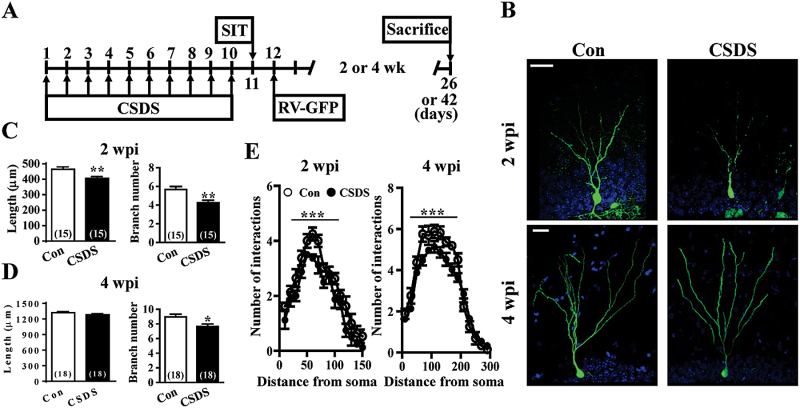
We next evaluated the impact of CSDS on the morphological maturation of adult-born DGCs by using a retrovirus-mediated birth-dating and labeling strategy (Duan et al., 2007). Engineered retroviruses expressing GFP were stereotaxically microinjected into the hilus of the DG. Sholl analysis for dendritic complexity of GFP+ DGCs was carried out 2 or 4 weeks postinjection (wpi) (Figure 2A). In accordance with previous findings (Zhao et al., 2006), the dendrites of adult-born DGCs became progressively more complex in the first 4 weeks after birth (Figure 2B). CSDS significantly reduced the radius interaction for both dendritic length and branch number in GFP+ DGCs at 2 wpi (Figure 2C). At 4 wpi, we did not detect an effect of CSDS on dendritic length of GFP+ DGCs, while a significant difference in branch number was still observed between control and CSDS mice (Figure 2D). Sholl analysis further revealed a significant decrease in the dendritic complexity of GFP+ DGCs in CSDS mice compared with control mice at 2 and 4 wpi (Figure 2E).
Figure 2.
Chronic social defeat stress (CSDS) results in a short-lived decrease in dendritic complexity of adult-born dentate granule cells (DGCs). (A) Schematic representation of the experimental designs for comparing individual differences in the effect of CSDS on morphogenesis of adult-born DGCs. Following a 10-day CSDS, mice were separated into susceptible and unsusceptible subpopulations at day 11. Control (Con) and susceptible mice to CSDS were then received intra-dentate gyrus (DG) injections of GFP-expressing retrovirus (RV-GFP) 24 hours after social interaction test (SIT). Mice were sacrificed 2 to 4 weeks after RV-GFP injection. (B) Representative images of adult-born DGCs expressing GFP (green) at 2 and 4 weeks postinjection (wpi) of retroviruses into the DG of Con and CSDS mice. All slices were counter-stained with 4’,6-diamidino-2-phenylindole (blue). Scale bar = 20 μm. (C-D) Quantitification of the dendritic length and branch number of adult-born DGCs at 2 (C) and 4 wpi (D) from Con and CSDS mice. (E) Sholl analysis of the dendritic tree of adult-born DGCs at 2 and 4 wpi from Con and CSDS mice. The total number of neurons examined is indicated by n in parenthesis. Data are presented as mean ± SEM. *P<.05, **P<.01, and ***P<.001 vs Con.
We also examined the impact of CSDS on the migration of adult-born DGCs. During neuronal differentiation, newborn DGCs migrate from the SGZ outwards in a radial manner into the DGC layer (supplemental Figure 2A). We confirmed that adult-born DGCs contribute almost exclusively to the inner two-thirds of the DGC layer (supplemental Figure 2B). At 2 wpi, the majority (70%) of GFP+ DGCs were distributed within the preexisting inner DGC layer, while some GFP+ DGCs (30%) had migrated into the middle DGC layer. By 4 wpi, a large percentage of the cells had progressed towards the inner and middle DGC layer. As shown in Figure S2C, a similar pattern of positioning of GFP+ DGCs was found between control and CSDS mice at 2 and 4 wpi. Furthermore, there were no significant differences between control and CSDS mice in the soma size of GFP+ DGCs at 2 and 4 wpi (supplemental Figure 2D).
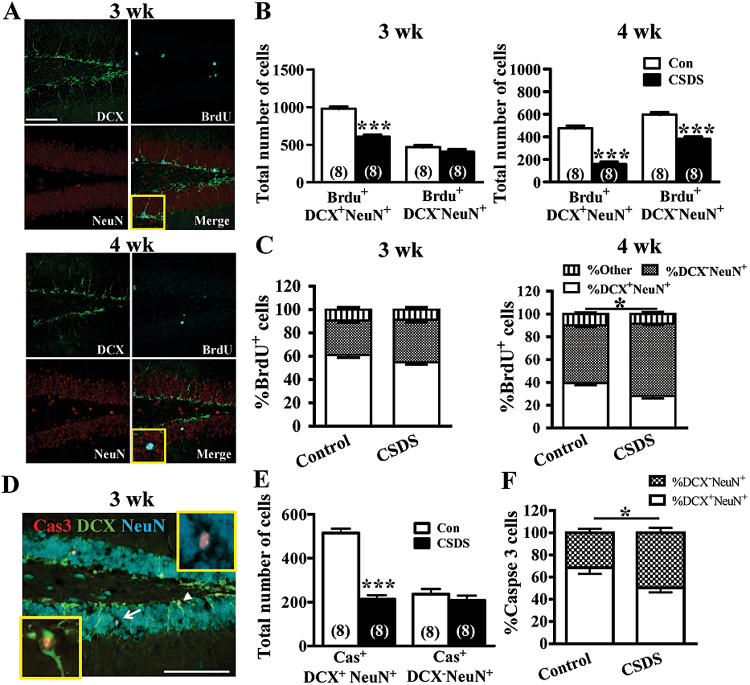
To determine whether CSDS may alter the maturation rate of adult-born DGCs, triple labeling for BrdU, NeuN, and doublecortin (DCX) was performed on hippocampal sections. Because DCX expression occurs transiently during the early stage of adult neurogenesis (Kempermann et al., 2004), we therefore characterized the relative maturity of BrdU+NeuN+ cells based on whether or not they express DCX (Figure 3A). As shown in Figure 3B, a significant decline in the number of immature BrdU+ DGCs (BrdU+DCX+NeuN+) was observed from 3 to 4 weeks after BrdU administration, indicating that the immature cells either die or mature out of the DCX stage (Wang et al., 2008). Unexpectedly, we observed that although CSDS mice demonstrated a significant reduction in the number of mature BrdU+ DGCs (BrdU+DCX-NeuN+) compared with control mice at 3 and 4 weeks after BrdU administration (Figure 3B), the proportion of BrdU+NeuN+ cells that ceased to express DCX was significantly increased from 50.2±4.6% in control mice to 63.8±4.3% (P<.05) in CSDS mice in the 4-week surviving DGCs (Figure 3C). There were no differences between control and CSDS mice in the proportions of BrdU+NeuN+ cells that were either DCX+ or DCX- at 3 weeks after BrdU administration. Because adult-born DGCs compete with neighboring newborn or mature DGCs for survival in the third week after their birth (Tashiro et al., 2006; Bergami and Berninger, 2012; Turnley et al., 2014), we further examined if the surviving DGCs in CSDS mice are less competitive than their control counterparts against mature DGCs. To test this possibility, we measured the extent of neuronal death based on the appearance of activated caspase 3 immunoreactivity in cytoplasm at 3 weeks after CSDS (Figure 3D). We found that significant fractions of DCX+NeuN+ and DCX-NeuN+ DGCs exhibited activated caspase 3 immunoreactivity in both CSDS and control mice, confirming that survival of adult-born DGCs is reduced at this critical stage of selection for stable integration (Tashiro et al., 2006). Although there was no difference in the number of activated caspase 3 immunoreactive DCX-NeuN+ DGCs between CSDS and control mice, a lower number of activated caspase 3 immunoreactive DCX+NeuN+ DGCs was observed in CSDS mice compared with control mice (Figure 3E). The proportion of DCX+NeuN+ DGCs expressing activated caspase 3 immunoreactivity was significantly reduced in CSDS mice compared with control mice (P<05) (Figure 3F).
Figure 3.
Chronic social defeat stress (CSDS) facilitates the maturation of adult-born dentate granule cells (DGCs). (A) Representative confocal images of control hippocampal sections triple stained for 5-bromo-2’-deoxyuridine (BrdU) (blue), doublecortin (DCX) (green), and neuronal specific nuclear protein (NeuN) (red) at 3 and 4 weeks after BrdU administration. Scale bar = 100 μm. (B) CSDS decreased the number of BrdU+NeuN+DCX+ and BrdU+NeuN+DCX- cells. (C) CSDS decreased the proportion of BrdU+NeuN+ cells that were DCX+ but increased the proportion that were DCX- at 4 weeks after BrdU administration. (D) Representative image of immuofluorescent triple stained for NeuN (blue), DCX (green), and activated caspase 3 (Cas, red) at 3 weeks after CSDS. Arrowhead and arrow indicate the Cas+DCX+NeuN+ and Cas+DCX-NeuN+ cell, respectively. Scale bar = 100 μm. (E) CSDS decreased the number of Cas+DCX+NeuN+ cells but not Cas+DCX-NeuN+ cells. (F) CSDS decreased the proportion of Cas+DCX+NeuN+ cells at 3 weeks after CSDS. The total number of neurons examined is indicated by n in parenthesis. Data are presented as mean ± SEM. ***P<.001 vs Con.
CSDS Promotes the Developmental Switch in Synaptic Plasticity of Adult-Born DGCs
To assess whether the morphological changes that resulted from CSDS led to alterations in physiological properties, we performed electrophysiological analysis of adult-born DGCs in acute slices from retrovirus-injected mice at 4 wpi when new neurons reach a more mature stage of development in both morphological (Zhao et al., 2006) and physiological properties (Laplagne et al., 2006). Biocytin was routinely included in the intracellular solution to allow posthoc identification of the recorded neurons. We included only DGCs, which showed colocalization of GFP and biocytin staining, for analysis (supplemental Figure 3A). We found that although adult-born GFP+ DGCs at 4 wpi showed comparable resting membrane potential (supplemental Figure 3B) and rate of rise of action potentials (supplemental Figure 3C) to those of GFP- mature DGCs, the mean amplitude of action potentials for GFP+ DGCs at 4 wpi was significantly less than GFP- mature DGCs (supplemental Figure 3D). There were no significant effects of CSDS on the spike amplitude of adult-born GFP+ and GFP- mature DGCs. In addition, GFP+ DGCs at 4 wpi displayed higher input resistances than GFP- mature DGCs (supplemental Figure 3E). A significant reduction in input resistances was found in adult-born GFP+ DGCs in slices from CSDS mice compared with control mice. To examine the impact of CSDS on excitatory synaptic transmission at MPP-DGC synapses, we examined synaptic strength by measuring stimulus-response curves of excitatory postsynaptic currents (EPSCs) in adult-born GFP+ DGCs. We found no differences between control and CSDS mice in the stimulus-response curve, maximal response, and EPSC waveform in adult-born GFP+ DGCs (supplemental Figure 3F). No significant differences were observed between control and CSDS mice in both amplitude and frequency of spontaneous mEPSCs in adult-born GFP+ DGCs (supplemental Figure 3G). To assess whether CSDS alters the presynaptic function of adult-born DGCs, we examined paired-pulse depression at the MPP-DGC synapses. As shown in supplemental Figure 3H, pairs of presynaptic fiber stimulation pulses delivered at different interpulse intervals evoked nearly identical amounts of paired-pulse ratio in slices from control and CSDS mice. Furthermore, no significant differences were observed between control and CSDS mice in both amplitude and frequency of spontaneous miniature inhibitory postsynaptic currents (mIPSCs) in adult-born GFP+ DGCs (supplemental Figure 3H).
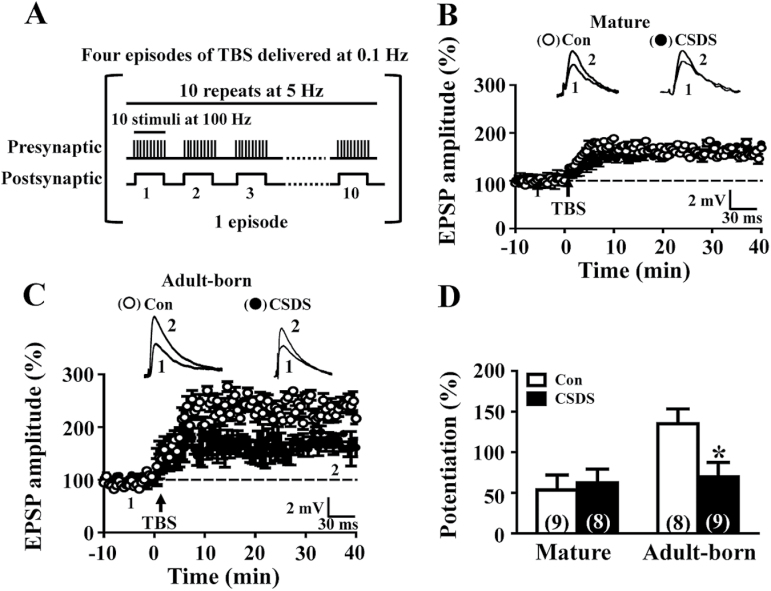
To examine the impact of CSDS on long-term synaptic plasticity, we analyzed the induction of LTP at the MPP-DGC synapses. A theta burst stimulation paradigm was used to elicit LTP (Figure 4A). In slices from control mice, the mean LTP magnitude for adult-born GFP+ DGCs at 4 wpi was significantly larger than that of GFP- mature DGCs (Figure 4B-D), consistent with our previous findings that immature DGCs exhibit enhanced LTP with increased potentiation magnitude and decreased induction threshold (Ge et al., 2007). We found that although CSDS did not change LTP magnitude of GFP- mature DGCs (Figure 4B, D), a significant decrease in LTP magnitude was observed in adult-born GFP+ DGCs (Figure 4C-D). Notably, LTP magnitude of adult-born GFP+ DGCs was comparable to that from GFP- mature DGCs in slices from CSDS mice, suggesting that CSDS can promote the acquisition of the mature LTP phenotype at synapses on adult-born DGCs.
Figure 4.
Chronic social defeat stress (CSDS) results in promoting acquisition of the mature LTP phenotype at synapses. (A) Theta burst stimulation protocol for LTP induction consists brief presynaptic activation of 10 bursts at 5 Hz paired with 100-pA postsynaptic current injection. The episode was applied 4 times at 0.1 Hz. (B) Summary of experiments showing LTP recorded from mature dentate granule cells (DGCs) in slices from control (Con) and CSDS mice. (C) Summary of experiments showing LTP recorded from adult-born GFP+ neurons at 4 weeks postinjection (wpi) in slices from Con and CSDS mice. (D) Bar graph comparing average potentiation measured during 30 to 40 minutes after theta burst stimulation. CSDS resulted in a significant decrease in LTP magnitude in adult-born DGCs (t (15) = 2.6, P=.02; unpaired Student’s t test). Representative traces of excitatory postsynaptic potentials (EPSPs) were taken at the time indicated by number. The total number of neurons examined is indicated by n in parenthesis. Data are presented as mean ± SEM. *P<.05 vs Con.
CSDS Reduces Synaptic GluN2B Subunit Expression in Adult-Born DGCs via an REST-Dependent Mechanism
We next investigated the molecular mechanisms underlying the effect of CSDS on LTP in adult-born DGCs. It is known that the induction of LTP at the MPP-DGC synapses is dependent on NMDAR activation regardless of the cell age (Ge et al., 2007). To test whether CSDS may alter the functional properties and subunit composition of synaptic NMDARs in adult-born DGCs, the relative current contribution in synaptic populations was assessed by an NMDAR/AMPAR ratio calculated from measurement of evoked EPSCs in both GFP- mature and adult-born GFP+ DGCs at 4 wpi. We observed a significantly higher NMDAR/AMPAR ratio at synapses onto adult-born GFP+ DGCs compared with that of GFP- mature DGCs (supplemental Figure 4A). However, we did not observe any change in the NMDAR/AMPAR ratio at synapses onto adult-born GFP+ DGCs or GFP- mature DGCs in CSDS mice compared with control mice (supplemental Figure 4B). We have previously shown that enhanced LTP in adult-born DGCs depends on developmentally regulated synaptic expression of GluN2B-containing NMDARs (Ge et al., 2007). We therefore examined the contribution of GluN2B-containing NMDARs to evoked EPSCs in both GFP- mature and adult-born GFP+ DGCs at 4 wpi. Application of ifenprodil (3 μM), a GluN2B-selective antagonist (Barth and Malenka, 2001), reduced the NMDAR-mediated EPSCs in adult-born GFP+ DGCs by 73.5±6.5%, whereas the same treatment led to only 25.9±4.4% reduction in GFP- mature DGCs (supplemental Figure 4B), consistent with the idea that GluN2B subunit is expressed predominantly during the early development of adult-born DGCs. Notably, a significant decrease in the expression of ifenprodil-sensitive NMDAR-mediated EPSCs was observed in adult-born GFP+ DGCs from CSDS mice compared with control mice (supplemental Figure 4B). Because GluN2B-containing NMDARs exhibit slower decay kinetics than their GluN2A-containing counterparts (Cull-Candy and Leszkiewicz, 2004), we also measured the decay time kinetics of NMDAR-mediated EPSCs. We confirmed that the decay of NMDAR-mediated EPSC was significantly slower in adult-born GFP+ DGCs than that of GFP- mature DGCs from control mice (supplemental Figure 4C). A decrease in the decay time kinetics of NMDAR-mediated EPSCs was observed in adult-born GFP+ DGCs from CSDS mice compared with those from control mice (supplemental Figure 4C).
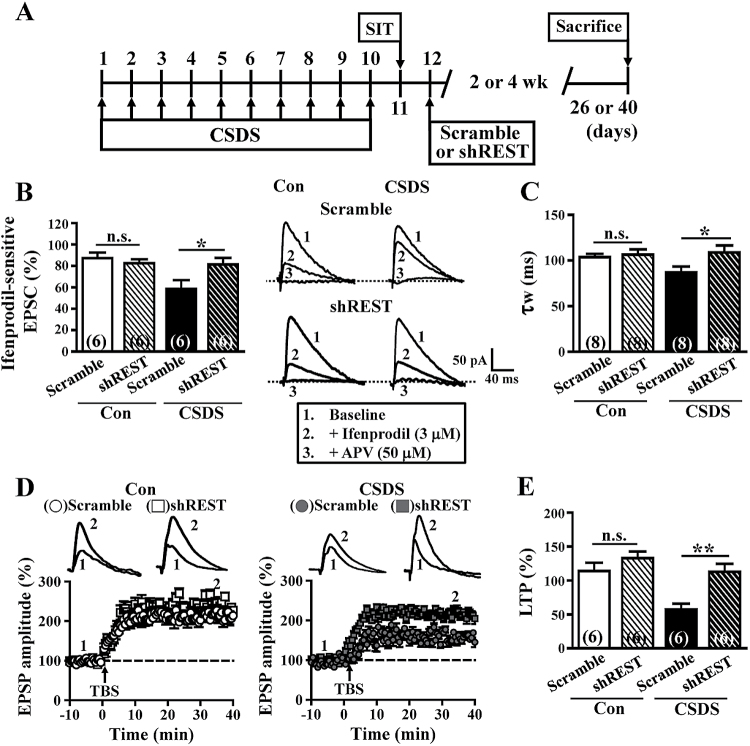
The GLI-Krüppel class C2H2 zinc finger protein REST is expressed in neural stem cells of the SGZ and declines during the differentiating stages (Gao et al., 2011). Recent work has shown that the REST-dependent epigenetic remodeling may promote the developmental switch of synaptic NMDAR subunit composition in mature DGCs (Rodenas-Ruano et al., 2012). To evaluate whether CSDS increases REST abundance in adult-born neurons, we examined REST mRNA expression by means of single-cell quantitative real-time PCR technique. We confirmed that the level of endogenous REST mRNA was significantly increased in adult-born GFP+ DGCs, but not in mature neurons, from CSDS mice compared with those from control mice (supplemental Figure 5A). To directly examine a possible causal role for REST in the CSDS-induced decline in GluN2B subunit expression in adult-born DGCs, we constructed several retroviruses expressing specific short-hairpin RNA (shRNA) against different regions of mouse REST. We found that 2 different shREST, but not the scrambled shRNA, specifically knocked down the expression of REST as shown by Western-blotting analysis in hippocampal neuronal cultures (supplemental Figure 5B). Having confirmed the knockdown efficiency of shRNA, shREST or scrambled shRNA retroviruses were stereotaxically microinjected into the hilus of the DG (Figure 5A). Consistent with an increase in the synaptic GluN2B subunit content, we found that NMDAR-mediated EPSCs of shREST-expressing GFP+ DGCs exhibited enhanced sensitivity to ifenprodil (3 μM) and slower decay time kinetics than those of scrambled control cells from CSDS mice (Figure 5B-C). Notably, no significant differences were observed between shREST-expressing GFP+ DGCs of CSDS mice and scrambled control GFP+ DGCs of control mice in the expression of ifenprodil-sensitive component and the decay time kinetics of NMDAR-mediated EPSCs.
Figure 5.
Knockdown of repressor element-1 silencing transcription factor (REST) rescued the effects of chronic social defeat stress (CSDS) on adult-born dentate granule cells (DGCs). (A) Schematic representation of the experimental timeline of retrovirus injection and assays. (B) Representative traces and summary data comparing the ifenprodil (3 μM)-sensitive N-methyl-D-aspartate receptors (NMDAR)-mediated EPSCs at +40 mV in adult-born DGCs infected with short-hairpin REST (shREST) or scrambled short-hairpin RNA (shRNA) retroviruses at 4 wpi in slices from control (Con) and CSDS mice. (C) Comparison of the weighted time constant (τw) of decay of NMDAR-mediated EPSCs at +40 mV in adult-born DGCs infected with shREST or scrambled shRNA retroviruses at 4 wpi in slices from Con and CSDS mice. (D) Comparison of the average theta burst stimulation-induced LTP magnitude in adult-born DGCs infected with shRNA-REST or scrambled shRNA retroviruses at 4 wpi in slices from Con and CSDS mice. (E) Bar graph comparing average potentiation measured during 30 to 40 minutes after theta burst stimulation. The total number of neurons examined is indicated by n in parenthesis. Data are presented as mean ± SEM. *P<.05 and **P<.01 vs scrambled shRNA. APV, D-2-amino-5-phosphonovalerate. n.s., not significant.
Finally, we assessed whether knockdown of REST in adult-born DGCs prevents CSDS-induced decline in LTP at 4 weeks of the cell age. Compared with scrambled shRNA-expressing cells, shREST-expressing GFP+ DGCs showed a higher LTP magnitude in CSDS mice (Figure 5D-E). However, we did not observe a difference between scramble shRNA- and shREST-expressing GFP+ DGCs from control mice in the magnitude of LTP. We also confirmed that knockdown of REST in adult-born DGCs rescued the effects of CSDS on the radius interaction for both dendritic length and branch number at 2 wpi (supplemental Figure 6A-B) and branch number at 4 wpi (supplemental Figure 6A, C). Together, these findings strongly suggest that CSDS triggers a cascade involving increased expression of REST, which leads to a decline in GluN2B subunits of the synaptic NMDARs, thereby promoting the maturation of LTP at the MPP-DGC synapses on adult-born DGCs.
Discussion
Adult neurogenesis is a complex multistep process that includes proliferation, fate specification, maturation, targeting, and functional integration of the newborn neurons into the preexisting neural circuits. These different developmental processes can be modified by various physiological, pathological, and pharmacological stimuli (Duman et al., 2001; Ming and Song, 2005). While chronic stress has been found to suppress the proliferation and survival of newborn DGCs in the adult hippocampus (Pham et al., 2003; Simon et al., 2005; Czéh et al., 2007; Ferragud et al., 2010; Van Bokhoven et al., 2011), little is known about its effects on the morphological and physiological maturation of adult-born neurons. Combining retrovirus-mediated birth-dating and labeling techniques and electrophysiological recordings, we have shown here for the first time that CSDS can promote the acquisition of the mature LTP phenotype at synapses on adult-born DGCs. We have identified that an acceleration of developmental switch in the composition of synaptic NMDARs from predominantly GluN2B to GluN2A may contribute to such enhanced synaptic maturation. Our findings also reveal an important role for REST in orchestrating the switch in synaptic NMDAR subunit composition after CSDS. Our data support a model in which CSDS increases REST expression, leading to a decline in GluN2B subunit expression at synapses, which in turn promotes the acquisition of the mature synaptic NMDARs and LTP phenotype.
While most previous studies have suggested that chronic stress reduces the survival of newborn neurons in the adult SGZ (Lee et al., 2006; Van Bokhoven et al., 2011), there is contradictory evidence that the survival of adult-born DGCs is enhanced after CSDS in mice that display persistent social avoidance (Lagace et al., 2010). The results of the present study concur with the preponderance of literature demonstrating decreased survival of adult-born DGCs after CSDS. Our findings seem to conflict with those that mice susceptible to CSDS have significantly enhanced survival of adult-born DGCs that were generated after defeated stress (Lagace et al., 2010). A conceivable explanation of these seemingly discrepant observations is the different BrdU administration regimens used between studies. In the study of Lagace et al. (2010), mice received an intraperitoneal injection of BrdU (150mg/kg) 24 hour after the last episode of CSDS, and the total number of BrdU-immunoreactive cells in the DG was calculated 4 weeks later. However, in our current study, mice were injected 6 times intraperitoneally with BrdU (50mg/kg) at 12-hour intervals starting 24 hours after SIT and BrdU+ cells in the DG were counted 4 weeks after BrdU administration. A typical neurogenesis study using BrdU labeling often involves multiple injections during the period of 24 to 48 hours (Kempermann et al., 1997). Considering that multiple injections may allow labeling of cells that are going through S-phase divisions across the day and thus incorporating BrdU at different time points (Cameron and McKay, 2001), our protocol may provide a quantitative estimate of a larger pool of dividing cells. Nevertheless, we cannot completely exclude the possibility that subtle differences in experimental variables may account, in part, for this apparent discrepancy.
Surprisingly, DCX+NeuN+ DGCs displayed a substantial decrease in death at 3 weeks after CSDS (Figure 3D-E). Although the molecular basis of how CSDS modulates the survival of adult-born DGCs at this stage remains unclear, one intriguing possibility is that the young DGCs in CSDS mice are somewhat less competitive than their control counteracts against neighboring newborn or mature DGCs, thus providing a survival advantage. Additional studies are needed to clarify this possibility.
The connectivity of a neuron is determined mainly by its dendritic arborization and synaptic contacts. Newborn DGCs exhibit features of dendrites as early as 2 weeks after birth and display overall morphological and functional characteristics of fully mature GCs at 6 weeks of cell age (Espósito et al., 2005; Zhao et al., 2006). Moreover, it has been suggested that the rate of adult-born DGC maturation correlates with the pattern of neuronal activity (Piatti et al., 2011). We demonstrated that CSDS decreased dendritic complexity of surviving adult-born DGCs, as shown by decreased dendritic length and branching. However, the suppressive effect of CSDS on dendrite maturation of adult-born DGCs is short-lived, and dendritic complexity returns to normal levels at about 4 weeks after CSDS. In addition, CSDS did not alter the relative positioning of adult-born DGCs in the DGC layer. In accordance with morphological observations, our electrophysiological data indicate that the intrinsic excitability and synaptic transmission of adult-born DGCs were not affected by CSDS. These data suggest that, although CSDS has effects on morphological properties of newborn DGCs during their initial development, some compensatory mechanisms may exist during late developmental stages to normalize the morphological and physiological changes of CSDS.
Adult-born DGCs have been shown to exhibit enhanced LTP at the MPP-DGC synapses with increased potentiation magnitude and decreased induction threshold during a critical period between 4 and 6 weeks of cell age (Snyder et al., 2001; Schmidt-Hieber et al., 2004; Ge et al., 2007). Such critical period plasticity is associated with developmentally regulated synaptic expression of GluN2B-containing NMDARs in adult-born DGCs (Ge et al., 2007) and is necessary for fine contextual discrimination (Kheirbek et al., 2012). Here we extend these findings by demonstrating that CSDS can accelerate the maturation of immature neurons to acquire the mature synaptic NMDARs and LTP phenotype. Our results also highlight the existence of a positive correlation between synaptic expression of GluN2B-containing NMDARs and enhanced LTP in adult-born DGCs. Because we did not observe significant change in the peak amplitude of NMDAR-mediated EPSCs of adult-born DGCs at 4 wpi by CSDS, it is reasonable to speculate that CSDS induces a loss of synaptic GluN2B-containing NMDARs and their replacement with GluN2A-containing NMDARs. These observations beg the question of how CSDS accelerates the developmental switch in synaptic NMDAR subunit composition from predominantly GluN2B to GluN2A. Our results suggest that REST is the principal regulator for governing the suppressive effect of CSDS on GluN2B subunit expression. We found that CSDS increases REST mRNA in adult-born DGCs and that knockdown of REST by shRNA rescued the decline in synaptic GluN2B subunit and LTP magnitude in adult-born DGCs of CSDS mice. Nonetheless, it is unclear how CSDS promotes REST gene expression in adult-born DGCs, and the molecular basis for the regulation of the developmental switch in synaptic NMDAR subunit composition by REST remains an open question. Additional investigation is warranted to determine whether the sustained high levels of basal corticosterone are responsible for CSDS- and REST-mediated downregulation of GluN2B subunit expression.
It has been shown that maternal deprivation, an animal model of early life stress, may impair REST activation and acquisition of the mature NMDAR phenotype in mature DGCs of rat hippocampus (Rodenas-Ruano et al., 2012). However, we demonstrate that CSDS accelerates the developmental switch in NMDARs in adult-born DGCs by increasing REST expression. The reason for these seemingly contradictory findings is unclear but may be related to differences in cell age targeted. Furthermore, the different stress protocols (maternal deprivation vs CSDS) and animal species (rat vs mouse) may account for the differences in REST activation obtained by Rodenas-Ruano et al. (2012) and our own. Regardless of the mode of action, these findings highlight a crucial role of REST in experience-dependent fine-tuning of synaptic NMDARs during neuronal development.
Recent work has shown that adult-born DGCs use GABA signaling to initiate functional glutamatergic synaptic transmission in response to an enriched environment (Chancey et al., 2013). Furthermore, Bergami and colleagues (2015) revealed a specific time window (2–6 weeks of age) during the maturation of adult-born DGCs for enriched environment-mediated remodeling of connections impinging on them. It is very likely that CSDS may also affect the connectivity of adult-born DGCs. Because we did not examine the influence of CSDS on the presynaptic connectivity of adult-born DGCs, at this point we cannot exclude the possibility that the impact of CSDS on LTP induction at the MPP-DGC synapses is mediated, at least in part, by an experience-dependent reorganization of the presynaptic inputs on adult-born DGCs. Future research will be required to evaluate whether REST may also play a role in experience-dependent remodeling of adult-born DGC connectivity.
In conclusion, we provide compelling evidence that CSDS decreases the survival of adult-born DGCs but accelerates the synaptic maturation of adult-born DGCs. Our data also support a sequential signaling model, in which CSDS can increase expression of REST in surviving adult-born DGCs, which leads to accelerated developmental switch in synaptic NMDAR subtype and thereby promotes acquisition of the mature LTP phenotype. Since adult neurogenesis has been implicated in various brain functions (Zhao et al., 2008; Ming and Song, 2011), delineating mechanism by which CSDS affect newborn DGC maturation should be critical for understanding the role of stress in influencing brain function.
Statement of Interest
None.
Supplementary Material
Acknowledgments
We thank Chih-Hao Yang for his technical assistance and members of the Hsu laboratory for helpful discussion and suggestions. This work was supported by research grants from the National Health Research Institute (NHRI-EX104-10336NI), the Ministry of Science and Technology (NSC102-2321-B-006-014), and the Ministry of Education (Aim for the Top University Project to the NCKU), Taiwan.
References
- Anacker C, Cattaneo A, Musaelyan K, Zunszain PA, Horowitz M, Molteni R, Luoni A, Calabrese F, Tansey K, Gennarelli M, Thuret S, Price J, Uher R, Riva MA, Pariante CM. (2013) Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc Natl Acad Sci USA 110:8708–8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain MJ, Dwyer SM, Rusak B. (2004) Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett 368:7–10. [DOI] [PubMed] [Google Scholar]
- Barth AL, Malenka RC. (2001) NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci 4:235–236. [DOI] [PubMed] [Google Scholar]
- Bergami M, Berninger B. (2012) A fight for survival: the challenges faced by a newborn neuron integrating in the adult hippocampus. Dev Neurobiol 72:1016–1031. [DOI] [PubMed] [Google Scholar]
- Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Göbel J, Yang SM, Conzelmann KK, Schinder AF, Götz M, Berninger B. (2015) A critical period for experience-dependent remodeling of adult-born neuron connectivity. Neuron 85:710–717. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. (2010) Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm Behav 58:769–779. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. (2001) Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435:406–417. [DOI] [PubMed] [Google Scholar]
- Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, Overstreet-Wadiche LS. (2013) GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci 33:6614–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. (2004) Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 255:re16. [DOI] [PubMed] [Google Scholar]
- Czéh B, Müller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. (2007) Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology 32:1490–1503. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. (2007) Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130:1146–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Nakagawa S, Malberg J. (2001) Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 25:836–844. [DOI] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. (2005) Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 25:10074–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferragud A, Haro A, Sylvain A, Velázquez-Sánchez C, Hernández-Rabaza V, Canales JJ. (2010) Enhanced habit-based learning and decreased neurogenesis in the adult hippocampus in a murine model of chronic social stress. Behav Brain Res 210:134–139. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ding P, Nashaat M, Yuan L, Ma J, Hammer RE, Hsieh J. (2011) The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells. J Neurosci 31:9772–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. (2006) GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. (2007) A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. (1997) Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17:2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E. (1998) Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA 95:3168–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joëls M, Lucassen PJ. (2004) Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging 25:361–375. [DOI] [PubMed] [Google Scholar]
- Hill MN, Kambo JS, Sun JC, Gorzalka BB, Galea LA. (2006) Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur J Neurosci 24:1845–1849. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27:447–452. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. (2012) NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci 32:8696–8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. (2010) Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci USA 107:4436–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Espósito MS, Piatti VC, Morgenstern NA, Zhao C, van P, raag H, Gage FH, Schinder AF. (2006) Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol 4:e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Kim SJ, Kim SW, Choi SH, Shin YC, Park SH, Moon BH, Cho E, Lee MS, Choi SH, Chun BG, Shin KH. (2006) Chronic mild stress decreases survival, but not proliferation, of new-born cells in adult rat hippocampus. Exp Mol Med 38:44–54. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M. (2013) Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J Neurosci 33:2961–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. (2003) Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28:1562–1571. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. (2003) Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 17:879–886. [DOI] [PubMed] [Google Scholar]
- Piatti VC, Davies-Sala MG, Espósito MS, Mongiat LA, Trinchero MF, Schinder AF. (2011) The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci 31:7715–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenas-Ruano A, Chávez AE, Cossio MJ, Castillo PE, Zukin RS. (2012) REST-dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors. Nat Neurosci 15:1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. (1999) Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci 19:10603–10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429:184–187. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. (2012) New neurons retire early. Nat Neurosci 15:1611–1612. [DOI] [PubMed] [Google Scholar]
- Simon M, Czéh B, Fuchs E. (2005) Age-dependent susceptibility of adult hippocampal cell proliferation to chronic psychosocial stress. Brain Res 1049:244–248. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. (2001) Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol 85:2423–2431. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. (2001) Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol 437:496–504. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. (2006) NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442:929–933. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Basrai HS, Christie KJ. (2014) Is integration and survival of newborn neurons the bottleneck for effective neural repair by endogenous neural precursor cells? Front Neurosci 8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van B, okhoven P, Oomen CA, Hoogendijk WJ, Smit AB, Lucassen PJ, Spijker S. (2011) Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci 33:1833–1840. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. (2008) Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci 28:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. (1991) Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.