Abstract
Aim:
Current ex vivo study compared fracture resistance of teeth instrumented using 5 endodontic files, filled with Gutta-percha and AH Plus.
Materials and Methods:
Sixty freshly extracted, single-rooted mandibular premolars were acquired and decoronated to obtain 15 mm segments. These samples were randomly divided into six groups (n = 10). Group 1 served as the control containing untreated samples (without instrumentation or filling). In Groups 2-6, samples were instrumented using rotary (Universal ProTaper and Revo-S), reciprocating (WaveOne and RECIPROC®), and self-adjusting file (SAF), respectively. Following instrumentation, the samples were filled by lateral compaction with Gutta-percha and AH Plus. A week later, after the sealer was completely set, a vertical load was applied to the specimen's canal in each group until fracture. The loads required for fracture were recorded, and statistical analysis was performed.
Results:
The mean fracture load differed significantly among the groups (P < 0.01; one-way ANOVA). Tukey's post-hoc tests revealed that the fracture resistance was similar in the control and SAF groups (P > 0.05) and was significantly higher than that of the 2 rotary and reciprocating groups (P < 0.01).
Conclusion:
The samples instrumented by the SAF exhibited a better fracture resistance.
Keywords: Fracture strength, instrumentation, ProTaper, RECIPROC, Revo-S, self-adjusting file, WaveOne
INTRODUCTION
Root canal system (RCS) instrumentation is an essential aspect of endodontic therapy, which aims to thoroughly debride it while maintaining the original shape of the root canal without harming dentine integrity. Instrumenting the RCS with motorized nickel-titanium (NiTi) files can weaken the dentin integrity, resulting in vertical root fractures (VRFs).[1,2,3] In addition, the predisposing factors for VRFs include the loss of tissue, dehydration of dentin, effects of irrigation solutions, and use of excessive pressure during root-filling procedures.[4] Over the last few decades, endodontic instrumentation has evolved on the line of technological advancements. The currently used motorized file systems consist of a solid metal core, with rotating blades, and flutes. These files are designed with increasing taper, resulting in active cutting, and relative removal of more dentine. In addition, an excessive taper results in more removal of dentine reducing the fracture strength.[5,6]
Another element directly related to the fracture resistance is the creation of microcracks in radicular dentine.[2,4,7] All the currently used rotary and reciprocating files create microcracks ranging from 18% to 60% in the roots instrumented.[1,2,7,8] The reciprocating files have 3 times more tendency to create microcracks when compared to the multiple sequences of rotary files.[8] Various studies have been reported on the fracture resistance of teeth and formation of microcracks in the radicular dentin.[2,4,6,9,10]
Recently introduced self-adjusting file (SAF) (ReDent-Nova, Ra’anana, Israel) is designed as hollow and flexible file that adapts itself to the root canal shape. It addresses higher percentages of the irregularly shaped root canals than the rotary/reciprocating files, providing a cleaner radicular dentin surface for successful three-dimensional obturation.[9] The SAF abrades the dentin (it has an abrasive surface), removing a thin uniform layer of dentin from the entire perimeter of the canal maintaining dentin integrity.[9] Also, the samples instrumented with this file exhibit no crack formation and higher fracture resistance.[2,4,9] Few studies have compared the efficiency of SAF with those of the reciprocating file systems.[11]
Hence, the purpose of our study was to compare the fracture resistance exhibited by teeth on instrumentation using 2 rotary files: ProTaper Universal (PT; Dentsply Maillefer, Ballaigues, Switzerland) and Revo-S (RS: Micro-Mega, Besançon, France), 2 reciprocating files: WaveOne (WO; Dentsply Maillefer, Ballaigues, Switzerland) and RECIPROC® (RC; VDW Silver, Munich, Germany) and the SAF (ReDent-Nova, Ra’anana, Israel).
MATERIALS AND METHODS
The methodology of the study was reviewed and approved by the local Ethical Committee. Intact human mandibular premolars comprising single root canals with completely formed apices were acquired and stored in a saliva substitute at 37°C until use. All samples were sectioned at or below the cemnto-enamel junction using a diamond coated bur under continuous water-cooling to obtain root segments with standard root canal length of 15 mm determined by inserting a size 15 K-file till the tip is visible in the apical foramen and withdrawn by 0.5 mm. All samples were subsequently examined under a stereomicroscope at ×10 magnification to detect craze lines or cracks. Teeth with such findings were excluded. After the above procedure a total of 60 samples were selected for the study.
Instrumentation for different groups
Group 1 control
The samples in this group remained untreated, and no instrumentation or filling was performed.
Group 2 ProTaper System
The samples in this group were instrumented using PT rotary files (Sx-F4; Dentsply Maillefer) with a preprogramed X Smart Plus (Dentsply Maillefer) at 300 rpm speed and 2-Ncm torque. The coronal third was preflared using Sx, followed by sequential files till working length (WL) S1 (#17.06), S2 (#20.06), F1 (#20.07), F2 (#25.08), F3 (#30.09), and F4 (#40.06). Before introducing a new file each time, the root canal was irrigated with 3 ml of 5.25% sodium hypochlorite (NaOCl) and recapitulated with a #15 K-file, and then the subsequent rotary file was introduced. Ethylenediaminetetraacetic acid (EDTA) paste (RC Help, Prime Dental Products, Mumbai, India) was used as lubricant for the file with every reinsertion.
Group 3: Revo-S system
The samples in this group were instrumented using sequential Revo-S rotary files. The root canal orifices were preflared using ENDOFLARE® (Micro-Mega). The canals were instrumented at a rotational speed of 300 rpm at a torque of 0.8 N/cm using XSmart Plus. The sequential files after orifice flaring were used in the following order:
SC1 (#25.06) was used until the two-third length of the root canal was reached,
SC2 (#25.04) and SU (#25.06) files were used up to the full WL. After this, AS30 (#30.06), AS35 (#35.06), and AS40 (#40.06) files were used till WL.
Before introducing each file, the root canal was irrigated with 5.25% NaOCl, the canal was recapitulated, and the subsequent file was introduced in the aforementioned sequence. EDTA Gel (RC Help) as used as lubricant for the file with an every reinsertion of the file.
Group 4: WaveOne system
The samples in this group were instrumented using WO large (#40.09) files, with a preprogramed X Smart Plus endo motor. A large file was selected according to the manufacturer's instructions (#25 K-file reached the apex passively). The WO file was introduced in the canal with reciprocating and pecking (in and out) motion. On meeting obstruction the file was removed, the canal was irrigated, recapitulated by a #15 K-file and the file was reintroduced into the canal. EDTA Gel (RC Help) as used as lubricant for the file. Instrumentation was continued in the aforementioned manner until the file reached the WL.
Group 5: RECIPROC® system
The samples in this group were instrumented using RC® R40 (#40.09) files, with XSmart Plus endo motor. The R40 was selected according to the manufacturer's instruction (#25 K-file reached the apex passively). The RC file was introduced in the canal with a reciprocating and pecking (in and out) motion, similar to that used in Group 4 and was continued till WL. EDTA Gel (RC Help) as used as lubricant for the file.
Group 6: Self-adjusting file system
The samples were instrumented using 2.0 mm SAF with transline motion (in-and-out). The file was operated with in-and-out pecking motion till the WL at 5000 vibrations/min using the EndoStation endodontic system (ReDent-Nova). The file was operated for 4 min with continuous irrigation with 5.25% NaOCl at a flow rate of 4 mL/min (as recommended by the manufacture). The irrigation solution was applied through the hollow file using a built-in peristaltic pump. This was followed by the removal of smear layer with 5 ml of 17% aqueous EDTA (Dent Wash) for 1-min and a final rinse with 5 ml of distilled water.
Final irrigation protocol for Groups 2-5
After instrumentation, a final flush was applied using 5 mL of 17% aqueous EDTA (Dent Wash, Prime Dental Products) for 1-min (for removal of smear layer) and 5 mL of 5.25% NaOCl for 1-min followed by the final rinse with 5 mL of distilled water.
Root canal obturation
After instrumentation (Groups 2-6), the canals were dried with sterile paper points. The samples were then filled using master Gutta-percha cones (apical fit confirmed using digital radiography). The dentinal wall was coated with AH plus sealer (Dentsply DeTrey, Konstanz, Germany) using a lentulo spiral, followed by placing the selected master-cone, and adding accessory cones using a #25 NiTi finger spreader. Excess Gutta-percha was sheared off using a hot hand plugger and access cavities were filled with composite resin and sealer was allowed to set completely for 7 days at 37°C and 100% humidity.
Fracture testing
The apical 5 mm of the samples were covered with wax to obtain a 0.2-0.3 mm thick layer. These samples were then mounted in copper rings (25 mm in height and 15 mm in diameter) and filled with self-cure acrylic resin, exposing 9 mm of the coronal part. With the onset of polymerization, the roots were removed and the cleaned off wax using a curette, followed by coating them with thin layer of polyvinylsiloxane impression material and embedding again into acrylic resin.
The acrylic blocks were then placed on the universal testing machine (Instron Corp, Canton, MA). The tip with a diameter of 3 mm was used. The tip was centered over the canal orifice, and a gradually increasing vertical force was exerted (1 mm/min) until fracture. The maximum force required to fracture each sample was recorded in Newton (N).
Statistical analysis
The fracture load for Group 1 to Group 6 was analyzed using one-way analysis of variance (ANOVA) and Tukey's multiple comparisons (SPSS, v20; SPSS Inc, Chicago, IL).
RESULTS
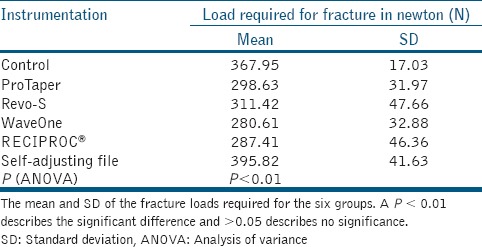
The mean loads required for the fracture were 367.95 N (±17.03) for Group 1, 298.63 N (±31.97) for Group 2, 311.42 N (±47.66) for Group 3, 280.61 N (±32.88) for Group 4, 287.41 N (±46.36) for Group 5, and 395.82 N (±41.63) for Group 6 [Table 1]. A significant difference in fracture resistance was observed among the groups (P < 0.01: ANOVA), and Tukey's post-hoc test was applied for multiple comparisons. Samples instrumented by SAF (Group 6) exhibited similar fracture strength when compared to the control group (P > 0.05; Tukey's post-hoc test) whereas the samples instrumented by rotary (Group 2 and 3) and reciprocating files (Group 4 and 5) exhibited significantly reduced fracture strength compared to the control group (P < 0.01; Tukey's post-hoc test). The results are plotted in Graph 1. The fracture resistance of the samples was reduced approximately by 23%, 18%, 31%, and 27% in Groups 2, 3, 4, and 5, respectively compared to the control group.
Table 1.
Mean load required in newton (N)
Graph 1.
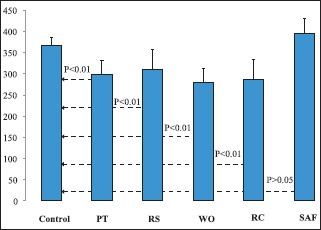
Meanwise distribution of the loads required (N) for fracture in all the five groups. The dotted arrow represents the P value after application of the multiple tests of comparison (post-hoc test) (P < 0.01 significant and P > 0.05 insignificant)
DISCUSSION
The results of the present study indicate that the instrumentation decreases the fracture resistance of teeth by either rotary or reciprocating files. The samples instrumented using the SAF exhibited better fracture resistance when compared to the other experimental groups (P < 0.01). Also, this group did not differ from the control (P > 0.05). Root canal instrumentation using the rotary and reciprocating files reduces the fracture resistance of the instrumented tooth up to 30%.[11] In the current study, compared with the samples in Group 1 control, the fracture resistance reduced approximately by 23% in Group 2, 18% in Group 3, 31% in Group 4, and 27% in Group 5. In all previous studies with respect to the fracture resistance or microcracks, the apical preparation was controlled till a #30 size and in the current study we controlled it till #40 for both rotary and reciprocating files. This could be the reason for similar fracture resistance exhibited by the groups instrumented with rotary/reciprocating files and can be concluded that the larger apical tip diameter of these files can result in reducing the fracture resistance of endodontically treated teeth.
Cracks formed during instrumentation may develop into fractures during retreatment or after long-term functional stresses such as chewing.[12] Rotary and reciprocating files create microcracks in the radicular dentin ranging from 15% to 60%. These files are associated tapers of varying sizes (0.06-0.09), which may contribute to the dentinal crack formation, which has been observed at various levels including the apical thirds.[1,2,4,8,9] These cracks become high-stress concentration areas and gradually propagate from these areas to the root canal surface, thus causing VRFs.[13,14]
Kim et al., reported that the rotary files generate a stress of 311-368 MPa on the outer surface of the dentin. The tensile strength of the radicular dentin is 106 MPa. The stress generated by such files is 3 times larger than the strength of the dentin, resulting in microcracks.[15] These files possess a solid metal core with flutes and blades for increased cutting. Also, the large-sized taper of these files result in excessive, unnecessary removal of the dentin, thus weakening the root, and such aggressively cutting the dentin may contribute to microcrack formation.[4,15,16] Reciprocating files create a larger number of microcracks,[8,15] which may be the reason for the reduced fracture resistance exerted by the teeth in Groups 4 and 5 in the current study. The reciprocal motion also enhances debris transportation toward the apex, which may increase torsional forces.[17] The discrepancy between the apical size of rotary/reciprocating files and the actual cross-section of the apical part of canals led to the recommendation to use larger files for apical preparations in order to address the entire perimeter of the canal wall. This often results in the excessive removal of dentin in the apical third reducing the strength.[9] To standardize the apical preparation we used all rotary and reciprocating files corresponding to size #40 with 0.06 taper for rotary and 0.09 for reciprocating files, and the larger 2 mm file of the SAF was used to compare.
SAF has been reported to cause no dentinal microcracks.[2,18] It is a hollow file composed of NiTi lattice. It is devoid of any metal core or flutes and blades; it does not cut the dentin. Instead, has an abrasive surface that abrades the dentin, restricting the removal of the intact dentin.[9,16,19] Kim et al., reported that the stress generated by the SAF was approximately 10 MPa,[20] which can be attributed to the very few-to-no microcracks created using SAF, thus increasing the fracture resistance of the treated teeth. Yoldas et al.,[2] and Liu et al.,[13] in their study found that the samples instrumented with SAF and hand files exhibited no microcracks in the radicular dentin. The less stress generation and no microcracks formed in the radicular dentin could be attributed to the higher fracture resistance of the teeth instrumented by the SAF in the study reported by Pawar et al.[4]
Obturating materials are used to improve the fracture resistance of an endodontically treated tooth.[21,22] Epoxy resin-based sealers adhere strongly to the root canal dentin and penetrate deeper into dentinal tubules,[23,24,25] thus increasing the retention of the filling material by inducing mechanical locking with the canal walls. Therefore, a combination of Gutta-percha and an AH Plus sealer was used in the present study.
Fracture resistance exhibited by the teeth instrumented using RS have not been reported in literature so far, and those using the SAF, WO, and RC have not been explored extensively and hence, this study was designed. But such studies still possess inherent limitations. Like the current ex vivo study was carried out only on single rooted teeth, standardized root lengths, with a compressed loading applied at a single point. Clinically related variables like occlusion, masticatory force, the level of alveolar bone attachment, and para-functional habits should also be considered.
CONCLUSION
Instrumentation of samples using the rotary and reciprocating files remarkably reduced the fracture resistance of the instrumented teeth compared with the control. Conversely, the fracture resistance of samples in the SAF group was similar to that of the samples in the control group. Additional studies are required to assess the short- and long-term impacts of instrumentation on microcrack formation and VRFs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Dr. Zvi Metzger serves as Scientific Consultant to Redent-Nova Co., manufacturer of the SAF System. All other authors deny any conflict of interest for the present study.
REFERENCES
- 1.Shemesh H, Bier CA, Wu MK, Tanomaru-Filho M, Wesselink PR. The effects of canal preparation and filling on the incidence of dentinal defects. Int Endod J. 2009;42:208–13. doi: 10.1111/j.1365-2591.2008.01502.x. [DOI] [PubMed] [Google Scholar]
- 2.Yoldas O, Yilmaz S, Atakan G, Kuden C, Kasan Z. Dentinal microcrack formation during root canal preparations by different NiTi rotary instruments and the self-adjusting file. J Endod. 2012;38:232–5. doi: 10.1016/j.joen.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Fuss Z, Lustig J, Tamse A. Prevalence of vertical root fractures in extracted endodontically treated teeth. Int Endod J. 1999;32:283–6. doi: 10.1046/j.1365-2591.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 4.Pawar AM, Pawar SM, Pawar MG, Kokate SR. Fracture resistance of teeth instrumented by the Self-Adjusting File, ProTaper NEXT and WaveOne. J Pierre Fauchard Acad. 2014;28:83–7. [Google Scholar]
- 5.Bergmans L, Van Cleynenbreugel J, Beullens M, Wevers M, Van Meerbeek B, Lambrechts P. Smooth flexible versus active tapered shaft design using NiTi rotary instruments. Int Endod J. 2002;35:820–8. doi: 10.1046/j.1365-2591.2002.00574.x. [DOI] [PubMed] [Google Scholar]
- 6.Capar ID, Altunsoy M, Arslan H, Ertas H, Aydinbelge HA. Fracture strength of roots instrumented with self-adjusting file and the ProTaper rotary systems. J Endod. 2014;40:551–4. doi: 10.1016/j.joen.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 7.Bier CA, Shemesh H, Tanomaru-Filho M, Wesselink PR, Wu MK. The ability of different nickel-titanium rotary instruments to induce dentinal damage during canal preparation. J Endod. 2009;35:236–8. doi: 10.1016/j.joen.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Bürklein S, Tsotsis P, Schäfer E. Incidence of dentinal defects after root canal preparation: Reciprocating versus rotary instrumentation. J Endod. 2013;39:501–4. doi: 10.1016/j.joen.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 9.Metzger Z. The self-adjusting file (SAF) system: An evidence-based update. J Conserv Dent. 2014;17:401–19. doi: 10.4103/0972-0707.139820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu R, Kaiwar A, Shemesh H, Wesselink PR, Hou B, Wu MK. Incidence of apical root cracks and apical dentinal detachments after canal preparation with hand and rotary files at different instrumentation lengths. J Endod. 2013;39:129–32. doi: 10.1016/j.joen.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Wu MK, van der Sluis LW, Wesselink PR. Comparison of mandibular premolars and canines with respect to their resistance to vertical root fracture. J Dent. 2004;32:265–8. doi: 10.1016/j.jdent.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Bier CA, Shemesh H, Tanomaru-Filho M, Wesselink PR, Wu MK. The ability of different nickel-titanium rotary instruments to induce dentinal damage during canal preparation. J Endod. 2009;35:236–8. doi: 10.1016/j.joen.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Hou BX, Wesselink PR, Wu MK, Shemesh H. The incidence of root microcracks caused by 3 different single-file systems versus the ProTaper system. J Endod. 2013;39:1054–6. doi: 10.1016/j.joen.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Adorno CG, Yoshioka T, Jindan P, Kobayashi C, Suda H. The effect of endodontic procedures on apical crack initiation and propagation ex vivo. Int Endod J. 2013;46:763–8. doi: 10.1111/iej.12056. [DOI] [PubMed] [Google Scholar]
- 15.Kim HC, Lee MH, Yum J, Versluis A, Lee CJ, Kim BM. Potential relationship between design of nickel-titanium rotary instruments and vertical root fracture. J Endod. 2010;36:1195–9. doi: 10.1016/j.joen.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Metzger Z, Teperovich E, Zary R, Cohen R, Hof R. The self-adjusting file (SAF). Part 1: Respecting the root canal anatomy — A new concept of endodontic files and its implementation. J Endod. 2010;36:679–90. doi: 10.1016/j.joen.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Pawar AM, Pawar MG, Metzger Z, Kokate SR. The self-adjusting file instrumentation results in less debris extrusion apically when compared to WaveOne and ProTaper NEXT. J Conserv Dent. 2015;18:89–93. doi: 10.4103/0972-0707.153057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hin ES, Wu MK, Wesselink PR, Shemesh H. Effects of self-adjusting file, Mtwo, and ProTaper on the root canal wall. J Endod. 2013;39:262–4. doi: 10.1016/j.joen.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Pawar AM, Pawar MG, Kokate SR. Meant to make a difference, the clinical experience of minimally invasive endodontics with the self-adjusting file system in India. Indian J Dent Res. 2014;25:509–12. doi: 10.4103/0970-9290.142552. [DOI] [PubMed] [Google Scholar]
- 20.Kim HC, Sung SY, Ha JH, Solomonov M, Lee JM, Lee CJ, et al. Stress generation during self-adjusting file movement: Minimally invasive instrumentation. J Endod. 2013;39:1572–5. doi: 10.1016/j.joen.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Ghoneim AG, Lutfy RA, Sabet NE, Fayyad DM. Resistance to fracture of roots obturated with novel canal-filling systems. J Endod. 2011;37:1590–2. doi: 10.1016/j.joen.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Karapinar Kazandag M, Sunay H, Tanalp J, Bayirli G. Fracture resistance of roots using different canal filling systems. Int Endod J. 2009;42:705–10. doi: 10.1111/j.1365-2591.2009.01571.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee KW, Williams MC, Camps JJ, Pashley DH. Adhesion of endodontic sealers to dentin and gutta-percha. J Endod. 2002;28:684–8. doi: 10.1097/00004770-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Sousa-Neto MD, Marchesan MA, Pécora JD, Junior AB, Silva-Sousa YT, Saquy PC. Effect of Er:YAG laser on adhesion of root canal sealers. J Endod. 2002;28:185–7. doi: 10.1097/00004770-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Mamootil K, Messer HH. Penetration of dentinal tubules by endodontic sealer cements in extracted teeth and in vivo. Int Endod J. 2007;40:873–81. doi: 10.1111/j.1365-2591.2007.01307.x. [DOI] [PubMed] [Google Scholar]


