Abstract
Background:
The entire effects of different bleaching regimens on the mechanical properties of composite resins have remained unknown. The purpose of this study was to evaluate the effects of different bleaching regimens on the flexural strength (FS) of hybrid composite resins.
Materials and Methods:
In this in vitro study, 80 bar-shaped specimens of hybrid composite resins were fabricated and randomly divided into four groups, 20 specimens in each group. First group (C) was considered as control. The other groups were treated by home bleaching (HB) agent, in-office bleaching (IB) agent, and the combination regimens (HIB), respectively. The FS was evaluated by three-point bending test by using a Universal Testing Machine. All data were analyzed by using Statistical Package for the Social Sciences (SPSS) software version 18, analysis of variance (ANOVA), and Turkey's post hoc statistical tests (α = 0.05).
Results:
The maximum mean value of FS was seen in HB group with significant differences to other groups (P < 0.05). Also, the minimum FS was observed in group HIB.
Conclusion:
Application of different bleaching regimens does not have any adverse effect on the FS of hybrid composite resins. However, the administration of HB regimens seemed to have lesser negative impact on the FS.
Keywords: Composite resin, flexural strength (FS), home bleaching (HB), in-office bleaching (IB)
INTRODUCTION
It has been reported that 21% of the people in the US and 28% of the adults in the UK are dissatisfied with their current tooth color.[1] Demands for an improved tooth appearance and color have made the tooth bleaching technique a popular dental procedure in the last few decades.[1]
Bleaching techniques are mostly classified by whether they involve vital or nonvital teeth or by whether the procedure is performed by using in-office or at-home components.[2] All tooth bleaching procedures use either hydrogen peroxide or carbamide peroxide as whitening agent.[3] The vital tooth bleaching technique seems to make surface alteration in dental substrate and composite restorative materials.[4,5] The surface alteration of composite resin might be associated with physical properties of the material.[6] The bleaching agents may alter the organic or inorganic structure of composite resins. It is speculated that the high oxidative capacity of the bleaching agents would be able to damage the organic molecules of polymeric chain, so the consequence would be degradation of composite resin.[7,8]
Flexural strength (FS), defined as the failure stress of a material as measured in bending, is generally considered a meaningful mechanical property for brittle materials that are much weaker in tension than in compression.[9]
Clinically composite restorations can be subjected to considerable flexural stresses. Therefore, in class I, II, III, and IV restorations, where the stresses are significant, high FS would be desirable.[10] Materials with low FS or stiffness would deform more under masticatory stresses, resulting in catastrophic destruction of the marginal seal in composite–dental tissue interfaces.[10]
Although bleaching is a safe procedure, it may endanger dental materials with more degradation characteristics.[10] Changes in the chemical and morphological structure of restorative materials should be considered when bleaching is administered as a whitening procedure.[11]
Previous literature mostly focused on the surface hardness of composite resin after bleaching procedure.[12,13,14] So, the aim of this study was to evaluate the effect of different bleaching regimens on FS of hybrid composite resin.
MATERIALS AND METHODS
Sample preparation
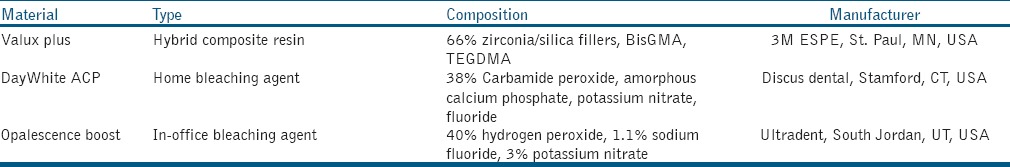
In this analytical–observational in vitro study, a silicon mold with 2 × 2 × 10 mm3 dimensions were used to prepare bar-shaped specimens. Then, 80 bar-shaped specimens were fabricated from a hybrid composite resin [Table 1] based on the manufacturer's instructions and ISO 4049 specifications.[15] The specimens were stored in deionized water at 37°C for 24 h. Afterwards, the specimens were polished on the four longest surfaces by using abrasive disks (Sof-Lex, 3M ESPE, St Paul, MN, USA) with medium, fine, and superfine roughness while rotating in one direction. The heights and widths of each polished specimens were measured by a digital micrometer (Mitutoyo Co., Tokyo, Japan) for three times. The specimens were again stored in deionized water at 37°C for 1 week.
Table 1.
The tested materials and exclusive information on them
Bleaching procedure
All surfaces of the specimens, expect one long surface, were covered with nail varnish. All the specimens were divided into four groups (N = 20) as follows:
C: Immersed only in distilled water (37°C) as the control group
Home bleaching (HB): Bleached by DayWhite ACP (Discus dental, Stamford, CT, USA) [Table 1], as home-bleaching agent, which was applied on the surfaces for 30 min per day.
In-office bleaching (IB): Bleached by Opalescence Boost (Ultradent, South Jordan, UT, USA) [Table 1], as IB agent, for 20 min each 4 days.
HIB: Bleached by a combination of previous agents by means of 30 min per day for the HB agent and 20 min each 4 days for the IB agent.
The bleaching procedure was accomplished in 14 consecutive days. After each bleaching interval, the specimens were rinsed by distilled water (37°C) to wash away any bleaching agent remnants in 1 min, and immersed in distilled water (37°C) which was renewed daily.
FS evaluation
The three-point bending test was administered by using a Universal Testing Machine (21046, Walter + bai, Switzerland) with crosshead speed of 0.5 mm/min. The force was applied until observing the fracture. The FS (σ) values (MPa) were calculated based on the following equation:[16]
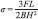
where F is the failure load (N), L is the distance between the jig supports (mm, 10 mm in this study), B and H are the width and height of the specimen, respectively (all in mm).
Statistical analysis
The recorded values were statistically analyzed by using one-way analysis of variance (ANOVA) and Tukey's post hoc tests and using Statistical Package for the Social Sciences (SPSS) software (version 18) (SPSS-Inc., Chicago, IL) at a significance level of 0.05.
RESULTS
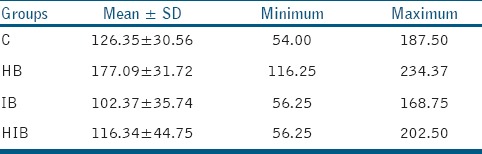
Table 2 represents the mean FS values of different groups. The mean FS values were the highest in HB group (177.09 ± 31.72 MPa), and were the lowest in IB group (102.37 ± 35.74 MPa). The lowest and highest FS values were observed in groups C (54.00 MPa) and IB (234.37 MPa), respectively. The one-way ANOVA test revealed significant differences among all study groups (P values <0.001). The Tukey's post hoc test revealed only significant difference of group HB with all other groups (all P values = 0.001).
Table 2.
Mean and standard deviation, maximum and minimum FS (MPa) of specimens
DISCUSSION
The demand for “white teeth” has been growing recently and tooth bleaching has been introduced as a noninvasive aesthetic approach among patients and clinicians.[17] The contemporary tooth bleaching mostly relies on the oxidation activation of hydrogen peroxide (or one of its precursors) through activation agents such as light or heat.[18]
The three-point bending test is still an appropriate choice for measuring composite's FS due to the lower standard deviation, coefficient of variation, and the less complex crack distribution.[19]
Present results reflected that there was no significant reduction in FS values of hybrid composite resin when IB regimes were used. Similar result was reported by Varanda et al. who evaluated the effect of using 20% and 35% hydrogen peroxide on the morphology of a microhybrid composite resin.[20] They observed no significant morphological changes after utilizing the bleaching agent. Nevertheless, different type and brands of composite resins, and using different test for evaluating physical properties might limit the true comparison of the two studies. These results might support the hypothesis that the filler volume seems to have less correlation with fracture as the crack propagation in the specimen is intergranular.[21] Also, the chemical bonds, promoted by silane coupling agent at resin–filler interface, might have an impact on the mechanical properties.[22] In present study, the dimensions of specimens (2 × 2 × 10 mm3) were in accordance with the ISO 4049 specification that aimed to provide the optimum rate of polymerization. However, the samples were prepared in 1 × 1 × 10 mm3 in that study.[20] Also, a hybrid composite resin was used in the current study versus to the microhybrid composite resin in that study. Bleaching agents seem to cause softening and reduction of the microhardness. Resin–filler interface might be debonded by the free radicals that were induced by peroxides. As a result, microscopic cracks would be observed beside probable increase of surface roughness.[23]
It was observed that urethane dimethacrylate (UDMA) resin monomers is less degraded compared to the bisphenol A-glycidyl methacrylate (Bis-GMA) resin monomers.[24] Moreover, the glass particles containing quartz or silica (like what was used in the present study) are less susceptible to hydrolytic attack than barium particles. So, the inorganic particles on the surface, especially in microhybrid composite resins, would be dissolved by the hydrogen peroxide significantly.
In the home bleached (HB) group, the FS of the hybrid composite resin was increased significantly, which coincide with the results of Firoozmand et al.[25] The conclusion of that study reflected that carbamide peroxide gel 35% had a positive effect on the FS of the microhybrid composite resin.[25] The result of utilizing carbamide peroxide on composite resins manifested remarkable increase in microhardness.[13,14]
CONCLUSION
Within the limitation of in vitro studies, it can be concluded that using tested HB agent (containing carbamide peroxide) might influence the FS of hybrid composite resin. However, tested in-office bleaching agents (containing hydrogen peroxide) would have not have a remarkable effect, neither to increase nor to decrease, on the FS of tested composite resin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Yu H, Li Q, Cheng H, Wang Y. The effects of temperature and bleaching gels on the properties of tooth-colored restorative materials. J Prosthet Dent. 2011;105:100–7. doi: 10.1016/S0022-3913(11)60007-3. [DOI] [PubMed] [Google Scholar]
- 2.Polydorou O, Mönting JS, Hellwig E, Auschill TM. Effect of in-office tooth bleaching on the microhardness of six dental esthetic restorative materials. Dent Mater. 2007;23:153–8. doi: 10.1016/j.dental.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Lima DA, De Alexandre RS, Martins AC, Aguiar FH, Ambrosano GM, Lovadino JR. Effect of curing lights and bleaching agents on physical properties of a hybrid composite resin. J Esthet Restor Dent. 2008;20:266–75. doi: 10.1111/j.1708-8240.2008.00190.x. [DOI] [PubMed] [Google Scholar]
- 4.Oltu U, Gürgan S. Effects of three concentrations of carbamide peroxide on the structure of enamel. J Oral Rehabil. 2000;27:332–40. doi: 10.1046/j.1365-2842.2000.00510.x. [DOI] [PubMed] [Google Scholar]
- 5.Potocnik I, Kosec L, Gaspersic D. Effect of 10% carbamide peroxide bleaching gel on enamel microhardness, microstructure, and mineral content. J Endod. 2000;26:203–6. doi: 10.1097/00004770-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Mourouzis P, Koulaouzidou EA, Helvatjoglu-Antoniades M. Effect of in-office bleaching agents on physical properties of dental composite resins. Quintessence Int. 2013;44:295–302. doi: 10.3290/j.qi.a29154. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein GR, Kiremidjian-Schumacher L. Bleaching: Is it safe and effective? J Prosthet Dent. 1993;69:325–8. doi: 10.1016/0022-3913(93)90114-4. [DOI] [PubMed] [Google Scholar]
- 8.Haywood VB. History, safety, and effectiveness of current bleaching techniques and applications of the nightguard vital bleaching technique. Quintessence Int. 1992;23:471–88. [PubMed] [Google Scholar]
- 9.Jayanthi N, Vinod V. Comparative evaluation of compressive strength and flexural strength of conventional core materials with nanohybrid composite resin core material an in vitro study. J Indian Prosthodont Soc. 2013;13:281–9. doi: 10.1007/s13191-012-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attin T, Hannig C, Wiegand A, Attin R. Effect of bleaching on restorative materials and restorations--a systematic review. Dent Mater. 2004;20:852–61. doi: 10.1016/j.dental.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Zuryati AG, Qian OQ, Dasmawati M. Effects of home bleaching on surface hardness and surface roughness of an experimental nanocomposite. J Conserv Dent. 2013;16:356–61. doi: 10.4103/0972-0707.114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon YH, Shin DH, Yun DI, Heo YJ, Seol HJ, Kim HI. Effect of hydrogen peroxide on microhardness and color change of resin nanocomposites. Am J Dent. 2010;23:19–22. [PubMed] [Google Scholar]
- 13.Malkondu O, Yurdaguven H, Say EC, Kazazoglu E, Soyman M. Effect of bleaching on microhardness of esthetic restorative materials. Oper Dent. 2011;36:177–86. doi: 10.2341/10-078-L. [DOI] [PubMed] [Google Scholar]
- 14.Sharafeddin F, Jamalipour G. Effects of 35% carbamide peroxide gel on surface roughness and hardness of composite resins. J Dent (Tehran) 2010;7:6–12. [PMC free article] [PubMed] [Google Scholar]
- 15.Geneva: 1992. International Standards Organisation. Dentistry-Resin based Filling Materials, ISO 4049: 1988/Cor 1 ISO; p. 26. [Google Scholar]
- 16.Janda R, Roulet JF, Latta M, Ruttermann S. The effects of thermocycling on the flexural strength and flexural modulus of modern resin-based filling materials. Dent Mater. 2006;22:1103–8. doi: 10.1016/j.dental.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Badole GP, Warhadpande MM, Bahadure RN, Badole SG. Aesthetic rehabilitation of discoloured nonvital anterior tooth with carbamide peroxide bleaching: Case series. J Clin Diagn Res. 2013;7:3073–6. doi: 10.7860/JCDR/2013/6303.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pintado-Palomino K, Tirapelli C. The effect of home-use and in-office bleaching treatments combined with experimental desensitizing agents on enamel and dentin. Eur J Dent. 2015;9:66–73. doi: 10.4103/1305-7456.149645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung SM, Yap AU, Chandra SP, Lim CT. Flexural strength of dental composite restoratives: Comparison of biaxial and three-point bending test. J Biomed Mater Res B Appl Biomater. 2004;71:278–83. doi: 10.1002/jbm.b.30103. [DOI] [PubMed] [Google Scholar]
- 20.Varanda E, Do Prado M, Simão RA, Dias KR. Effect of in-office bleaching agents on the surface roughness and morphology of different dental composites: An AFM study. Microsc Res Tech. 2013;76:481–5. doi: 10.1002/jemt.22190. [DOI] [PubMed] [Google Scholar]
- 21.Loughran GM, Versluis A, Douglas WH. Evaluation of sub-critical fatigue crack propagation in a restorative composite. Dent Mater. 2005;21:252–61. doi: 10.1016/j.dental.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Blackham JT, Vandewalle KS, Lien W. Properties of hybrid resin composite systems containing prepolymerized filler particles. Oper Dent. 2009;34:697–702. doi: 10.2341/08-118-L. [DOI] [PubMed] [Google Scholar]
- 23.Wattanapayungkul P, Yap AU, Chooi KW, Lee MF, Selamat RS, Zhou RD. The effect of home bleaching agents on the surface roughness of tooth-colored restoratives with time. Oper Dent. 2004;29:398–403. [PubMed] [Google Scholar]
- 24.Dadoun MP, Bartlett DW. Safety issues when using carbamide peroxide to bleach vital teeth — a review of the literature. Eur J Prosthodont Restor Dent. 2003;11:9–13. [PubMed] [Google Scholar]
- 25.Firoozmand LM, Pagani C. Influence of bleaching treatment on flexural resistance of hybrid materials. Acta Odontol Latinoam. 2009;22:75–80. [PubMed] [Google Scholar]


