Abstract
Separate sexes have evolved on numerous independent occasions from hermaphroditic ancestors in flowering plants. The mechanisms of sex determination is known for only a handful of such species, but, in those that have been investigated, it usually involves alleles segregating at a single locus, sometimes on heteromorphic sex chromosomes. In the genus Mercurialis, transitions between combined (hermaphroditism) and separate sexes (dioecy or androdioecy, where males co-occur with hermaphrodites rather than females) have occurred more than once in association with hybridisation and shifts in ploidy. Previous work has pointed to an unusual 3-locus system of sex determination in dioecious populations. Here, we use crosses and genotyping for a sex-linked marker to reject this model: sex in diploid dioecious M. annua is determined at a single locus with a dominant male-determining allele (an XY system). We also crossed individuals among lineages of Mercurialis that differ in their ploidy and sexual system to ascertain the extent to which the same sex-determination system has been conserved following genome duplication, hybridisation and transitions between dioecy and hermaphroditism. Our results indicate that the male-determining element is fully capable of determining gender in the progeny of hybrids between different lineages. Specifically, males crossed with females or hermaphrodites always generate 1:1 male:female or male:hermaphrodite sex ratios, respectively, regardless of the ploidy levels involved (diploid, tetraploid or hexaploid). Our results throw further light on the genetics of the remarkable variation in sexual systems in the genus Mercurialis. They also illustrate the almost identical expression of sex-determining alleles in terms of sexual phenotypes across multiple divergent backgrounds, including those that have lost separate sexes altogether.
Introduction
The mechanisms of sex determination (s.d.) employed by animals are remarkably diverse. They include: XY systems with male heterogamety; XY systems with a polymorphic X; XY systems with more than one Y; ZW systems with female heterogamety; haplodiploidy in which males are unfertilised haploid organisms and females are fertilised diploids; systems in which males result from the loss of one genome after fertilisation; polygenic systems; environmental s.d.; and s.d. with both genetic and environmental components (Bull, 1983; Werren and Beukeboom, 1998; Uller et al., 2007; Charlesworth and Mank, 2010; Janousek and Mrackova, 2010; Beukeboom and Perrin, 2014). Remarkably, quite different s.d. systems have often been adopted by closely related species, indeed even by different populations of the same species (for example, fish (Kallman, 1973) and amphibians (Ogata et al., 2008)). Such variation points to frequent transitions between one s.d. system and another, possibly as a result of responses to sex-ratio selection, intra- and inter-genomic conflict, or turnover of sex chromosomes following their degeneration when recombination is suppressed (Werren and Beukeboom, 1998; Uller et al., 2007; Van Doorn and Kirkpatrick, 2007, 2010; Blaser et al., 2013; Beukeboom and Perrin, 2014).
Although not as extreme as that found in animals, s.d. mechanisms also vary among plants (Juarez and Banks, 1998; Ming et al., 2007; Janousek and Mrackova, 2010; Ming et al., 2011). Fully environmental s.d. is known for several homosporous ferns, where sex expression of gametophytes depends on interplant signalling through hormone release and perception (Banks, 1994, 1997; Korpelainen, 1998; Desoto et al., 2008). Most plants with separate sexes, however, appear to possess genetic s.d., with either heteromorphic or homomorphic sex chromosomes (Ming et al., 2011). Species with heteromorphic sex chromosomes include both those with an XY (for example, Cycas revoluta—(Segawa et al., 1971; Hizume et al., 1998); Cannabis sativa—(Sakamoto et al., 1998; Rode et al., 2005; Sakamoto et al., 2005); Silene latifolia—(Correns, 1928; Westergaard, 1958; Filatov, 2005; Nicolas et al., 2005; Marais et al., 2008; Qiu et al., 2013) and S. diclinis—(Nicolas et al., 2005; Howell et al., 2009) or ZW s.d. (for example, Gingko biloba—(Lan et al., 2008); S. otitis—(Slancarova et al., 2013)). In Humulus japonicus (Grabowska-Joachimiak et al., 2011) and some Rumex species (Ono, 1935; Navajas-Perez et al., 2009; Steflova et al., 2013), gender is determined by the ratio of X chromosomes to autosomes. The genus Rumex is particularly interesting, because s.d. varies substantially among species and even within species (for example, Hough et al., 2014). In species with homomorphic sex chromosomes, sex is likely controlled by a single locus, or several tightly linked loci, with the heterogametic sex being either male (for example, Asparagus officinalis—(Telgmann-Rauber et al., 2007); Sagittaria latifolia—(Dorken and Barrett, 2004) and Spinacia oleracea—(Deng et al., 2013)) or female (for example, Fragaria virginiana—(Spigler et al., 2008) and Populus trichocarpa—(Tuskan et al., 2012)).
Until recently, the European wind-pollinated annual plant Mercurialis annua appeared to stand out as an exception among other dioecious angiosperms that appear always to have single-locus s.d. On the basis of multi-generational crossing data, Louis (1989) and Durand and Durand (1991) posited a model of s.d. for M. annua involving three independently segregating loci, whereby individuals carrying a dominant allele at locus A and a dominant allele at either one of the two B loci develop as males, and genotypes homozygous for the recessive allele at the A locus or homozygous recessive at both B loci develop as females. Multi-locus systems of s.d. are known from several animal species, including the swordtail fish Xiphophorus helleri (Woolcock et al., 2006), the European sea bass Dicentrarchus labrax (Vandeputte et al., 2007) and the housefly Musca domestica (Dubendorfer et al., 2002; Kozielska et al., 2006), but M. annua is cited as the only plant species known to display such a system (Dellaporta and Calderonurrea, 1993; Grant et al., 1994; Ainsworth, 2000; Janousek and Mrackova, 2010). The discovery of the single sex-linked SCAR marker OPB01-1562 (that is, a Sequence Characterized Amplified Region of 1562 bp in length) in dioecious M. annua (Khadka et al., 2002), which was found in all males tested but not in females, casts doubt on the three-locus model. However, sampling has thus far been limited to individuals predominantly from Belgian populations of the species (Khadka et al., 2002), which are known to be genetically depauperate (Obbard et al., 2006b). It is thus possible that alleles segregate for sex at more than one locus in other populations.
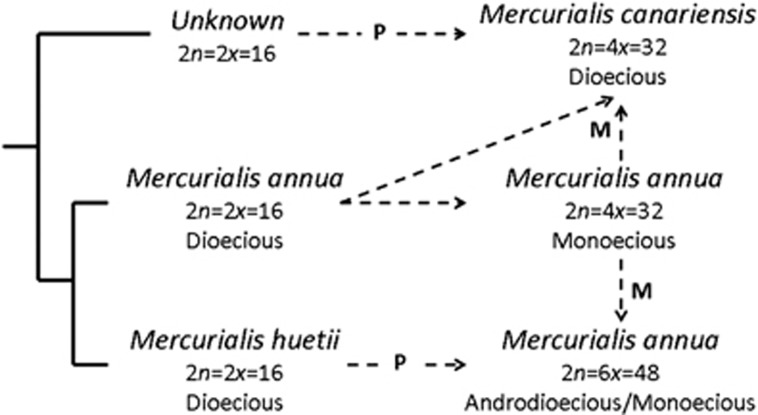
Sex determination in Mercurialis is potentially particularly interesting because separate sexes have been gained and lost more than once within the genus (Durand, 1963; Durand and Durand, 1992; Krahenbuhl et al., 2002; Obbard et al., 2006a). Although transitions between hermaphroditism and dioecy have been frequent in flowering plants, including reversals from dioecy to hermaphroditism, little is known about what happens to sex-determination loci, or to other genes in the genome that express their effect, when separate sexes are lost. For instance, would a male-determining allele still be expressed as a male phenotype in a genomic context that otherwise only expresses hermaphrodites? In Mercurialis, dioecy has broken down to yield monoecy, or functional hermaphroditism, in association with tetraploidisation, perhaps under selection for reproductive assurance. Evidently, separate sexes then re-evolved from monoecy when the tetraploid lineage hybridised with the closely related dioecious M. huetii (Figure 1, Krahenbuhl et al., 2002; Obbard et al., 2006a). Obbard et al. (2006a) hypothesised that the expression of males in the resulting hexaploid populations of M. annua might be associated with the introgression into a formerly monoecious tetraploid background of male-determining elements from M. huetii; but this hypothesis remains to be tested. It would be revealing to know whether, and how, the sex-determining alleles from one lineage would be expressed in the genomic background of another, not only in the cross hypothesised to have given rise to androdioecy, but also among the other lineages that have either combined or separate sexes.
Figure 1.
Hypothesised relationships between the annual lineages of Mercurialis. Filled lines indicate phylogenetic relationships between species and dashed arrows represent proposed hybridisation and/or polyploidisation events; M indicates proposed maternal parentage and P represents proposed paternal parentage. Figure based on Obbard et al. (2006a).
All species of Mercurialis are wind-pollinated and show similar inflorescence differences between males and females or hermaphrodites (Durand, 1963; Durand and Durand, 1991). Males have sessile staminate flowers arranged in clusters along erect axillary peduncles held above the plant; female flowers are almost exclusively held on short axillary pedicels and hermaphrodites (monoecious individuals) appear to be modified females, with subsessile axillary female flowers surrounded by a cluster of male flowers (Pannell et al., 2008). Dioecious M. annua is widely distributed across Europe, the Middle East and North Africa; M. huetii has a narrow range in northeastern Spain and southern France; tetraploid M. annua occurs in coastal Morocco south of Casablanca; hexaploid M. annua occurs further north in Morocco and in coastal areas throughout the Iberian Peninsula as far as Galicia and Catalonia (where it meets diploid M. annua, forming sterile hybrids; (Buggs and Pannell, 2006)); and tetraploid M. canariensis has been found in Tenerife in the Canary Islands (Obbard et al., 2006c). Higher ploidy levels of M. annua (always monoecious) occur in Tunisia, Corsica and Sardinia (Durand, 1963; Durand and Durand, 1992), and have been occasionally found in the contact zones between diploid and hexaploid M. annua in northern Spain. Apart from the three-locus model for s.d. in diploid M. annua (Louis, 1989; Durand and Durand, 1991) and a simple single-locus system for hexaploid androdioecious M. annua inferred from open pollinated crosses (Pannell, 1997a), very little is known about s.d. in the genus.
Here, we investigate the genetic mechanism of s.d. in the clade of annual species of the genus Mercurialis, which have undergone the shifts in ploidy and sexual system referred to above. First, we test the three-locus model of s.d. in diploid dioecious M. annua (Louis, 1989; Durand and Durand, 1991) by assessing sex-ratio variation among families with a range of different parents. Deviation from equality would not be consistent with a simple one-locus model, and would suggest a more complex mechanism of s.d., such as the three-locus model. To assess s.d. for individuals sampled widely across the geographic distribution of M. annua, we counted sex ratios from both open-pollinated families of half-sibs from a large number of populations and conducted crosses between targeted individuals from three widely separated populations (Israel, the UK and Spain). For the full-sib crosses, we crossed selected dams to multiple sires to allow more precise inference of the mechanism of s.d. for families whose sex ratios deviate from 1:1.
Second, we conduct crosses among several different lineages of M. annua to determine whether the expression of sex-determining alleles in terms of sexual phenotypes has been conserved across the clade of annual Mercurialis species. The segregation of males at an equal frequency with females or hermaphrodites in hybrid progeny would be consistent with the functionality of the male-determining allele in genetic backgrounds with different ploidy levels or in backgrounds in which separate sexes have been lost. Our between-lineage crosses included: diploid dioecious M. annua; tetraploid M. annua (the putative autopolyploid monoecious derivative of diploid M. annua); diploid dioecious M. huetii (the sister species of diploid M. annua) and hexaploid androdioecious M. annua (a putative allopolyploid derivative of a cross between M. huetii and tetraploid M. annua). Finally, we ask whether the sex-linked SCAR marker identified by Khadka et al. (2002) consistently segregates with males across the geographic range of diploid M. annua, as would be expected for a simple one-locus, but not a multi-locus, model, and we assess its presence or absence in the other lineages of M. annua investigated here. Our study thus characterises s.d. across a clade of several plant species that differ in ploidy and in which separate sexes are ancestral, were lost and were then evidently regained. Our data also allow inferences concerning the likely origins of combined versus separate sexes in the species complex, as well as the associated ploidy levels.
Materials and methods
Sex ratios of half- and full-sib families for dioecious M. annua
We assessed variation in the sex ratio among half-sib and full-sib families for consistency with a simple one-locus versus more complex models of s.d. Half-sib seed families were collected from 48 female plants of dioecious M. annua from wild populations from extremes across the species' range: 12 from Israel; 11 from the UK and 25 from Spain. The seeds of each family were sown in separate seed trays and raised to flowering (approximately 6 weeks), and the progeny sex ratios were recorded.
Full-sib families were generated by crossing individuals both within and between three populations sampled from across the species' range: HaGoshrim (Israel), Sestri Levante (Italy) and Tarragona (Spain). One male and four female plants from each population were selected at random and raised to flowering in individual pots in the same glasshouse. Each of the 12 females were crossed with the male from each of the three populations, thus generating three full-sib seed families per dam, each with a different sire. All details for these crosses are given in Supplementary Table S2.
To conduct the crosses, the 12 dams were exposed consecutively to pollen from each of the three different sires. Specifically, the dams were grown in a small mating array at the Wytham Field Station near Oxford, well separated from any other Mercurialis individuals, into which was introduced the first sire. Plants were allowed to mate for 8 weeks, after which point all seeds were collected, and the dams pruned back to above their basal node. Upon resprouting, the second sire was introduced and the process was repeated. The plants were subsequently exposed to the third sire in the same fashion. The 36 seed families obtained were raised to flowering for sex ratio determination. Eight further crosses were undertaken several months later using progeny from a single seed family that showed a biased sex ratio (see Results), with four dams crossed with each of two sires from the same family.
Sex expression in hybrids of parents from different lineages
We performed reciprocal crosses to assess the expression of gender in hybrids produced by crossing individuals from different lineages, including those with different sexual systems and ploidy levels. Crosses were produced between: diploid M. annua and M. huetii; tetraploid M. annua and M. huetii; diploid M. annua and tetraploid M. annua; and diploid M. annua and hexaploid M. annua. For diploid M. annua × hexaploid M. annua crosses, hexaploid males and hexaploid monoecious individuals were separately used as sires, thus giving a total of nine separate crosses, each performed by 10 dams and 10 sires together in separate compartments in the glasshouse. Genotypes for the crosses were established from a single population of known ploidy for each lineage (ploidy had been previously assessed by (Obbard et al., 2006a)). All plants were maintained for 10 weeks to allow open pollination. We prevented monoecious dams from siring progeny in their array by removing all of their male flowers every 3 days prior to anthesis. Although each array had multiple potential sires and dams, crossing was only possible in any given array in one specified direction, for example, one species was the sire and one species the dam. Combining individuals insured against failures due to mortality of individual plants and assured large numbers of progeny for sex ratio determination. For almost all species pairs (see Table 1), crosses were performed reciprocally, using different sets of plants for each crossing direction.
Table 1. Crosses performed between different lineages of the annual clade of Mercurialis and the results obtained.
| Maternal lineage | Paternal lineage | Males | Females | Hermaph. | Sex ratio | G | Pollen viability | Female fertility | Hybrid -isation |
|---|---|---|---|---|---|---|---|---|---|
| M. annua (2x) | M. huetii (2x) | 190 | 258 | 0 | 0.424 | 10.4a | 0.195 (0.072) | 0.061 (0.019) | 99.3 |
| M. huetii (2x) | M. annua (2x) | 222 | 189 | 0 | 0.540 | 2.65 | 0.186 (0.081) | 0.034 (0.012) | 99.3 |
| M. annua (4x) | M. huetii (2x) | 96 | 0 | 84 | 0.533 | 0.80 | — | — | 21.7 |
| M. huetii (2x) | M. annua (4x) | 0 | 0 | 521 | 0.000 | — | 0.186 (0.068) | 0.001 (0.001) | 98.5 |
| M. annua (2x) | M. annua (4x) | 0 | 0 | 88 | 0.000 | — | 0.206 (0.050) | 0.005 (0.003) | 77.2 |
| M. annua (4x) | M. annua (2x) | 5 | 0 | 6 | 0.455 | 0.09 | — | — | 16.2 |
| M. annua (2x) | M. annua (6x), male | 40 | 0 | 35 | 0.533 | 0.33 | 0.161 (0.050) | 0.006 (0.005) | 94.9 |
| M. annua (2x) | M. annua (6x), hermaphrodite | 0 | 0 | 31 | 0.000 | — | 0.132 (0.051) | 0.001 (0.001) | 81.6 |
| M. annua (6x) | M. annua (2x) | 5 | 0 | 9 | 0.357 | 1.16 | — | — | 46.7 |
Sex ratios, pollen viability, female fertility and hybridisation success (the percentage of hybrids out of total progeny produced) are displayed for each cross.
Pollen viability represented as the mean proportion of pollen grains stained with lactophenol blue (± one standard error) per cross. Female fertility was measured as the mean proportion of female flowers visibly setting seed (± one standard error) per cross. M. annua (2x) and M. huetii (2x) are dioecious, M. annua (4x) is monoecious, and M. annua (6x) is androdioecious. See text for further details.
Denotes values of G significant at P<0.05.
The seeds produced in each array were pooled, sown and raised to flowering. Hybrids of M. annua × M. huetii crosses could easily be distinguished from pure-bred individuals by their intermediate morphology. For all other crosses, which involved parents of different ploidy, we verified morphological identification of hybrids using flow cytometry for a sample of up to 20 progeny of each gender per cross, following methods of Buggs and Pannell (2006). Differences in fruit morphology between pure-bred and hybrid progeny (largely due to hybrid sterility) were very clear, and we found a 100% association between our morphological assessment of ploidy and results from flow cytometry, in agreement with Buggs and Pannell (2006). All hybrids were scored for sex, and sex ratios were computed for each cross. Several months later, we performed further crosses to obtain sex ratios for an F2 generation, using the same approach.
Sex ratio analysis
Sex ratios for all maternal families were determined by counting individuals on the basis of their expression of easily recognisable sex phenotypes, that is, by the production of either pistillate (female) or staminate (male) flowers by females or males, respectively, or both pistillate and staminate flowers (for monoecious individuals). Males also produced characteristic pedunculate inflorescences. Sex phenotypes could be scored for all plants, including sterile individuals, which nonetheless produced flowers of one or both genders. The number of progeny counted per family varied widely, largely as a function of the size reached by the mother.
Sex ratios were tested for bias using replicated goodness-of-fit tests (G-tests; (Sokal and Rohlf, 1995)), with a partitioning of heterogeneity into that due to variation among seed families (Ghet) and that due to a bias in the overall sex ratio (Gpooled). We used the Dunn-Šidák method to account for inflated Type I error due to multiple tests (Sokal and Rohlf, 1995). In addition to G-tests, we used a generalised linear model to test whether the sex ratios of field-collected half-sib seed families were influenced by their country of origin, with the family sex ratio as the response variable, country of origin fitted as a fixed factor and specifying a binomial error structure.
We used a generalised linear mixed effects model to determine: whether specific mothers or fathers used in crosses influenced sex ratios; whether the population of origin of mother plants influenced sex ratios; and whether the sex ratios from crosses involving parents from different combinations of populations differed significantly (as only one father was sampled per population, we were unable to differentiate between population- and individual-level paternal effects on sex ratios). Family sex ratio was again used as the response variable, with the mother's population of origin fitted as a fixed factor, the father's identity fitted as a random factor, the mother's identity fitted as a random factor nested within the mother's population of origin and specifying a binomial error structure. All analyses were conducted in R, version 2.8.0 (http://www.r-project.org).
Assessment of male and female fertility in hybrid progeny
Pollen viability and female fertility were also estimated in hybrid progeny and parent plants of hybrids. Between 9 and 21 plants per gender per parent lineage/hybrid cross were sampled for assessment of fertility, except in the case of M. huetii for which plant mortality reduced numbers to 6 male and 4 female plants. Pollen viability was estimated by staining with lactophenol blue and scoring a sample of 100 pollen grains per plant (Stone et al., 1995), whilst female fertility was estimated by counting the proportion of female flowers per plant that were visibly setting seed.
As the fertility data did not meet the assumptions of analysis of variance, Kruskal-Wallis tests were used to assess differences in mean pollen viability and female fertility between hybrids and parent lineages. Comparisons were made between different hybrid groups and parent lineages, as well as more broadly between all hybrid and all parent plants.
Inheritance and segregation with gender of the sex-linked SCAR marker
To verify that the SCAR marker OPB01-1562 is sex-linked across the species range of M annua, we assessed its presence in males and absence in females for all 15 parent plants used in dioecious M. annua controlled crosses, as well as four male and four female progeny selected at random from each of the 36 full-sib seed families obtained from these crosses. We also checked the presence of the male-linked SCAR marker in plants from lineages with different sexual systems and ploidy levels, using eight individuals of each gender of M. huetii, M. canariensis, and tetraploid and hexaploid (androdioecious) M. annua.
For each individual, we extracted genomic DNA from fresh and dried leaf material using a modified CTAB procedure (Doyle and Doyle, 1987). Ground leaves were incubated in 2 × CTAB at 65 °C, before being purified with two chloroform:isoamyl alcohol (24:1) extractions. Following precipitation using propan-2-ol at −20 °C, samples were washed in 70% ethanol, dried and resuspended in Tris-EDTA buffer, then stored at −20 °C until required. The reagents for all polymerase chain reaction (PCR) reactions were as follows: 1 × PCR buffer (supplied by Yorkshire Bioscience, Heslington, York, UK); 100 μM four dNTPs mix; 2 mM MgCl2; 0.16 μM of each primer; 1.0 units Taq DNA polymerase (Yorkshire Bioscience) and 10 ng DNA in 25 μl volume. PCR amplification conditions were as follows: 1 cycle of 94 °C, 90 s; 40 cycles of 94 °C, 30 s, 58 °C, 30 s, 72 °C, 90 s; and a final cycle of 72 °C, 5 min. PCR products were visualised by ethidium bromide staining after electrophoresis on 1.5% agarose gels.
All individuals were assessed for the presence of both the SCAR marker OPB01-1562, previously found to amplify only in males, and a 766 bp marker, believed to be linked to OPB01-1562 and that amplified in both males and females (Khadka et al., 2002). Both markers were identified by PCR amplification with primer pairs B1F01/B1R01 and B1F01/B1R06, respectively, following Khadka et al. (2002). Presence of the SCAR marker in males but not females of diploid M. annua would confirm its sex linkage. Similarly, co-segregation of the SCAR marker with maleness in other Mercurialis lineages would be further evidence that the marker is linked to an element that determines sex in different genetic backgrounds. Absence of the SCAR marker in both males and females of other lineages than diploid M. annua would be consistent with divergence in its primer region among lineages. Presence in both males and females would suggest a rupture in sex-linkage between diverging lineages, or could be the result of amplification of paralogous sequences.
Results
Sex ratios of open-pollinated half-sib seed families of dioecious M. annua
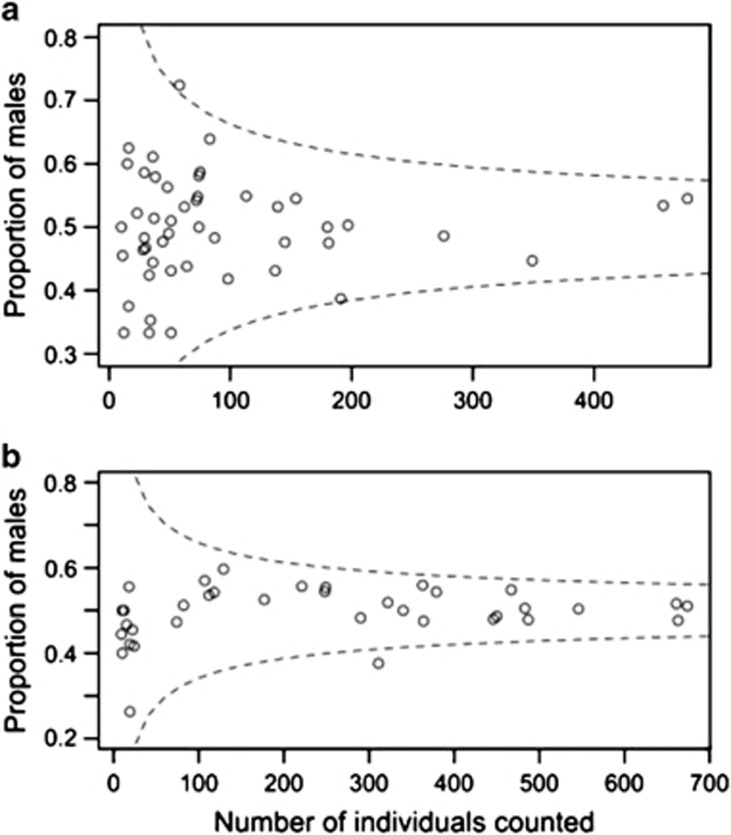
Summed across all half-sib families, dioecious M. annua showed no evidence of deviation from a 1:1 sex ratio (Gpooled 1=0.04, P=0.836), but families differed significantly in their sex ratios (Ghet 47=77.8, P=0.003). Case-by-case comparisons using the Dunn-Šidák method for multiple tests identified one family with a significantly male-biased sex ratio (seed family 34a1, sex ratio=0.724, G1=12.1, P=0.024) and one that showed a marginally significant female-biased sex ratio (seed family BS10, sex ratio=0.387, G1=9.76, P=0.082; Figure 2, and see Supplementary Table S1). Omitting these two seed families from the analysis reduced the heterogeneity in the data, which was no longer significant (Ghet 45=55.7, P=0.131). Half-sib family sex ratios were not influenced by their country of origin (χ22=4.43, P=0.109).
Figure 2.
The sex ratios of (a) 48 field-collected half-sib dioecious M. annua seed families and (b) 36 full-sib dioecious M. annua seed families from controlled crosses plotted against seed family size. Dashed lines represent the boundaries of the 0.05 acceptance region for tests of individual seed family sex ratios for departures from 1:1 (Dunn-Šidák method). Raw data and site localities are presented in Supplementary Tables S1 and S2.
Sex ratios of full-sib seed families of dioecious M. annua from controlled crosses
There was no significant deviation from 1:1 in the overall sex ratio across all 36 full-sib seed families from controlled crosses of dioecious M. annua (Gpooled 1=1.33, P=0.248), but here too, we found significant heterogeneity among families (Ghet 35=59.8, P=0.006). When a single seed family with a strongly female-biased sex ratio was removed from the dataset (cross SpainG2 × IsraelM2; sex ratio=0.376, G1=19.3, P<0.001; Dunn-Šidák method for multiple tests; Figure 2 and Supplementary Table S2), significant heterogeneity was lost (Ghet 34=37.9, P=0.298), and the overall sex ratio across all families became very slightly male-biased (sex ratio=0.511, Gpooled 1=4.02, P=0.045). Eight further crosses using four female and two male progeny from the female-biased family showed no significant deviation from a 1:1 sex ratio (Gtotal 8=7.98, P=0.435; see Supplementary Table S3).
Full-sib family sex ratios were not influenced by the identity of the mother (χ21=0.03, P=0.868) or father (χ21=2.11, P=0.146), nor by the population of origin of the mother (χ22=1.82, P=0.403) or by the combination of populations from which parents originated (χ21=3.22, P=0.073).
Hybrid progeny fertility and sex ratios
All crosses between M. annua and M. huetii, both dioecious diploids, yielded only male and female hybrid progeny (Table 1). However, the specific sex ratio depended on the direction of the cross, with a female-biased sex ratio produced when M. annua was the mother (sex ratio=0.424, G1=10.4, P=0.001), but an equal sex ratio when M. huetii was the mother (sex ratio=0.540, G1=2.65, P=0.103). Sex ratios were equal in all F2 progeny from these crosses.
All hybrid progeny obtained from the female M. huetii × tetraploid monoecious M. annua cross were morphologically monoecious (that is, hybrids produced both male and female flowers, though with very low fertility). In contrast, hybrids obtained by crossing male M. huetii with monoecious tetraploid M. annua as the mother were either male or hermaphroditic, with a 1:1 sex ratio (G1=0.80, P=0.371). Hybrid progeny from crosses between diploid M. annua and tetraploid M. annua showed a similar pattern; when tetraploid M. annua was the father, all hybrid progeny were hermaphroditic, whereas when diploid M. annua was the father, hybrids were either male or hermaphroditic, with a 1:1 sex ratio (G1=0.09, P=0.763; Table 1).
Crosses between diploid dioecious M. annua and hexaploid androdioecious M. annua produced similar sex ratios. Thus, when diploid M. annua females were crossed with hexaploid M. annua hermaphrodites as the male parent, all hybrid progeny were hermaphroditic, but when hexaploid males acted as sire, male and hermaphrodite progeny were produced in a 1:1 sex ratio (G1=0.33, P=0.564). Finally, when males of dioecious diploid M. annua acted as sire, hybrids were either male or hermaphroditic, again with a sex ratio of 1:1 (G1=1.16, P=0.282; Table 1).
Both male and female fertility was substantially lower in hybrids than in their parents (pollen viability: H1=48.1, P<0.001; female fertility: H1=85.7, P<0.001; Table 1). Interestingly, the proportion of female flowers setting seed was significantly greater in hybrid progeny obtained from crosses between diploid M. annua and M. huetii (in both directions) than in other hybrid progeny (H1=23.7, P<0.001), with female fertility not differing significantly between hybrid progeny from all other crosses assessed (H3=3.04, P=0.385; Table 1).
With the exception of hybrids of diploid M. annua and M. huetii, further crosses using hybrids failed to produce any viable seeds. Monoecious plants obtained from M. huetii × tetraploid M. annua, tetraploid M. annua × M. huetii, tetraploid M. annua × diploid M. annua and hexaploid M. annua × diploid M. annua crosses set no seeds (maternal lineage denoted first), whilst monoecious progeny obtained from diploid M. annua × tetraploid M. annua, diploid × hexaploid male M. annua and diploid M. annua × hexaploid monoecious M. annua hybridisations produced just a single seed per cross, all of which failed to germinate.
Conservation, inheritance and segregation with gender of OPB01-1562
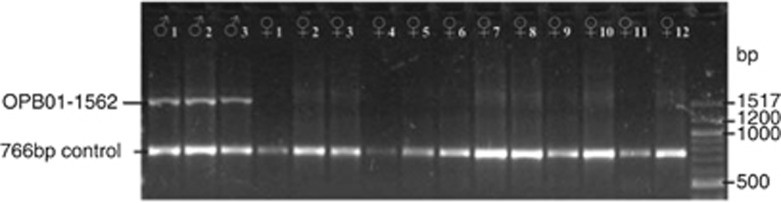
Of the 15 dioecious M. annua plants used as parents in controlled crosses, all three males, but none of the 12 females, amplified the DNA (SCAR) marker OPB01-1562 (Figure 3). Of the 288 progeny tested from these crosses (four male and four female progeny from each of the 36 crosses), all male individuals, but no females, amplified OPB01-1562. In contrast, no M. huetii, M. canariensis, tetraploid or hexaploid M. annua individuals amplified OPB01-1562, irrespective of the gender of plants. All individuals tested amplified the 766 bp marker.
Figure 3.
PCR amplification of OPB01-1562 and a 766 bp marker in the 15 parent plants used in controlled crosses of dioecious M. annua.
Discussion
Sex determination in dioecious Mercurialis annua
The results of our analysis of family sex ratios point to a simple model of s.d., with the sex ratios of only two seed families deviating significantly from a 1:1 sex ratio after accounting for multiple tests. If these families displayed a biased sex ratio as a result of the segregation of sex-ratio distorters, one might expect F2 crosses to show evidence of continued bias. However, this was not the case in the full-sib family for which further crosses were undertaken, suggesting the bias observed was unlikely to be due to genetic modifiers. The 100% co-segregation of the DNA marker OPB01-1562 with male gender, and 0% segregation with female gender, among 36 crosses and over 300 individuals is also strongly consistent with a single-locus mechanism of s.d. in dioecious M. annua, with a dominant male determinant analogous to an XY chromosomal system. OPB01-1562 is putatively linked to a dominant male-determining allele, with males the heterogametic sex and females homozygous recessive at this locus.
We can offer no convincing explanation for the sex ratios presented by Louis (1989) and Durand and Durand (1991) that imply the existence of modifier B loci as part of a three-locus system of s.d. It is possible that such modifier loci do segregate in some populations, but such populations, if they exist, are probably rare. It is well established that phytohormones have a key role in sex expression in M. annua (Louis, 1989; Durand and Durand, 1991). In particular, auxins and cytokinins have been identified as influencing gender development in the species, two groups of hormones that act antagonistically and have long been known to regulate plant growth, development and sexual expression (Skoog and Miller, 1957; Yamasaki et al., 2005). In M. annua, exogenous application of cytokinin causes males to produce female flowers (Louis and Durand, 1978; Durand and Durand, 1991). The three-locus mechanism was proposed to explain differences in ‘male strength' (the degree of resistance to feminisation by the exogenous application of cytokinins) between male M. annua individuals, with B1 and B2 together inducing complete resistance to feminisation, B1 alone conferring intermediate resistance, and B2 alone conferring low resistance (Louis, 1989). Moreover, endogenous auxin and cytokinin levels were reported to be correlated with different allelic combinations of the three sex genes (Hamdi et al., 1989; Louis et al., 1990). It is possible that the single sex-determining locus identified in this study represents the A gene of the three-locus model, with a dominant allele at this locus necessary for male development, and that B genes (perhaps at numerous loci) regulate auxin and/or cytokinin production, perhaps influencing sex allocation and inflorescence architecture, which are highly variable in M. annua (Pannell, 1997b; Pannell et al., 2008), but are not typically able to determine gender themselves.
Deviation from 1:1 sex ratios in some families
Although almost all families we investigated showed 1:1 progeny sex ratios, consistent with single-locus s.d., two families showed significant and substantial deviations, one male-biased (sex ratio=0.724) and one female-biased (sex ratio=0.376). Mechanisms that bias the sex ratios of individual seed families are well documented in other dioecious species and can operate in spite of strict genetic sex-determining systems (reviewed in De Jong and Klinkhamer, 2002; Barrett et al., 2010). For example, Rumex acetosa (Rychlewski and Zarzycki, 1975), Rumex nivalis (Stehlik and Barrett, 2005; Stehlik et al., 2008) S. latifolia (Taylor, 1994) and Urtica dioica (De Jong and Klinkhamer, 2002; De Jong et al., 2005; Glawe and De Jong, 2007, 2009) all display family-level sex ratio heterogeneity despite the presence of chromosomal or, in the case of dioecious U. dioica (De Jong et al., 2005), evidently single-locus sex-determining systems.
Two major mechanisms have been proposed to account for biases in seed family sex ratios, namely: (i) certation, the differential performance of male- and female-determining pollen grains due to the accumulation of mutations in degenerate Y chromosomes (Correns, 1903); and (ii) meiotic drive/segregation distortion, a form of intragenomic conflict leading to a bias in the ratio of male- to female-determining gametes produced away from 1:1 (Taylor and Ingvarsson, 2003). For certation to occur, a degree of chromosome degeneration is believed to be required to produce differences in the performance of X- versus Y-bearing microgametophytes. Whether M. annua possesses a substantial non-recombining region on the Y chromosome is not yet known, but there are no clearly heteromorphic sex chromosomes in the species (Durand, 1963). Certation seems unlikely to be a mechanism operating with important effects in M. annua, and in any case it would be incapable of explaining the male-biased sex ratio observed.
Alternatively, the biased sex ratios we observed might be attributable to selfish genetic elements that promote their own transmission, as has been hypothesised to explain female-biased sex ratios in dioecious S. latifolia. Through the use of reciprocal crosses, Taylor (1994) indicated that the extent of the female bias is influenced largely by a Y-linked sex ratio modifier that increases the proportion of males in the progeny to counteract the effects of X-linked and cytoplasmic feminising genes. A similar mechanism might have given rise to the female-biased sex ratio observed in the family sired by the male from HaGoshrim in our study. Meiotic drivers would, however, seem to be inconsistent with the finding of simple 1:1 sex ratios in the F2 progenies from this family. The fact that removal of the significantly female-biased sex ratio in the full-sib crosses resulted in significant male bias in the remaining crosses is difficult to explain, but might be attributable to sex-ratio distorters. Reciprocal crosses, similar to those used by Taylor (1994) in Silene, might help to establish whether sex ratio distorters are present in dioecious M. annua, but the bulk of our data indicates that they are not widespread.
Sex determination in the other annual Mercurialis lineages and their hybrid crosses
The results of our crosses among the different lineages of the annual clade of Mercurialis are consistent with a simple genetic mechanism of s.d. that has been conserved during diversification of the clade (see Figure 4). According to the proposed single-locus model, maleness in diploid dioecious M. annua results from the expression of a dominant male-determining allele that males should transmit to half of the progeny they sire. Although we have not tested this model in M. huetii using within-species crosses, the male determinant was transmitted to half of the M. huetii progeny sired by diploid M. annua males, with the resulting male and female progeny being largely fertile. Given that reciprocal crosses yielded much the same results, it is highly likely that sex in M. huetii is also determined by a single-locus XY system (notwithstanding the somewhat female-biased sex ratio, which might be due to sex-ratio distorters). The fact that the SCAR marker did not amplify in M. huetii, nor in the M. annua polyploids, likely points to divergence at the primer sites in these lineages. It will be useful to identify more conserved sex-linked markers in future work to investigate the potential homology of the sex-determining loci in species and their hybrids across the clade.
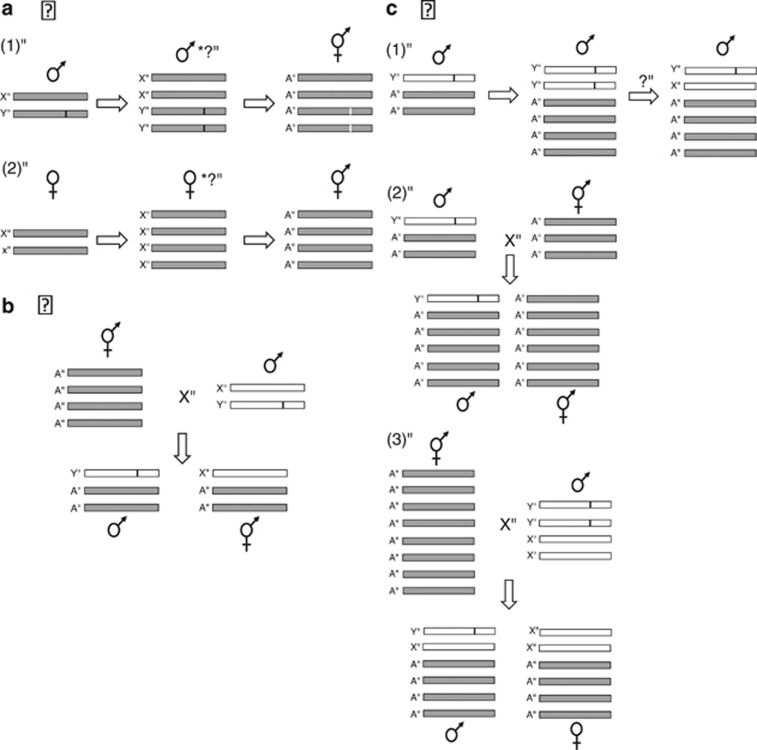
Figure 4.
Models for sex determination in Mercurialis annua in the context of autopolyploidisation and allopolyploidisation with M. huetii. (a) Two possible scenarios for the breakdown of dioecy through autopolyploidisation. Model 1: genome duplication of a diploid male individual of M. annua, yielding a tetraploid male with two copies of the male-determining element, with possible ‘leaky' gender expression (the production of some female flowers, denoted by an asterisk). Subsequent selection brings about the evolution of a fully monoecious phenotype. Model 2: genome duplication of a female individual with leaky gender expression and subsequent selection of monoecy. (b) Hybridisation between monoecious tetraploid M. annua and a diploid male individual of M. huetii, yielding a 1:1 sex ratio of triploid offspring. A similar scenario can be envisaged for hybridisation between any of the Mercurialis lineages studied, with the active expression of the male-determining element in the hybrid progeny. (c) Three possible scenarios for the evolution of androdioecy in hexaploid M. annua through allopolyploid hybridisation between monoecious tetraploid M. annua and diploid M. huetii. Model 1: genome duplication of a triploid hybrid male, yielding a male with two copies of the male-determining element, all of whose sons would be XY males for the segregating chromosome pair. Model 2: the union of an unreduced male gamete of a triploid hybrid (produced through the crossing of a diploid male M. huetii with a tetraploid monoecious M. annua) with an unreduced female gamete of another triploid hybrid (this time a monoecious individual also produced by crossing M. huetii with tetraploid M. annua). Model 3: the union of a tetraploid egg produced by an originally tetraploid individual with a duplicated (octoploid) genome and a diploid sperm arising from an originally diploid M. huetii male with a duplicated (tetraploid) genome. Grey and white bars denote chromosomes from M. annua and M. huetii, respectively. Black and white markers indicate an active or a silenced male-determining element, respectively. X, Y and A represent X, Y and autosomal chromosomes, respectively.
Hexaploid males of androdioecious M. annua also sired 50% sons in their crosses with diploid females. This too is consistent with a single-locus system of s.d. (Figure 4), confirming the inferences made by Pannell (1997a) based on the outcome of mating among hexaploid individuals. That study also found evidence for an influence of density on s.d., but more recent work has failed to replicate the finding (Sanchez-Vilas and Pannell, 2012). Sex expression in hexaploid M. annua is highly variable among populations, particularly in terms of inflorescence architecture (unpublished results). It is thus possible that some populations have evolved a sensitivity to density in their sex expression whereas others have not. A broader survey of among-population variation in phenotypic plasticity of sex expression would be useful to address this hypothesis.
The confirmation of single-locus s.d. in androdioecious M. annua is also consistent with the conclusion from the segregation of both isozyme (Obbard et al., 2006a) and microsatellite loci (Korbecka et al., 2010) that the hexaploid genome has become diploidised across many loci, including the sex-determining locus. Obbard et al. (2006a) hypothesised that the sex-determining locus in hexaploid M. annua was introgressed from M. huetii when a male hybridised with a tetraploid monoecious individual, yielding hexaploid individuals following a further round of genome duplication. Our results are consistent with that interpretation because the OPB01-1562 SCAR marker is not found in hexaploid males, but more work needs to be carried out before it is firmly established. If it is correct, it provides an appealing explanation for the origin of androdioecy in Mercurialis, which is otherwise known to be difficult to evolve (Lloyd, 1975; Charlesworth and Charlesworth, 1978; Charlesworth, 1984; Pannell, 2002).
It is noteworthy that females never segregated in the progeny of any crosses involving hermaphrodites, regardless of the ploidy backgrounds involved or of whether the hermaphrodites were sires (in which case progeny were all hermaphrodites) or dams (in which case progeny sired by males were either hermaphrodites or males). A similar result was found for reciprocal crosses between hermaphroditic Bryonia alba and dioecious B. dioica (Correns, 1928), and crosses between females of dioecious S latifolia and individuals of its hermaphroditic relative S. viscosa also found that progeny had partially restored male function and were thus (albeit male-sterile) hermaphrodites (Zluvova et al., 2005). This latter study points to the substitution by hermaphrodites of a stamen-promoting function in progeny lacking the Y chromosome on which the stamen-promoting function gene otherwise resides. The conclusion is consistent with models of the evolution of sex chromosomes that invoke the fixation of recessive male-sterility mutations on X chromosomes and dominant male-promoting factors (in addition to female-function suppressors) on the Y chromosome (Charlesworth and Charlesworth, 1978; Charlesworth, 2002a). It is too early to know whether a similar model can explain s.d. in M. annua, but this seems unlikely. Results of artificial selection (Pujol and Pannell, 2008) and natural selection experiments (Dorken and Pannell, 2009) both suggest that allocation to male function by hermaphrodites (which are essentially male-flower-producing females) is a quantitative trait under the influence of many loci. The results of our crosses here are consistent with this hypothesis (though cannot rule out the classical model invoking major sex-linked loci), with male-flower production by females or hermaphrodites governed by one or more non-recessive alleles fixed or segregating at one or more sex-allocation loci. In this case, crosses between hermaphrodites and females would yield only hermaphrodite progeny, as observed.
Revealingly, crosses between hermaphrodites of M. annua and males yielded only hermaphrodites and males with normal male inflorescence architecture and no progeny with any sort of intermediate male-female inflorescence architecture. (Recall that male inflorescences are pedunculate, with flowers held on inflorescence stalks, whereas pistillate flowers produced by females are usually subsessile in the leaf axils.) It would seem that the dominant male-determining allele in Mercurialis completely suppresses female floral or inflorescence development, and that its absence allows female development with the production of male flowers governed by (quantitative) loci elsewhere in the genome. We are currently testing this hypothesis using intraspecific crosses (yielding fertile progeny) among individuals with different inflorescences and sex allocations.
The precise paths leading to genome duplication in the M. annua species complex are not yet known; here, analysis of sequence variation of both active and quiescent sex-determining or sex-linked loci will be revealing. In the light of results presented here, we judge model 2 in Figure 4a to be the most likely scenario for the derivation of tetraploid monoecious individuals, that is, the genome duplication of a female rather than a male diploid plant, with subsequent selection of individuals with ‘leaky' gender expression. Leaky females that produce a few male flowers (Pannell, 2001) would enjoy an advantage under selection for reproductive assurance, resulting in the evolution of full monoecy; in this context, it is significant that monoecious individuals of M. annua typically have a female-like morphology and lack the pedunculate inflorescence typical of males. In contrast, the male determinant in hexaploid androdioecious M. annua would seem to be most likely derived from M. huetii following allopolyploid hybridisation between monoecious M. annua and a male individual of M. huetii (Figures 4b and c, and see Obbard et al., 2006a). However, it is not known whether two copies of the male-determinant were initially incorporated into the hexaploid genome, with one subsequently becoming silenced (allowing the segregation of males and hermaphrodites), or whether a single male-determining element was incorporated (see Figure 4). Comparative genome analysis of the annual Mercurialis lineages will throw light onto these questions.
Concluding remarks
Recent work on s.d. in a number of dioecious plants, notably S. latifolia, has yielded results consistent with the leading model for the evolution of sex chromosomes, which involved recessive male-sterility and female-promoting elements on the X chromosome and dominant male-promoting elements on the Y chromosome (Charlesworth and Charlesworth, 1978; Charlesworth, 2002b). Very little is yet known about the architecture of sex chromosomes of M. annua, but it is clear from our study and previous work (Khadka et al., 2002) that a single locus (or set of linked loci) determines gender, with males being the heterogametic sex, as in S. latifolia. The fact that interspecific or interploidy crosses between hermaphrodites and females yield progeny with a male function would be consistent with this model. However, a number of observations suggest that the classic model might not, in fact, apply in the case of M. annua.
First, in the genus Mercurialis, the polarity of sexual-system transitions differs from that assumed for the classic model. In the M. annua species complex, dioecy is the ancestral sexual system, and functional hermaphroditism (technically monoecy) is derived from dioecy rather than the reverse (Krahenbuhl et al., 2002; Obbard et al., 2006a). And second, patterns of phenotypic variation in hermaphroditic sex allocation (Pannell, 1997a, 1997b; Pannell et al., 2014) and the results of selection experiments on pollen production (Pujol and Pannell, 2008; Dorken and Pannell, 2009) suggest that male function in hermaphrodites is a quantitative trait for which many loci are probably responsible, rather than a single male-sterility mutation. Indeed, females of dioecious M. annua occasionally produce small numbers of staminate flowers with fully viable pollen (Yampolsky, 1919), suggesting incomplete suppression of a male function in XX individuals, and this tendency also seems to be a quantitative trait (G. Cossard and J.R. Pannell, unpublished data). Such leakiness in the expression of gender is very common in dioecious species, especially those derived from monoecy where male and female functions can be leaky (Lloyd, 1980; Lloyd and Bawa, 1984; Delph, 2003; Ehlers and Bataillon, 2007). Determining whether the genetic architecture of s.d. in such species is different from the classic model will throw important light on its broad generality.
Data Archiving
All data are available in the supplementary tables.
Acknowledgments
K. Ridout, C. Roux and G. Cossard commented on the manuscript. We thank the BBSRC, UK, and Linacre College, Oxford, for a studentship grant awarded to JRWR, and grants from the NERC, UK, and National Science Foundation, Switzerland, to JRP.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Heredity website (http://www.nature.com/hdy)
Supplementary Material
References
- Ainsworth C (2000). Boys and girls come out to play: The molecular biology dioecious plants. Ann Bot 86: 211–221. [Google Scholar]
- Banks JA (1994). Sex-determining genes in the homosporous fern Ceratoptris. Development 120: 1949–1958. [DOI] [PubMed] [Google Scholar]
- Banks JA (1997). Sex determination in the fern Ceratopteris. Trends Pl Sci 2: 175–180. [Google Scholar]
- Barrett SCH, Yakimowski SB, Field DL, Pickup M (2010). Ecological genetics of sex ratios in plant populations. Philos Trans R Soc B-Biol Sci 365: 2549–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukeboom LW, Perrin N (2014). The Evolution of Sex Determination. Oxford University Press: Oxford. [Google Scholar]
- Blaser O, Grossen C, Neuenschwander S, Perrin N (2013). Sex-chromosome turnovers induced by deleterious mutation load. Evolution 67: 635–645. [DOI] [PubMed] [Google Scholar]
- Buggs RJA, Pannell JR (2006). Rapid displacement of a monoecious plant lineage is due to pollen swamping by a dioecious relative. Curr Biol 16: 996–1000. [DOI] [PubMed] [Google Scholar]
- Bull JJ (1983). Evolution of Sex Determining Mechanisms. Benjamin/Cummings: Menlo Park, CA. [Google Scholar]
- Charlesworth D (1984). Androdioecy and the evolution of dioecy. Biol J Linn Soc 23: 333–348. [Google Scholar]
- Charlesworth D (2002. a). Plant sex determination and sex chromosomes. Heredity 88: 94–101. [DOI] [PubMed] [Google Scholar]
- Charlesworth D (2002. b). What maintains male-sterility factors in plant populations? Heredity 89: 408–409. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B (1978). A model for the evolution of dioecy and gynodioecy. Amer Nat 112: 975–997. [Google Scholar]
- Charlesworth D, Mank JE (2010). The birds and the bees and the flowers and the trees: lessons from genetic mapping of sex determination in plants and animals. Genetics 186: 9–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correns C (1903). Über die dominierenden Merkmale der Bastarde. Berichte der Deutschen Botanischen Gesellschaft 21: 133–147. [Google Scholar]
- Correns C (1928). Bestimmung, Verteilung und Vererbung des Geschlechts bei den höheren Pflanzen. Handbuch der Vererbungswissenschaft 2: 1–138. [Google Scholar]
- De Jong TJ, Klinkhamer PGL (2002). Sex ratios in dioecious plants. In: Hardy ICW (ed.) Sex Ratios: Concepts and Research Methods. Cambridge University Press: Cambridge 349–364. [Google Scholar]
- De Jong TJ, Nell HW, Glawe GA (2005). Heritable variation in seed sex ratio of the stinging nettle (Urtica dioica). Plant Biol 7: 190–194. [DOI] [PubMed] [Google Scholar]
- Dellaporta SL, Calderonurrea A (1993). Sex determination in flowering plants. Plant Cell 5: 1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delph LF (2003). Sexual dimorphism in gender plasticity and its consequences for breeding system evolution. Evol Devel 5: 34–39. [DOI] [PubMed] [Google Scholar]
- Deng CL, Qin RY, Cao Y, Gao J, Li SF, Gao WJ et al. (2013). Microdissection and painting of the Y chromosome in spinach (Spinacia oleracea). J Plant Res 126: 549–556. [DOI] [PubMed] [Google Scholar]
- Desoto L, Quintanilla LG, Mendez M (2008). Environmental sex determination in ferns: effects of nutrient availability and individual density in Woodwardia radicans. J Ecol 96: 1319–1327. [Google Scholar]
- Dorken ME, Barrett SCH (2004). Sex determination and the evolution of dioecy from monoecy in Sagittaria latifolia (Alismataceae). Proc Biol Sci 271: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorken ME, Pannell JR (2009). Hermaphroditic sex allocation evolves when mating opportunities change. Curr Biol 19: 514–517. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL (1987). A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochemistry Bulletin 19: 11–15. [Google Scholar]
- Dubendorfer A, Hediger M, Burghardt G, Bopp D (2002). Musca domestica, a window on the evolution of sex-determining mechanisms in insects. Int J Dev Biol 46: 75–79. [PubMed] [Google Scholar]
- Durand B (1963). Le complèxe Mercurialis annua L. s.l.: une étude biosystématique. Ann Sci Nat Bot Paris 12: 579–736. [Google Scholar]
- Durand B, Durand R (1991). Sex determination and reproductive organ differentiation in Mercurialis. Plant Sci 80: 49–66. [Google Scholar]
- Durand R, Durand B (1992). Dioecy, monoecy, polyploidy and speciation in annual mercuries. Bull Soc Bot France Lett Bot 139: 377–399. [Google Scholar]
- Ehlers BK, Bataillon T (2007). ‘Inconstant males' and the maintenance of labile sex expression in subdioecious plants. New Phytol 174: 194–211. [DOI] [PubMed] [Google Scholar]
- Filatov DA (2005). Evolutionary history of Silene latifola sex chromosomes revealed by genetic mapping of four genes. Genetics 170: 975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glawe GA, De Jong TJ (2007). Inheritance of progeny sex ratio in Urtica dioica. J Evol Biol 20: 133–140. [DOI] [PubMed] [Google Scholar]
- Glawe GA, De Jong TJ (2009). Complex sex determination in the stinging nettle Urtica dioica. Evol Ecol 23: 635–649. [Google Scholar]
- Grabowska-Joachimiak A, Mosiolek M, Lech A, Goralski G (2011). C-Banding/DAPI and in situ hybridization reflect karyotype structure and sex chromosome differentiation in Humulus japonicus Siebold & Zucc. Cytogenet Genome Res 132: 203–211. [DOI] [PubMed] [Google Scholar]
- Grant S, Houben A, Vyskot B, Siroky J, Pan WH, Macas J et al. (1994). Genetics of sex determination in flowering plants. Developmental Genetics 15: 214–230. [Google Scholar]
- Hamdi S, Yu LX, Cabre E, Delaigue M (1989). Gene expression in Mercurialis annua flowers: In vitro translation and sex genotype specificity: Male-specific complementary DNA cloning and hormonal dependence of a corresponding specific RNA. Mol Gen Genet 219: 168–176. [Google Scholar]
- Hizume M, Kurose N, Shibata F, Kondo K (1998). Molecular cytogenetic studies on sex chromosomes and proximal heterochromatin containing telomere-like sequence in Cycas revoluta. Chromosome Science 2: 63–72. [Google Scholar]
- Hough J, Hollister JD, Wang W, Barrett SCH, Wright SI (2014). Genetic degeneration of old and young Y chromosomes in the flowering plant Rumex hastatulus. Proc Natl Acad Sci USA 111: 7713–7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell EC, Armstrong SJ, Filatov DA (2009). Evolution of neo-sex chromosomes in Silene diclinis. Genetics 182: 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janousek B, Mrackova M (2010). Sex chromosomes and sex determination pathway dynamics in plant and animal models. Biol J Linn Soc 100: 737–752. [Google Scholar]
- Juarez C, Banks JA (1998). Sex determination in plants. Curr Opin Plant Biol 1: 68–72. [DOI] [PubMed] [Google Scholar]
- Kallman KD. The sex determining mechanism of the Platyfish, Xiphophorus maculatus. Schröder JH. Genetics and Mutagenesis of Fish. Berlin/Heidelberg/New York: Springer-Verlag,. (1973). 19–28. [Google Scholar]
- Khadka DK, Nejidat A, Tal M, Golan-Goldhirsh A (2002). DNA markers for sex: Molecular evidence for gender dimorphism in dioecious Mercurialis annua L. Mol Breed 9: 251–257. [Google Scholar]
- Korbecka G, Rymer PD, Harris SA, Pannell JR (2010). Solving the problem of ambiguous paralogy for marker loci: microsatellite markers with diploid inheritance in allohexaploid Mercurialis annua (Euphorbiaceae). J Hered 101: 504–511. [DOI] [PubMed] [Google Scholar]
- Korpelainen H (1998). Labile sex expression in plants. Biological Reviews of the Cambridge Philosophical Society 73: 157–180. [Google Scholar]
- Kozielska M, Pen I, Beukeboom LW, Weissing FJ (2006). Sex ratio selection and multi-factorial sex determination in the housefly: a dynamic model. J Evol Biol 19: 879–888. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl M, Yuan YM, Kupfer P (2002). Chromosome and breeding system evolution of the genus Mercurialis (Euphorbiaceae): implications of ITS molecular phylogeny. Plant Syst Evol 234: 155–170. [Google Scholar]
- Lan TY, Chen RY, Li XL, Dong FP, Qi YC, Song WQ et al. (2008). Microdissection and painting of the W chromosome in Ginkgo biloba showed different labelling patterns. Botanical Studies 49: 33–37. [Google Scholar]
- Lloyd DG (1975). The maintenance of gynodioecy and androdioecy in angiosperms. Genetica 45: 325–339. [Google Scholar]
- Lloyd DG (1980). The distribution of gender in four angiosperm species illustrating two evolutionary pathways to dioecy. Evolution 34: 123–134. [DOI] [PubMed] [Google Scholar]
- Lloyd DG, Bawa KS (1984). Modification of the gender of seed plants in varying conditions. Evol Biol 17: 255–338. [Google Scholar]
- Louis J-P, Durand B (1978). Studies with the dioecious angiosperm Mercurialis annua L. (2n=16). Correlation between genic and cytoplasmic male sterility, sex segregation and feminising hormones (cytokinins). Mol Gen Genet 165: 309–322. [Google Scholar]
- Louis JP (1989). Genes for the regulation of sex differentiation and male fertility in Mercurialis annua L. J Hered 89: 104–111. [Google Scholar]
- Louis JP, Augur C, Teller G (1990). Cytokinins and differentiation processes in Mercurialis annua. Genetic regulation, regulations with auxins, indoleacetic acid oxidases, and sexual expression patterns. Plant Physiology 94: 1535–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais GaB, Nicolas M, Bergero R, Chambrier P, Kejnovsky E, Monéger F et al. (2008). Evidence for degeneration of the Y chromosome in the dioecious plant Silene latifolia. Curr Biol 18: 545–549. [DOI] [PubMed] [Google Scholar]
- Ming R, Bendahmane A, Renner SS. Sex chromosomes in land plants. In: Merchant SS, Briggs WR, Ort D (eds). Annual Review of Plant Biology. Palo Alto: AnnualReviews.,. (2011). 62, 485–514. [DOI] [PubMed] [Google Scholar]
- Ming R, Wang JP, Moore PH, Paterson AH (2007). Sex chromosomes in flowering plants. Amer J Bot 94: 141–150. [DOI] [PubMed] [Google Scholar]
- Navajas-Perez R, Schwarzacher T, Rejon MR, Garrido-Ramos MA (2009). Molecular cytogenetic characterization of Rumex papillaris, a dioecious plant with an XX/XY1Y2 sex chromosome system. Genetica 135: 87–93. [DOI] [PubMed] [Google Scholar]
- Nicolas M, Marais G, Hykelova V, Janousek B, Laporte V, Vyskot B et al. (2005). A gradual process of recombination restriction in the evolutionary history of the sex chromosomes in dioecious plants. Plos Biol 3: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obbard DJ, Harris SA, Buggs RJA, Pannell JR (2006. a). Hybridization, polyploidy, and the evolution of sexual systems in Mercurialis (Euphorbiaceae). Evolution 60: 1801–1815. [PubMed] [Google Scholar]
- Obbard DJ, Harris SA, Pannell JR (2006. b). Sexual systems and population genetic structure in an annual plant: testing the metapopulation model. Amer Nat 167: 354–366. [DOI] [PubMed] [Google Scholar]
- Obbard DJ, Pannell JR, Harris SA (2006. c). Mercurialis canariensis (Euphorbiaceae), a new endemic to the Canary Islands. Kew Bull 61: 99–106. [Google Scholar]
- Ogata M, Hasegawa Y, Ohtani H, Mineyama M, Miura I (2008). The ZZ/ZW sex-determining mechanism originated twice and independently during evolution of the frog, Rana rugosa. Heredity 100: 92–99. [DOI] [PubMed] [Google Scholar]
- Ono T (1935). Chromosomen und Sexualität von Rumex acetosa. Science reports of the Tohoku Imperial University IV 10: 41–210. [Google Scholar]
- Pannell J (1997. a). Mixed genetic and environmental sex determination in an androdioecious population of Mercurialis annua. Heredity 78: 50–56. [DOI] [PubMed] [Google Scholar]
- Pannell J (1997. b). Variation in sex ratios and sex allocation in androdioecious Mercurialis annua. J Ecol 85: 57–69. [DOI] [PubMed] [Google Scholar]
- Pannell JR (2001). A hypothesis for the evolution of androdioecy: the joint influence of reproductive assurance and local mate competition in a metapopulation. Evol Ecol 14: 195–211. [Google Scholar]
- Pannell JR (2002). The evolution and maintenance of androdioecy. Annu Rev Ecol Syst 33: 397–425. [Google Scholar]
- Pannell JR, Dorken ME, Pujol B, Berjano R (2008). Gender variation and transitions between sexual systems in Mercurialis annua (Euphorbiaceae). Int J Plant Sci 169: 129–139. [Google Scholar]
- Pannell JR, Eppley SM, Dorken ME, Berjano R (2014). Regional variation in sex ratios and sex allocation in androdioecious Mercurialis annua. J Evol Biol 27: 1467–1477. [DOI] [PubMed] [Google Scholar]
- Pujol B, Pannell JR (2008). Reduced responses to selection after species range expansion. Science 321: 96. [DOI] [PubMed] [Google Scholar]
- Qiu S, Bergero R, Charlesworth D (2013). Testing for the footprint of sexually antagonistic polymorphisms in the pseudoautosomal region of a plant sex chromosome pair. Genetics 194: 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode J, In-Chol K, Saal B, Flachowsky H, Kriese U, Weber WE et al. (2005). Sex-linked SSR markers in hemp. Plant Breeding 124: 167–170. [Google Scholar]
- Rychlewski J, Zarzycki K (1975). Sex ratio in seeds of Rumex acetosa L as a result of sparse or abundant pollination. Acta Biol Crac Ser Bot 18: 101–114. [Google Scholar]
- Sakamoto K, Abe T, Matsuyama T, Yoshida S, Ohmido N, Fukui K et al. (2005). RAPID markers encoding retrotransposable elements are linked to the male sex in Cannabis sativa L. Genome 48: 931–936. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Akiyama Y, Fukui K, Kamada H, Satoh S (1998). Characterization, genome sizes and morphology of sex chromosomes in hemp (Cannabis sativa L.). Cytologia (Tokyo) 63: 459–464. [Google Scholar]
- Sanchez-Vilas J, Pannell JR (2012). Do plants adjust their sex allocation and secondary sexual morphology in response to their neighbours? Ann Bot 110: 1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa M, Kishi S, Tatuno S (1971). Sex chromosomes of Cycas revoluta. Japanese Journal of Genetics 46: 33 &. [Google Scholar]
- Skoog F, Miller CO (1957). Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symposia of the Society for Experimental Biology 11: 118–130. [PubMed] [Google Scholar]
- Slancarova V, Zdanska J, Janousek B, Talianova M, Zschach C, Zluvova J et al. (2013). Evolution of sex determination systems with heterogametic males and females in Silene. Evolution 67: 3669–3677. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ (1995). Biometry. W.H. Freeman and Company: New York. [Google Scholar]
- Spigler RB, Lewers KS, Main DS, Ashman TL (2008). Genetic mapping of sex determination in a wild strawberry, Fragaria virginiana, reveals earliest form of sex chromosome. Heredity 101: 507–517. [DOI] [PubMed] [Google Scholar]
- Steflova P, Tokan V, Vogel I, Lexa M, Macas J, Novak P et al. (2013). Contrasting patterns of transposable element and satellite distribution on sex chromosomes (XY1Y2) in the dioecious plant Rumex acetosa. Genome Biol Evol 5: 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik I, Barrett SCH (2005). Mechanisms governing sex-ratio variation in dioecious Rumex nivalis. Evolution 59: 814–825. [PubMed] [Google Scholar]
- Stehlik I, Friedman J, Barrett SCH (2008). Environmental influence on primary sex ratio in a dioecious plant. Proc Natl Acad Sci U S A 105: 10847–10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JL, Thomson JD, Dent-Acosta SJ (1995). Assessment of pollen viability in hand-pollination experiments: a review. Amer J Bot 82: 1186–1197. [Google Scholar]
- Taylor DR (1994). Sex ratio in hybrids between Silene alba and Silene dioica: evidence for Y-linked restorers. Heredity 74: 518–526. [DOI] [PubMed] [Google Scholar]
- Taylor DR, Ingvarsson PK (2003). Common features of segregation distortion in plants and animals. Genetica 117: 27–35. [DOI] [PubMed] [Google Scholar]
- Telgmann-Rauber A, Jamsari A, Kinney MS, Pires JC, Jung C (2007). Genetic and physical maps around the sex-determining M-locus of the dioecious plant asparagus. Mol Genet Genomics 278: 221–234. [DOI] [PubMed] [Google Scholar]
- Tuskan GA, Difazio S, Faivre-Rampant P, Gaudet M, Harfouche A, Jorge V et al. (2012). The obscure events contributing to the evolution of an incipient sex chromosome in Populus: a retrospective working hypothesis. Tree Genet Genomes 8: 559–571. [Google Scholar]
- Uller T, Pen I, Wapstra E, Beukeboom LW, Komdeur J (2007). The evolution of sex ratios and sex-determining systems. Trends Ecol Evol 22: 292–297. [DOI] [PubMed] [Google Scholar]
- Van Doorn GS, Kirkpatrick M (2007). Turnover of sex chromosomes induced by sexual conflict. Nature 449: 909–912. [DOI] [PubMed] [Google Scholar]
- Van Doorn GS, Kirkpatrick M (2010). Transitions Between Male and Female Heterogamety Caused by Sex-Antagonistic Selection. Genetics 186: 629–U294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte M, Dupont-Nivet M, Chavanne H, Chatain B (2007). A polygenic hypothesis for sex determination in the European sea bass, Dicentrarchus labrax. Genetics 176: 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Beukeboom LW (1998). Sex determination, sex ratios, and genetic conflict. Annu Rev Ecol Syst 29: 233–261. [Google Scholar]
- Westergaard M (1958). The mechanism of sex determination in dioecious plants. Adv Genet 9: 217–281. [DOI] [PubMed] [Google Scholar]
- Woolcock B, Kazianis S, Lucito R, Walter RB, Kallman KD, Morizot DC et al. (2006). Allele-specific marker generation and linkage mapping on the Xiphophorus sex chromosomes. Zebrafish 3: 23–37. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Fujii N, Takahashi H (2005). Hormonal regulation of sex expression in plants. Vitamins and Hormones 72: 79–110. [DOI] [PubMed] [Google Scholar]
- Yampolsky C (1919). Inheritance of sex in Mercurialis annua. Amer J Bot 6: 410–442. [Google Scholar]
- Zluvova J, Lengerova M, Markova M, Hobza R, Nicolas M, Vyskot B et al. (2005). The inter-specific hybrid Silene latifolia x S. viscosa reveals early events of sex chromosome evolution. Evol Dev 7: 327–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.