Abstract
Objectives. We evaluated time trends in sharing needles and other injection equipment from 1994 to 2013 among injection drug users in the Seattle, Washington area.
Methods. We combined data from 4 sources: the Risk Activity Variables, Epidemiology, and Network (RAVEN) study, recruited from institutional settings; the Kiwi study, recruited from jails; National HIV Behavioral Surveillance system (NHBS) surveys, which used respondent-driven sampling; and surveys at needle-exchange sites.
Results. Levels of needle sharing were higher in the earlier studies: RAVEN, 1994 to 1997 (43%) and Kiwi, 1998 to 2002 (61%). In the NHBS surveys, the initial level of 44% in 2005 declined to 31% in the period 2009 to 2012. Across needle-exchange surveys (2009–2013) the level was 21%. There was a parallel reduction in sharing other injection equipment. These trends persisted after control for sociodemographic and risk-associated variables. There was a contemporaneous increase in the number of needles distributed by local needle exchanges and a decline in the number of reported HIV cases among injection drug users.
Conclusions. The apparent long-term reduction in sharing injection equipment suggests substantial success in public health efforts to reduce the sharing of injection equipment.
Injection drug users (IDUs) are at elevated risk for infection with HIV and HCV. In 2011, there were an estimated 5000 new HIV cases among IDUs and IDUs who were also men who have sex with men (MSM) in the United States, which together constituted 10% of the total cases.1 Most of these cases are likely to derive from sharing injection equipment,2 though some are a product of sexual contact.3,4 An estimated 17 000 new infections of HCV occurred in 2010.5 Parenteral contact through sharing injection equipment is the most common risk factor for HCV infection.5,6
Reducing the level of sharing contaminated injection equipment would be expected to reduce the transmission of these viruses. The Centers for Disease Control and Prevention includes access to sterile syringes among its list of evidence-based HIV prevention interventions.7 A 2013 panel of infectious disease policy experts recommended improving access to sterile injection equipment for IDUs who cannot or will not stop injecting drugs to reduce the transmission of HCV,8 which is more readily transmitted by blood contact than is HIV.2,9 Information on long-term trends in the sharing of injection equipment can help guide and evaluate public health measures to reduce the burden of HIV and HCV disease.
Because of the resources and long-term commitment demanded, there has been only a modest number of long-term studies of time trends in injection equipment sharing, many of them dating from the earlier periods in the HIV epidemic.10–20 In this report we present data from the Seattle, Washington, area over the period 1994 to 2013 on time trends in sharing needles and in sharing injection equipment other than needles, the latter of which has been implicated in HCV transmission, and likely promotes HIV transmission as well.21,22 We combined data from 3 Seattle-area National HIV Behavioral Surveillance (NHBS) surveys with data from other local surveys of IDU: the Risk Activity Variables, Epidemiology, and Network (RAVEN) study, the Kiwi study, and from 3 surveys of needle-exchange users at exchanges run by Public Health–Seattle & King County. We include results of multivariate analyses evaluating time trends in sharing needles and sharing other injection equipment after control for differences in the study populations in sociodemographic and drug-related characteristics associated with such sharing.
METHODS
The 1994 to 1997 RAVEN study recruited participants from a variety of institutional settings with a random number–based algorithm.23 The study consisted of 2 groups. The first group was recruited from 4 methadone-maintenance centers. The second group was recruited from a drug detoxification center, jail, a drug treatment evaluation agency, and a community-based organization that targeted services to IDUs. The 2 groups were administered different questionnaires, which both had some changes over the course of the study. The 2 groups contributed varying proportions of participants across the years of the study.
The Kiwi study recruited participants in 1998 to 2002 from the King County jail in downtown Seattle and, starting in 2000, from the jail in Kent (in southern King County).24 Participants were recruited from persons in the jail’s booking area at randomly selected time intervals, or from persons who volunteered for HIV testing at the jail health clinic. A revised version of the study questionnaire was administered to participants from both jails interviewed after August 2001.
The NHBS surveys recruited IDUs in 2005, 2009, and 2013 with respondent-driven sampling (RDS), a modified form of snowball sampling in which participants are given coupons with which to recruit their peers.25 Although quite similar protocols and questionnaires were used in each survey, differences in sociodemographics and risk behavior have been reported between the 2005 and 2009 NHBS study populations,26 as well as between the 2005 NHBS and the previous RAVEN and Kiwi populations.27
We also used data from surveys conducted at needle exchanges run by Public Health–Seattle & King County in 2009, 2011, and 2013. These surveys attempted to interview all persons exchanging needles during defined time periods. In all years, about 60% of participants came from the exchange in downtown Seattle, about 30% from the exchange on Capitol Hill (Seattle’s traditional hip and gay neighborhood), and smaller proportions from other needle exchanges.
To ensure a more consistent sample, we restricted this analysis to participants in all surveys aged 18 years or older and residents of King County, which includes Seattle. Facility in English was required. Survey eligibility required injection in the previous 12 months in NHBS, RAVEN, and Kiwi; needle-exchange users are presumed to be current injectors.
Sharing Injection Equipment
Differences in wording of the study questionnaires among all studies and between questionnaire versions within individual studies are detailed in Appendix A (available as a supplement to the online version of this article at http://www.ajph.org). We note here some of the most important differences. In RAVEN and Kiwi, the referent period was 6 months, in NHBS 12 months, and in the needle-exchange surveys 3 months. RAVEN, Kiwi, and NHBS studies assessed sharing needles in terms of receptive needle sharing—that is, sharing a needle that had previously been used by someone else. The needle-exchange surveys combined both receptive and distributive sharing in a single question.
We defined sharing injection equipment other than needles as sharing cookers, cottons, or water, or backloading (dividing up drugs through use of a common syringe). In the Kiwi study, data on sharing water were not collected; those Kiwi participants surveyed by the earlier questionnaire, in addition, lacked data on sharing cottons.
Covariates
We characterized the study populations in terms of a collection of sociodemographic and drug-associated characteristics that might plausibly be associated with sharing injection equipment: age, race/ethnicity, gender, area of residence, education, homelessness, drug most frequently injected, injection frequency, and a variable combining MSM and amphetamine-injection status, the last of which has been found to identify a key Seattle-area IDU subpopulation.28
We were necessarily restricted to variables collected in all surveys and defined in ways that allowed comparable categorization across surveys (Table 1). We described area of residence in terms of 5 areas of King County, defined by zip code. Data on education were lacking in the 2009 needle-exchange survey. We could only evaluate injection frequency in terms of daily versus less-than-daily injection across the different studies. We defined the variable for combined MSM and amphetamine injection status on the basis of reporting male-to-male sex in the previous 6 months (in RAVEN and Kiwi) or 12 months (in NHBS and the needle-exchange surveys) and reporting amphetamines as the drug most frequently injected.
TABLE 1—
Sociodemographic and Drug-Related Characteristics Across Seattle-Area Studies of Injection Drug Users: Seattle, WA, 1994–2013
| Characteristic | RAVEN (n = 2780), % | Kiwi (n = 1692), % | NHBS (n = 1555), % | Needle Exchange Surveys (n = 1279), % | P |
| Age, y | < .001 | ||||
| 18–29 | 23 | 27 | 13 | 29 | |
| 30–29 | 39 | 39 | 23 | 26 | |
| 40–49 | 32 | 29 | 31 | 25 | |
| ≥ 50 | 6 | 5 | 33 | 21 | |
| Race/ethnicity | < .001 | ||||
| White | 64 | 64 | 58 | 69 | |
| Black | 23 | 15 | 18 | 8 | |
| Hispanic | 5 | 8 | 8 | 10 | |
| Other race | 6 | 12 | 4 | 7 | |
| Multiple races | 3 | 1 | 12 | 6 | |
| Gender | < .001 | ||||
| Male | 64 | 77 | 67 | 72 | |
| Female | 36 | 23 | 33 | 29 | |
| Area of residence | < .001 | ||||
| North Seattle | 23 | 19 | 17 | 25 | |
| Downtown Seattle | 24 | 24 | 44 | 30 | |
| South Seattle | 29 | 21 | 22 | 21 | |
| South King County | 18 | 24 | 13 | 16 | |
| East King County | 7 | 12 | 4 | 8 | |
| Education | < .001 | ||||
| < high-school graduate | 26 | 27 | 26 | 19 | |
| High-school graduate | 39 | 40 | 40 | 31 | |
| > high-school graduate | 34 | 33 | 33 | 50 | |
| Homelessness | < .001 | ||||
| No | 48 | 70 | 51 | 71 | |
| Yes | 52 | 30 | 49 | 29 | |
| Drug most frequently injected | < .001 | ||||
| Heroin | 66 | 50 | 81 | 84 | |
| Speedballs | 15 | 11 | 7 | 1 | |
| Cocaine | 13 | 11 | 3 | 2 | |
| Amphetamines | 7 | 28 | 10 | 13 | |
| Daily injection frequency | < .001 | ||||
| No | 41 | 60 | 32 | 32 | |
| Yes | 59 | 40 | 68 | 68 | |
| MSM | < .001 | ||||
| No | 96 | 95 | 90 | 88 | |
| Yes, not amphetamine injector | 2 | 3 | 5 | 5 | |
| Yes, amphetamine injector | 1 | 3 | 5 | 7 |
Note. MSM = men who have sex with men; NHBS = National HIV Behavioral Surveillance system; RAVEN = Risk Activity Variables, Epidemiology, and Network study.
Statistical Methods
We evaluated differences among study populations and across years within the individual study populations by the Pearson χ2 test. We evaluated linear time trends by a linear-by-linear χ2 test (Ptrend). We did not adjust the NHBS surveys by RDS-specific methods to control for recruitment biases in RDS.29
We combined all 4 study populations into a single analysis file for statistical evaluation of time trends and multivariate analyses. We used general estimating equation analyses to investigate possible confounding of time trends by differences among the study populations. These analyses employed a robust variance estimate and a Poisson model with a log-link function; grouping was on the basis of unique individual participant identification numbers, yielding an analysis essentially lacking grouping structure. We report results incorporating a categorical variable for year and covariates that were found to be significantly (P < .05) associated with sharing injection equipment when included together in an analysis. We included missing values for covariates as a separate category of the covariate. We chose the 1994 RAVEN data as the reference for comparison. Statistical tests for simple linear trend incorporated a continuous term for year with the relevant covariates. We conducted analyses in SPSS version 20 (IBM, Somers, NY).
RESULTS
There were significant differences across the different studies in all measured covariates (P < .001; Table 1). The NHBS study population was the oldest and the needle-exchange survey population was notably older than either RAVEN or Kiwi. RAVEN had the highest proportion of Black participants. Kiwi had the lowest proportion of female participants. Needle-exchange survey participants reported the highest educational levels. Kiwi participants were less likely to report daily injection and more likely to report amphetamines as the drug they most frequently injected. Both NHBS and needle-exchange participants were more likely than RAVEN or Kiwi participants to report heroin as the drug they most frequently injected. The proportion of participants who were characterized as MSM amphetamine injectors was higher in the later studies.
Within the individual studies there were also differences in the characteristics of the study populations over time. RAVEN participants had statistically significant differences across years in age, race/ethnicity, area of residence, education, homelessness, drug most frequently injected, injection frequency, and combined MSM and amphetamine injection status (Table A, available as a supplement to the online version of this article at http://www.ajph.org). For Kiwi, differences were found for race/ethnicity, area of residence, education, drug most frequently injected, injection frequency, and combined MSM and amphetamine injection status. Within NHBS, differences across years were seen in age, gender, area of residence, homelessness, drug most frequently injected, injection frequency, and combined MSM and amphetamine injection status; the needle-exchange surveys differed in area of residence and drug most frequently injected.
Needle Sharing
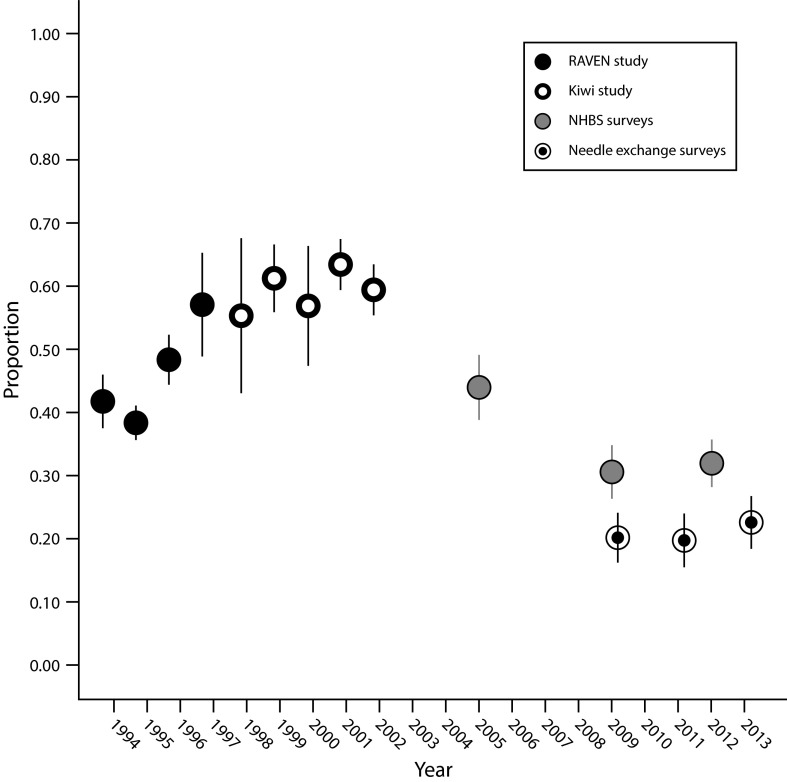
The time trend in the proportion of participants reporting needle sharing is illustrated in Figure 1, and detailed in Table 2. In broad brush, there is an apparent change from higher levels of sharing in the earlier RAVEN (43% overall) and Kiwi studies (61%), to substantially lower levels in the 2 later years of the NHBS surveys (31%) and in the needle-exchange surveys (21%). The proportion of participants reporting sharing needles in 2005 (44%), the first year of the NHBS surveys, was intermediate between the level in the preceding Kiwi study and that in later NHBS surveys. A test for simple linear trend across the full time span 1994 to 2013 was statistically significant (Ptrend < .001).
FIGURE 1—
Time Trends in the Proportion of Participants Reporting Sharing Needles Across Seattle-Area Surveys of Injection Drug Users: 1994–2013
Note. NHBS = National HIV Behavioral Surveillance system; RAVEN = Risk Activity Variables, Epidemiology, and Network study.
TABLE 2—
Time Trends in Sharing Needles and Injection Equipment Other Than Needles Across Seattle-Area Surveys of Injection Drug Users, With Multivariate Results: Seattle, WA, 1994–2013
| Needle Sharing |
Sharing Injection Equipment Other Than Needles |
||||||
| Year | Survey | % | No./Total No. | PRa (95% CI) | % | No./Total No. | PRb (95% CI) |
| 1994 | RAVENc | 42 | 210/503 | 1 (Ref) | 73 | 343/468 | 1 (Ref) |
| 1995 | RAVEN | 38 | 512/1335 | 0.87 (0.77, 0.98) | 78 | 984/1270 | 1.05 (0.99, 1.12) |
| 1996 | RAVEN | 48 | 313/648 | 1.07 (0.94, 1.21) | 81 | 514/633 | 1.10 (1.03, 1.17) |
| 1997 | RAVEN | 57 | 85/149 | 1.24 (1.05, 1.48) | 88 | 130/148 | 1.16 (1.07, 1.26) |
| 1998 | Kiwi | 55 | 37/67 | 1.29 (1.01, 1.65) | 74 | 51/69 | 1.01 (0.87, 1.18) |
| 1999 | Kiwi | 61 | 216/353 | 1.54 (1.35, 1.76) | 79 | 279/355 | 1.10 (1.02, 1.19) |
| 2000 | Kiwi | 57 | 62/109 | 1.48 (1.22, 1.80) | 77 | 85/111 | 1.11 (0.99, 1.24) |
| 2001 | Kiwi | 64 | 358/564 | 1.62 (1.44, 1.84) | 83 | 462/559 | 1.21 (1.13, 1.30) |
| 2002 | Kiwi | 60 | 345/580 | 1.51 (1.34, 1.72) | 83 | 457/548 | 1.20 (1.12, 1.28) |
| 2003 | . . . | . . . | . . . | . . . | . . . | . . . | . . . |
| 2004 | . . . | . . . | . . . | . . . | . . . | . . . | . . . |
| 2005 | NHBS | 44 | 163/371 | 1.09 (0.93, 1.28) | 75 | 279/371 | 1.05 (0.97, 1.14) |
| 2006 | . . . | . . . | . . . | . . . | . . . | . . . | . . . |
| 2007 | . . . | . . . | . . . | . . . | . . . | . . . | . . . |
| 2008 | . . . | . . . | . . . | . . . | . . . | . . . | . . . |
| 2009 | NHBS | 31 | 154/505 | 0.73 (0.62, 0.87) | 65 | 327/504 | 0.88 (0.81, 0.96) |
| 2009 | Needle exchange | 20 | 88/436 | 0.47 (0.38, 0.58) | 42 | 181/431 | 0.59 (0.37, 0.96) |
| 2010 | . . . | . . . | . . . | . . . | . . . | . . . | |
| 2011 | Needle exchange | 20 | 72/366 | 0.47 (0.37, 0.59) | 43 | 156/366 | 0.59 (0.52, 0.67) |
| 2012 | NHBS | 32 | 215/674 | 0.74 (0.63, 0.86) | 62 | 419/673 | 0.83 (0.77, 0.90) |
| 2013 | Needle exchange | 23 | 103/456 | 0.52 (0.43, 0.63) | 45 | 204/455 | 0.62 (0.55, 0.69) |
Note. CI = confidence interval; NHBS = National HIV Behavioral Surveillance system; PR = prevalence ratio; RAVEN = Risk Activity Variables, Epidemiology, and Network study.
Based on general estimating equation analyses including terms for age, race/ethnicity, gender, homelessness, injection frequency, drug most frequently injected, and combined men who have sex with men and amphetamine injector status. Education and area of residence were not significantly associated with needle sharing.
Based on general estimating equation analyses including terms for age, gender, education, homelessness, drug most frequently injected, and combined men who have sex with men and amphetamine injector status. Race/ethnicity and area of residence were not significantly associated with sharing injection equipment other than needles.
The referent period for both needle sharing and sharing injection equipment other than needles was 6 months in RAVEN and Kiwi, 12 months in NHBS, and 3 months in the needle-exchange surveys.
Within the 1994–1997 RAVEN study, there was significant variation in needle sharing across years (P < .001) but not a monotonic linear trend (Figure 1). RAVEN participants recruited in drug-treatment centers were less likely to report needle sharing than were other participants (39% vs 49%; P < .001). However, in-treatment and out-of-treatment groups of RAVEN followed parallel time trends (Figure A, available as a supplement to the online version of this article at http://www.ajph.org), so that the variation across the years of the RAVEN study is not simply a product of varying contributions of the 2 groups of the study over time.
Kiwi participants (1998–2002) reported the highest level of needle sharing. There was no indication of a time trend within Kiwi (Ptrend = 0.83; Figure 1), nor of a difference in needle sharing between Kiwi participants recruited from the jails in downtown Seattle and Kent (P = .11; Figure A).
Needle exchange (2009–2013) participants reported lower levels of sharing needles (20%–23%) than contemporaneously recruited NHBS participants (31%–32%). There was no evidence for a change over time in the proportion of needle-exchange survey participants reporting needle sharing (Ptrend = .37).
In multivariate analyses, we found significant and independent associations with needle sharing for year, age, race/ethnicity, gender, homelessness, and injection frequency (P < .001 for each variable), as well as drug most frequently injected (P = .003) and combined MSM and amphetamine injection status (P = .006). The temporal pattern of the prevalence ratios for needle sharing in the multivariate analyses adjusted for these variables closely approximated that of the unadjusted proportions (Table 2; Figure B, available as a supplement to the online version of this article at http://www.ajph.org). A test for simple linear trend across the full time period 1994 to 2013 in the multivariate analysis was significant (Ptrend < .001).
Sharing of Injection Equipment Other Than Needles
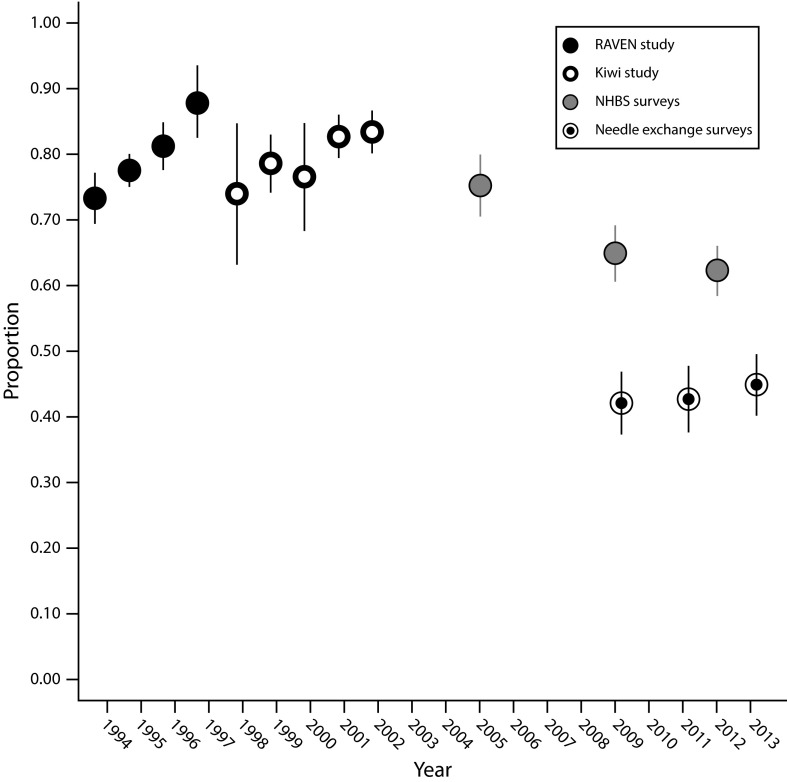
Sharing injection equipment other than needles was reported at substantially higher levels than needle sharing in all study populations. The pattern of changes over time was similar to that for needle sharing (Table 2; Figure 2), though the reduction was less pronounced. The proportion sharing injection equipment other than needles was higher across RAVEN (78% overall), Kiwi (81%), and the 2005 NHBS surveys (75%) than in the 2 later NHBS surveys (63% combined) and the needle-exchange surveys (43%). A test for simple linear trend across the full time span 1994 to 2013 was statistically significant (Ptrend < .001).
FIGURE 2—
Time Trends in the Proportion of Participants Reporting Sharing Injection Equipment Other Than Needles Across Seattle-Area Surveys of Injection Drug Users: 1994–2013
Note. NHBS = National HIV Behavioral Surveillance system; RAVEN = Risk Activity Variables, Epidemiology, and Network study.
In multivariate analyses, year, age, homelessness, injection frequency, and drug most frequently injected were significantly and independently associated with sharing injection equipment other than needles (P < .001) and were included in the model, as well as gender (P = .047), education (P = .02), and combined MSM and amphetamine injection status (P = .01). The temporal pattern for sharing injection equipment other than needles in the multivariate analysis was similar to that of the unadjusted data (Table 2; Figure C, available as a supplement to the online version of this article at http://www.ajph.org). A test for simple linear trend across the full time period 1994 to 2013 was significant (Ptrend < .001) in the multivariate analysis.
DISCUSSION
We combined information from 4 studies over the time interval 1994 to 2013 and report an apparent decline in the sharing of injection equipment among Seattle-area IDUs. The decline persisted in analyses that controlled for the effects of age, race/ethnicity, gender, education, area of residence, homelessness, injection frequency, drug most frequently injected, and combined MSM and amphetamine injection status. The decline is apparent both with respect to sharing needles and to sharing injection equipment other than needles.
The varying recruitment methodologies of the 4 studies we combined could well have biased their study populations. The in-treatment group in RAVEN might be expected to represent a lower-risk population of IDUs as they were undertaking efforts to address addiction. The changes over time in the characteristics of the RAVEN study population could be a product of progressive exhaustion of lower-risk IDUs over the course of the study, as RAVEN ultimately recruited 2780 of the 15 000 IDUs (19%) estimated to reside in the Seattle area.30 It is plausible that incarcerated Kiwi participants would have higher levels of risk behavior than other samples of IDUs. Needle-exchange participants could well constitute a study population inherently less likely to share injection equipment, as their presence at the needle exchange demonstrates their motivation to reduce injection risk and at the same time supplies them with sterile injection equipment with which to do so.
Nonetheless, 2 study populations recruited by different methods (RAVEN and Kiwi) in the earlier years reported higher sharing levels than 2 study populations (the later NHBS surveys and the needle-exchange surveys) recruited in the later years. A transition from higher to lower levels of injection equipment sharing was observed within the course of the NHBS surveys. Although we cannot logically exclude the possibility that the observed decline in injection equipment sharing is purely an artifact of different recruitment methods, combined with high inherent variability in the RDS-recruited NHBS surveys,31–33 we believe that the most parsimonious interpretation of these data is the occurrence of a substantial reduction in sharing injection equipment over the time period investigated.
Over the time course of the present study, the number of needles distributed through exchanges operated or supported by Public Health–Seattle & King County increased from approximately 800 000 per year in 1994 to well over 5 million per year in 2013 (Figure D, available as a supplement to the online version of this article at http://www.ajph.org). It is plausible that provision of needles on this scale would result in measurable reductions in needle sharing. A reduction in injection equipment sharing broadly contemporaneous with an increase in needles distributed at needle exchanges in turn supports the utility of needle exchange. Needle exchange use has been associated with lower levels of sharing needles and other injection equipment.34,35
Over the same time period, there was a decline in King County in the number of newly diagnosed HIV cases among IDUs reported to the HIV/AIDS Reporting System, from 62 in 1994 to 23 in 2013 (Figure E, available as a supplement to the online version of this article at http://www.ajph.org). The widespread adoption in the mid-to-late 1990s of effective antiretroviral treatments, and the consequent reduction in viral load among HIV-positive IDUs, undoubtedly contributed to this reduction. Nonetheless, a contemporaneous reduction in sharing injection equipment may well have played a role in the decline in HIV case numbers, particularly in the years before widespread antiretroviral adoption. Declining HIV incidence contemporaneous with reductions in sharing injection equipment in the years before widespread antiretroviral therapy has been reported in Chicago, Illionois20; San Francisco, California17,36,37; New York, New York10,38; Baltimore, Maryland13,16; and Amsterdam, The Netherlands18,39 (Table B, available as a supplement to the online version of this article at http://www.ajph.org).
Limitations
Our results should be interpreted in light of several considerations. The primary outcomes, sharing needles and sharing injection equipment other than needles, were evaluated in terms of any sharing within a relatively long referent period. Quite a range of sharing frequencies would be treated as identical by this construct, so these variables offer relatively crude means of measurement.
The variation in the referent period in the different studies could have influenced the measures of injection equipment sharing. The shorter 6-month referent period in RAVEN and Kiwi studies, relative to the 12 months in NHBS, would be expected to yield lower levels of sharing injection equipment, so that our data would tend to understate the decline from RAVEN and Kiwi to the NHBS surveys. The shorter 3-month referent period in the needle-exchange surveys could have contributed to the lower sharing levels seen in the needle-exchange surveys compared with the contemporaneous NHBS surveys. The referent periods for needle sharing and for sharing other injection equipment were identical in each of the studies, so these considerations apply with equal force to both variables.
In the Kiwi study, the lack of data on sharing water and cottons could have resulted in spuriously lower levels of sharing injection equipment other than needles. The effect of these omissions is likely to be modest. In the subset of Kiwi participants for whom data on cotton sharing was available, only 2 of 645 participants were scored as sharing injection equipment other than needles solely on the basis of sharing cottons. Among RAVEN and NHBS participants, only 9% of participants were categorized as sharing injection equipment other than needles solely on the basis of sharing either water or cottons. In any case, underreporting the Kiwi levels of such sharing would lead to an underestimate of the decline in sharing injection equipment other than needles relative to the later surveys.
In the needle-exchange surveys, the inclusion of both receptive and distributive needle sharing would be expected to increase the reported rate of needle sharing. Again, this would be expected to lead to an understatement of the decline in needle sharing in the needle-exchange surveys relative to earlier surveys.
Although we employed multivariate analyses to control for the effects of differing representation of characteristics across the different study populations, only variables available across surveys could be employed, there was some variation in how they were evaluated, and we may have missed other characteristics that materially affect injection equipment sharing.
Finally, our analyses are based on self-reported data and are subject to underreporting because of social desirability bias. This social desirability bias could vary in the differing study populations; for instance, persons in the drug-treatment programs used to recruit some RAVEN participants may be more inclined to minimize their reports of needle sharing than other injectors as this would suggest a failure of treatment.
Conclusions
We observed an apparent long-term reduction in the sharing of injection equipment among Seattle-area IDUs. Although the limitations on analysis and interpretation arising from the combination of several independently structured and administered studies are very real, we note that our data offer information that is not readily available through other means. In the absence of an effective vaccine against HIV, alternative and less definitive means of reducing HIV transmission must be employed. On the face of it, promoting behavioral risk reduction among IDUs would seem a difficult proposition in light of the chaotic and marginal circumstances of the lives of many IDUs. Our data suggest that such efforts can achieve measurable effects. The observed reduction in sharing injection equipment is contemporaneous with an increase in the volume of needles distributed by local needle exchanges and with a reduction in the number of reported HIV cases among IDUs. Although we cannot demonstrate with certainty that these relations are causal, our data suggest substantial success in public health efforts to reduce the sharing of injection equipment and the burden of HIV and HCV disease.
ACKNOWLEDGMENTS
Funding for this research came from a cooperative agreement with the Centers for Disease Control and Prevention (5U1BPS003250).
Note. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
HUMAN PARTICIPANT PROTECTION
RAVEN, Kiwi, and National HIV Behavioral Surveillance system were approved by the institutional review board of the University of Washington or the State of Washington. Needle-exchange surveys were determined to be program evaluations and not subject to review by the institutional review board of the State of Washington.
REFERENCES
- 1. Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2011. Vol. 23. 2013. Available at: http://www.cdc.gov/hiv/library/reports/surveillance/index.html. Accessed November 17, 2015.
- 2.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28(10):1509–1519. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kral AH, Bluthenthal RN, Lorvick J, Gee L, Bacchetti P, Edlin BR. Sexual transmission of HIV-1 among injection drug users in San Francisco, USA: risk-factor analysis. Lancet. 2001;357(9266):1397–1401. doi: 10.1016/S0140-6736(00)04562-1. [DOI] [PubMed] [Google Scholar]
- 4.Strathdee SA, Galai N, Safaiean M et al. Sex differences in risk factors for HIV seroconversion among injection drug users: a 10-year perspective. Arch Intern Med. 2001;161(10):1281–1288. doi: 10.1001/archinte.161.10.1281. [DOI] [PubMed] [Google Scholar]
- 5.Viral Hepatitis Surveillance United States, 2010. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 6.Vandelli C, Renzo F, Romano L et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gatroenterol. 2004;99(5):855–859. doi: 10.1111/j.1572-0241.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. High-impact HIV prevention: CDC’s approach to reducing HIV infections in the United States. 2011. Available at: http://www.cdc.gov/hiv/pdf/policies_NHPC_Booklet.pdf. Accessed November 17, 2015.
- 8.Valdiserri R, Khalsa J, Dan C et al. Confronting the emerging epidemic of HCV infection among young injection drug users. Am J Public Health. 2014;104(5):816–821. doi: 10.2105/AJPH.2013.301812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsui T, Iwan K, Kazuo M et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology. 1992;16(5):1109–1114. [PubMed] [Google Scholar]
- 10.Des Jarlais C, Perlis T, Friedman SR et al. Behavioral risk reduction in a declining HIV epidemic: injection drug users in New York City, 1990–1997. Am J Public Health. 2000;90(7):1112–1116. doi: 10.2105/ajph.90.7.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruneau J, Daniel M, Abrahamowicz M, Zang G, Lamothe F, Vincelette J. Trends in human immunodeficiency virus incidence and risk behavior among injection drug users in Montreal, Canada: a 16-year longitudinal study. Am J Epidemiol. 2011;173(9):1049–1058. doi: 10.1093/aje/kwq479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topp L, Day CA, Iversen J, Wand H, Maher L. Fifteen years of HIV surveillance among people who inject drugs: the Australian Needle and Syringe Program survey 1995–2009. AIDS. 2011;25(6):835–842. doi: 10.1097/QAD.0b013e32834412cc. [DOI] [PubMed] [Google Scholar]
- 13.Nelson KE, Galai N, Safaeian M, Strathdee SA, Celentano DD, Vlahov D. Temporal trends in the incidence of human immunodeficiency virus infection and risk behavior among injection drug users in Baltimore, Maryland, 1988–1998. Am J Epidemiol. 2002;156(7):641–653. doi: 10.1093/aje/kwf086. [DOI] [PubMed] [Google Scholar]
- 14.Falster K, Kaldor JM, Maher L. Hepatitis C virus acquisition among injecting drug users: a cohort analysis of a national repeated cross-sectional survey of needle and syringe program attendees in Australia, 1995–2004. J Urban Health. 2009;86(1):106–118. doi: 10.1007/s11524-008-9330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabbatini A, Carulli B, Villa M, Corrêa Leite ML, Nicolosi A. Recent trends in the HIV epidemic among injecting drug users in Northern Italy, 1993–1999. AIDS. 2001;15:2181–2185. doi: 10.1097/00002030-200111090-00014. [DOI] [PubMed] [Google Scholar]
- 16.Mehta SH, Galai N, Astemborski J et al. HIV incidence among injection drug users in Baltimore, Maryland (1988–2004) J Acquir Immune Defic Syndr. 2006;43(3):368–372. doi: 10.1097/01.qai.0000243050.27580.1a. [DOI] [PubMed] [Google Scholar]
- 17.Watters JK. Trends in risk behavior and HIV seroprevalence in heterosexual injection drug users in San Francisco, 1986–1992. J Acquir Immune Defic Syndr. 1994;7(12):1276–1281. [PubMed] [Google Scholar]
- 18.van Ameijden EJC, Coutinho RA. Maximum impact of HIV prevention measures targeted at injecting drug users. AIDS. 1998;12(6):625–633. doi: 10.1097/00002030-199806000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Longshore D, Annon J, Anglin MD. Long-term trends in self-reported HIV risk behavior: injection drug users in Los Angeles, 1987 through 1995. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(1):64–72. doi: 10.1097/00042560-199805010-00010. [DOI] [PubMed] [Google Scholar]
- 20.Wiebel WW, Jimenez A, Johnson W et al. Risk behavior and HIV seroincidence among out-of-treatment injection drug users: a four-year prospective study. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(3):282–289. doi: 10.1097/00042560-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91(1):42–46. doi: 10.2105/ajph.91.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorpe LE, Ouellet LJ, Hershow R et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155(7):645–653. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 23.Hagan H, McGough JP, Thiede H, Weiss NS, Hopkins S, Alexander ER. Syringe exchange and risk of infection with hepatitis B and C viruses. Am J Epidemiol. 1999;149(3):203–213. doi: 10.1093/oxfordjournals.aje.a009792. [DOI] [PubMed] [Google Scholar]
- 24.Thiede H, Romero M, Bordelon K, Hagan H, Murrill CS. Using a jail-based survey to monitor HIV and risk behaviors among Seattle area injection drug users. J Urban Health. 2001;78(2):264–278. doi: 10.1093/jurban/78.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–199. [Google Scholar]
- 26.Burt RD, Hagan H, Sabin K, Thiede H. Evaluating respondent-driven sampling in a major metropolitan area: comparing injection drug users in the 2005 Seattle area national HIV Behavioral Surveillance System survey with participants in the RAVEN and Kiwi studies. Ann Epidemiol. 2010;20(2):159–167. doi: 10.1016/j.annepidem.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burt RD, Thiede H. Evaluating consistency in repeat surveys of injection drug users recruited by respondent-driven sampling in the Seattle area: results from the NHBS-IDU1 and NHBS-IDU2 surveys. Ann Epidemiol. 2012;22(5):354–363. doi: 10.1016/j.annepidem.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burt RD, Thiede H. Evidence for risk reduction among amphetamine-injecting men who have sex with men: results from the National HIV Behavioral Surveillance surveys in the Seattle area 2008–2012. AIDS Behav. 2014;18(10):1998–2008. doi: 10.1007/s10461-014-0769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heckathorn DD. Respondent-driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Soc Probl. 2002;49(1):11–34. [Google Scholar]
- 30.Friedman SR, Tempalski B, Cooper H et al. Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. J Urban Health. 2004;81(3):377–400. doi: 10.1093/jurban/jth125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goel S, Salganik M. Assessing respondent-driven sampling. Proc Natl Acad Sci U S A. 2010;107(15):6743–6747. doi: 10.1073/pnas.1000261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wejnert C, Pham H, Krishna N, Le B, DiNenno E. Estimating design effect and calculating sample size for respondent-driven sampling studies of injection drug users in the United States. AIDS Behav. 2012;16(4):797–806. doi: 10.1007/s10461-012-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burt RD, Thiede H. Assessing differences in groups randomized by recruitment chain in a respondent-driven sample of Seattle-area injection drug users. Ann Epidemiol. 2014;24(11):861–867.e14. doi: 10.1016/j.annepidem.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bluthenthal RN, Kral AH, Gee L, Erringer EA, Edlin BR. The effect of syringe exchange use on high-risk injection drug users: a cohort study. AIDS. 2000;14(5):605–611. doi: 10.1097/00002030-200003310-00015. [DOI] [PubMed] [Google Scholar]
- 35.Holtzman D, Barry V, Ouellet LJ et al. The influence of needle exchange programs on injection risk behaviors and infection with hepatitis C virus among young injection drug users in select cities in the United States, 1994–2004. Prev Med. 2009;49(1):68–73. doi: 10.1016/j.ypmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Kral AH, Lorvick J, Ciccarone D et al. HIV prevalence and risk behaviors among men who have sex with men and inject drugs in San Francisco. J Urban Health. 2005;82(1 suppl 1):i43–i50. doi: 10.1093/jurban/jti023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kral AH, Lorvick J, Edlin B. Sex- and drug-related risk among populations of younger and older injection drug users in adjacent neighborhoods in San Francisco. J Acquir Immune Defic Syndr. 2000;24(2):162–167. doi: 10.1097/00126334-200006010-00011. [DOI] [PubMed] [Google Scholar]
- 38.Des Jarlais DC, Marmor M, Friedmann P et al. HIV incidence among injection drug users in New York City, 1992–1997: evidence for a declining epidemic. Am J Public Health. 2000;90(3):352–359. doi: 10.2105/ajph.90.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Ameijden EJC, van den Hoek JAR, Coutinho RA. The harm reduction approach and risk factors for HIV seroconversion in injecting drug users, Amsterdam. Am J Epidemiol. 1992;136(2):236–243. doi: 10.1093/oxfordjournals.aje.a116489. [DOI] [PubMed] [Google Scholar]