Abstract
Objectives. We compared an evidence-based model of group prenatal care to traditional individual prenatal care on birth, neonatal, and reproductive health outcomes.
Methods. We performed a multisite cluster randomized controlled trial in 14 health centers in New York City (2008–2012). We analyzed 1148 pregnant women aged 14 to 21 years, at less than 24 weeks of gestation, and not at high obstetrical risk. We assessed outcomes via medical records and surveys.
Results. In intention-to-treat analyses, women at intervention sites were significantly less likely to have infants small for gestational age (< 10th percentile; 11.0% vs 15.8%; odds ratio = 0.66; 95% confidence interval = 0.44, 0.99). In as-treated analyses, women with more group visits had better outcomes, including small for gestational age, gestational age, birth weight, days in neonatal intensive care unit, rapid repeat pregnancy, condom use, and unprotected sex (P = .030 to < .001). There were no associated risks.
Conclusions. CenteringPregnancy Plus group prenatal care resulted in more favorable birth, neonatal, and reproductive outcomes. Successful translation of clinical innovations to enhance care, improve outcomes, and reduce cost requires strategies that facilitate patient adherence and support organizational change.
Bundling health care services—integrating prevention and treatment—is a strategy to meet “triple aim” goals: enhanced health care quality, improved health outcomes, and lower cost.1,2 The institutional benefits of bundling health care include reduced infrastructure and cost, the opportunity to provide additional services, and collaborative partnerships. Patient benefits include integrated services and reduced barriers to care.
Pregnancy is an important window of opportunity, with frequent health care contact. Nonetheless, adverse birth outcomes remain leading causes of US infant morbidity and mortality3 and are concentrated among disadvantaged groups.4 Pregnant adolescents also have higher rates of sexually transmitted infection (STI) than do their nonpregnant counterparts and those who are nulliparous.5 Taken together, adolescent women from socially disadvantaged groups face adverse reproductive and sexual health disparities.
Yet, bundled preventive interventions are not as common as are those that address individual risk factors.6 Regarding pregnancy, interventions among pregnant adolescents target either reproductive or sexual health, both with limited effectiveness. Clinical interventions such as progesterone administration and cervical cerclage prevent preterm birth in singleton gestations with previous preterm birth or short cervix.7 However, one half of women who deliver preterm have no known risks.8 An independent review of prenatal care models found only 1 randomized controlled trial (RCT) that demonstrated improved health outcomes.9 This study, from our research team, compared CenteringPregnancy group prenatal care to standard individual prenatal care. Our previous research documented that women randomized to group prenatal care had a 33% lower rate of preterm delivery.10 We also documented improved outcomes among women randomized to group prenatal care that bundled reproductive health promotion (CenteringPregnancy Plus): greater than 50% reduction in rapid repeat pregnancy among all women and incident STI among adolescents.11
Translating evidence to routine health care practice is a National Institutes of Health priority.12 It is important to determine whether clinical interventions with demonstrated efficacy can be implemented to produce clinical benefits comparable to those observed during efficacy studies. First developed in 1968 to improve well-child care, group care consists of the same components of individual care visits coupled with education and skills building and takes place in a group of patients.13 Previous research across a range of health conditions suggests many clinical and psychosocial benefits, including improved patient self-management, adherence, satisfaction, and clinical outcomes.14 More time between patients and health care providers results in more patient-centered care.
We conducted a multisite cluster RCT to assess the clinical effectiveness of group prenatal care bundled with reproductive health promotion compared with the clinical effectiveness of standard individual prenatal care. Cluster randomized trials can evaluate changes in service provision under conditions of actual use and are characterized by their multilevel nature15: in this case, pregnant women clustered into prenatal care settings. We hypothesized that women at clinical sites randomly assigned to deliver group prenatal care would have better reproductive and sexual health outcomes than those of women at sites randomized to individual care and that greater exposure to group prenatal care would be associated with better outcomes. Specifically, a priori outcomes included gestational age at delivery, infant birth weight, and small for gestational age as well as incident STI, rapid repeat pregnancy, and behavioral risk factors (e.g., condom use). We also included admission to and days in the neonatal intensive care unit (NICU).
METHODS
We conducted a cluster RCT16 in 14 clinical sites, including 4 community health centers and 10 hospitals in New York City that serve predominantly low-resource women. We calculated the sample size using Optimal Design for Multilevel and Longitudinal Research, version 0.23 (http://www.wtgrantfoundation.org). On the basis of results from our previous study (d = 0.21–0.39), with power at 0.80 and α set to 0.05, we needed 14 sites and 90 patients per site to detect a small or medium effect size of d = 0.25. We determined these estimates by an intraclass correlation coefficient (ICC) of 0.001.
We initially selected clinical sites on the basis of a convenience sample. They could not be conducting group prenatal care, but they had to be committed to changing their practice to offer group prenatal care (including clinical leadership support) and have sufficient space. Sites were aware that they would be trained to offer group care either immediately (intervention condition) or after completion of study recruitment (delayed intervention condition). To minimize selection bias, we identified health centers and recruited before randomization. We randomized sites using a computer-generated sequence in stratified blocks to account for lags in recruitment and the time required for training and implementation. We enrolled participants between 2008 and 2011, with follow-up 1 year postpartum completed in 2012. We then offered training and support to sites randomized to individual care to implement group care.
Patient Selection and Recruitment
Research staff referred adolescents aged 14 to 21 years attending an initial prenatal care visit at participating clinical sites to the study or recruited them directly. We focused on adolescents because they are the most vulnerable to adverse perinatal and reproductive health outcomes. Inclusion criteria were pregnancy at less than 24 weeks gestation, pregnancy not considered high risk, ability to speak English or Spanish, and willingness to participate in group prenatal care. To reduce poststratification patient selection bias,17 we asked all women to consent to receive group prenatal care, if available at their site, without specifying at recruitment whether group care was available.
Women completed structured interviews at 4 time points: during second (mean ±SD = 18.72 ±3.29 weeks gestational age) and third (mean ±SD = 29.99 ±5.28 weeks gestational age) trimester as well as postpartum at 6 (mean ±SD = 26.07 ±5.21 weeks) and 12 (mean ±SD = 57.30 ±13.50 weeks) months. We completed interviews in English (77.7%) or Spanish (22.3%) using audio handheld assisted personal interview technology. Respondents listened over headphones to spoken questions displayed on computer screens. They were paid $20 per interview. Trained staff reviewed maternal and child medical records by using case report forms to extract uniform data.
Intervention
We implemented CenteringPregnancy Plus group prenatal care. Members of the research team at Yale University and at the Centering Healthcare Institute (Boston, MA) provided training and resources. Training included (1) onsite organizational preparation to assist with conversion of health systems to accommodate group care (e.g., staffing, scheduling); (2) 2-day basic training with clinical and administrative staff that included group prenatal care and research overviews, logistics and planning (e.g., recruitment, scheduling), curriculum review, and skills building on facilitative leadership and HIV and STI prevention; (3) webcasts to review session content; and (4) advanced facilitation training for providers who had run an entire group series.
Described in detail previously,10,11,18 CenteringPregnancy Plus begins with standard clinical intake (a history and a physical) conducted individually. Thereafter, all care occurs within the group except concerns requiring privacy or urgent medical attention. Groups included 8 to 12 women of the same gestational age, and a credentialed clinician (e.g., obstetrician, midwife) and cofacilitator (e.g., nurse, medical assistant) facilitated the groups. There were 10 sessions lasting 120 minutes that followed the clinical guidelines of the American Congress of Obstetricians and Gynecologists.
At arrival, participants engaged in self-management activities, including taking weight and blood pressure, charting progress in health records, and completing brief self-assessment surveys to trigger discussion. The clinician completed fundal height and heart rate monitoring in a private area within the group space. Twenty hours of group prenatal care provided much more extensive care, including facilitated discussions on many issues related to pregnancy, childbirth, and postpartum (Massey et al.18 provide a detailed description). Structured reproductive health promotion activities were delivered during 4 of the 10 sessions and included activities to improve sexual self-efficacy, HIV knowledge, interpersonal sexual communication, perceived risk, and social norms.
Study Endpoints
We specified primary outcomes a priori during clinical trial registration (http://www.clinicaltrials.gov, NCT00628771). Primary birth and neonatal outcomes consisted of gestational age at delivery dichotomized as term or preterm (< 37 weeks); infant birth weight dichotomized as normal or low birth weight (< 2500 g); small for gestational age (< 10th percentile in weight for gestational age); and breastfeeding initiation.
We also examined NICU rates and length of stay as a major driver of cost. Primary reproductive outcomes included incident STI 12 months postpartum, which was diagnosed via urine-based ligase chain reaction; rapid repeat pregnancy within 12 months; and 2 behavioral indicators of risk in the past 6 months (i.e., percentage condom use, number of unprotected sexual intercourse occasions).
Statistical Analyses
We performed primary analyses on our prespecified hypotheses using intention-to-treat principles, with study condition (group vs individual care) as the predictor. We employed multilevel mixed models to control for interdependence owing to site clustering (e.g., gestational age ICC = 0.002; condom use ICC = 0.013), with effect of site modeled as a random effect.15 We conducted linear models for continuous variables, logistic models for dichotomous variables, and models with Poisson distribution for count data and skewed distributions (e.g., gestational age in days, days in NICU).
In real-world clinical settings, where nonadherence is expected,19 significant deviations in study protocol may result in biased estimates of the magnitude of treatment effects. When nonadherence to study protocol is substantial, effects of treatment as assigned and as received should be estimated separately.20 Therefore, we conducted planned as-treated analyses to identify potential associations between exposure and outcomes. We classified patients according to the amount of intervention received, with the number of group visits (range = 0–10) as the primary predictor. We assigned patients at sites randomized to individual care and those at sites randomized to group care but who attended no group visits a “0.” Unadjusted models included only the effect of number of group prenatal visits attended.
Adjusted models included the number of group visits, controlling for characteristics associated with group attendance; specifically, those who attended more group visits were more likely to be born outside the United States, to live with their family of origin, to be nulliparous, to have begun intervention earlier in pregnancy, and to have attended fewer individual care visits (all P ≤ .02). For outcomes with baseline data (e.g., percentage condom use, unprotected sexual intercourse), adjusted models also included baseline scores. Finally, we used post hoc analyses to evaluate the impact of receiving at least the minimal intervention dose: 50% of the group prenatal visits scheduled (5 of 10). We conducted analyses using SPSS Statistics for Windows, version 21.0 (IBM Corp, Armonk, NY).
RESULTS
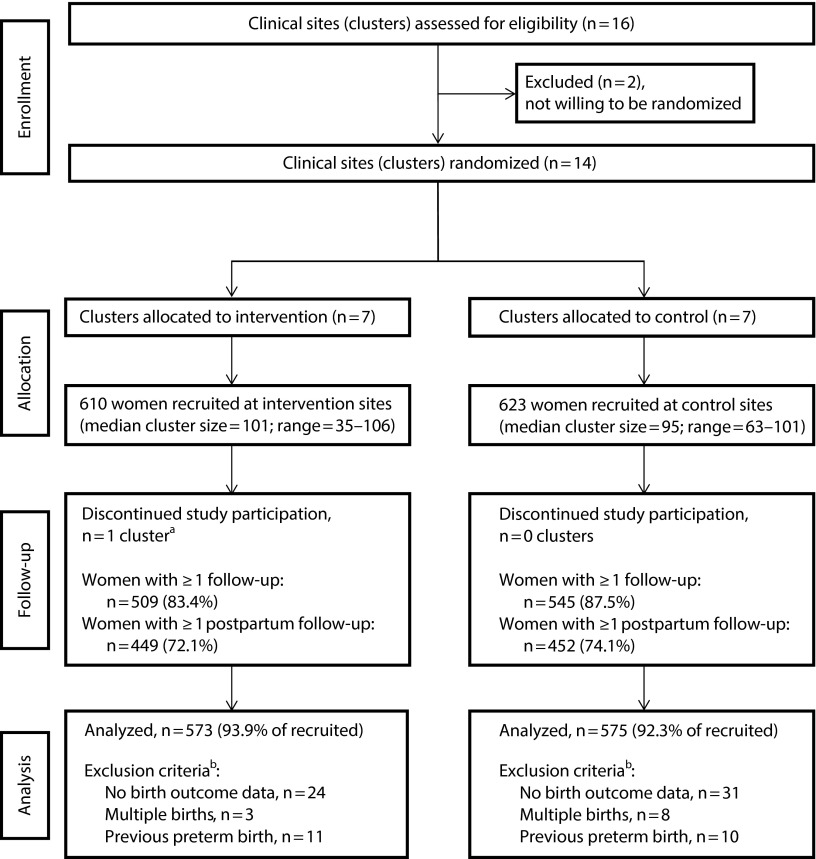
As shown in the CONSORT (Consolidated Standards of Reporting Trials) diagram (Figure 1), 16 clinical sites were originally included; 14 agreed to randomization, and 13 remained in the study. One site randomized to intervention never conducted group prenatal care and dropped out after recruiting only 35 patients. Two small sites (1 per condition) recruited 63 and 67 women, respectively. All other sites recruited at least 80 women (80–106), with 610 women recruited at intervention sites and 623 women recruited at delayed intervention sites. Follow-up rates were comparable.
FIGURE 1—
CONSORT Diagram for Cluster Randomized Controlled Trials: Group Prenatal Care Study, New York, NY, 2008–2011
Note. CONSORT = Consolidated Standards of Reporting Trials.
aThere were 35 women enrolled at this cluster or clinical site before discontinuation. We included these women in all analyses per intention-to-treat principles.
bOne woman in each condition met > 1 exclusion criterion.
Participant Characteristics
Of the 1549 eligible women, 1233 enrolled (participation rate = 80%). Those who agreed to participate were more likely to be Black (38% vs 27%; χ2(1) = 23.36; P < .001) and were slightly younger (mean ±SD = 18.63 ±1.73 vs 19.00 ±1.67; t(1548) = −3.46; P = .001). We limited analyses to women with birth outcomes data, singleton birth, and no history of preterm birth. The final analytic sample included 1148 women.
After controlling for intraclass correlation (patients within clinical settings), the only difference was that women at sites randomized to group care were more likely to be married (14.9% vs 10.5%; Table 1). There were no significant differences in any clinical characteristics, such as parity, gestational age at study entry, or history of STI.
TABLE 1—
Participant Sociodemographic and Clinical Characteristics at Study Entry: Group Prenatal Care Study; New York, NY; 2008–2011
| Characteristic | Group Care (n = 573), % (No.) or Mean ±SD | Individual Care (n = 575), % (No.) or Mean ±SD | P |
| Race/ethnicitya | |||
| Latina | 56.2 (322) | 59.3 (341) | .76 |
| Black, non-Latina | 33.2 (190) | 34.3 (197) | .76 |
| White or other, non-Latina | 10.6 (61) | 6.4 (37) | .37 |
| Enrolled in schoolb | 42.3 (240) | 48.4 (278) | .10 |
| Ageda 14–21 y | 18.7 ±1.8 | 18.6 ±1.7 | .68 |
| Education,b 1–16 y | 10.6 ±2.5 | 11.0 ±2.0 | .10 |
| Enrolled in schoolb | 42.3 (240) | 48.4 (278) | .10 |
| Interviewed in Spanisha | 28.7 (164) | 16.0 (92) | .61 |
| Relationship statusc | |||
| Single, never married | 52.2 (291) | 59.1 (333) | .30 |
| Living with partner | 31.2 (174) | 27.5 (155) | .52 |
| Married | 14.9 (83) | 10.5 (59) | .03 |
| Separated or divorced | 0.5 (3) | 1.4 (8) | .65 |
| Widowed | 1.1 (6) | 1.4 (8) | .86 |
| Nulliparousc | 85.1 (464) | 86.6 (484) | .68 |
| Prior miscarriagec | 10.5 (60) | 11.0 (63) | .92 |
| Prepregnancy BMIc is 13.4–59.0 kg/m2 | 24.7 ±6.5 | 24.2 ±6.2 | .19 |
| Gestational age | |||
| Entry to carec at 3–28 wk | 13.0 ±4.8 | 12.8 ±4.7 | .67 |
| Study entrya at 3–28 wk | 14.3 ±5.1 | 14.7 ±5.2 | .34 |
| Substance use in pregnancy | |||
| Smokingb | 5.3 (30) | 5.1 (29) | .76 |
| Drinking alcoholb | 8.0 (46) | 6.6 (38) | .50 |
| History of STId | 34.0 (168) | 34.7 (181) | .80 |
Note. BMI = body mass index; STI = sexually transmitted infection.
0% missing data.
< 1% missing data.
1%–6% missing data.
11% missing data.
Intention-to-Treat Analysis
Participants in group prenatal care were less likely to deliver a baby small for gestational age than were those in individual care (11.0% vs 15.8%, respectively; Table 2)—a 34% risk reduction (odds ratio [OR] = 0.66; 95% confidence interval [CI] = 0.44, 0.99; P = .04). These results were stable after adjusting for both clinical sites and prenatal care groups. Survival analyses indicated that there were also differences in the timing of births for small for gestational age (χ2(1) = 5.79; P = .02). Specifically, women in group prenatal care were less likely to have a small-for-gestational-age infant, and when they did the child was born at a later gestational age.
TABLE 2—
Intention-to-Treat Results, Group Versus Individual Prenatal Care: Group Prenatal Care Study; New York, NY; 2009–2012
| Outcome | Group Care (n = 573), %a (No.) | Individual Care (n = 575), %a (No.) | OR (95% CI) |
| Birth or neonatal | |||
| Preterm birth is at < 37 wk | 10.1 (57) | 10.1 (57) | 1.00 (0.68, 1.47) |
| Low birth weight, < 2500 g | 8.7 (48) | 9.8 (55) | 0.86 (0.50, 1.48) |
| Small for gestational age | 11.0 (60) | 15.8 (88) | 0.66 (0.44, 0.99) |
| Breastfeeding initiation | 88.8 (325) | 87.2 (279) | 1.18 (0.63, 2.21) |
| Admitted to NICUb | 15.4 (82) | 17.3 (83) | 0.86 (0.41, 1.80) |
| Reproductive health | |||
| Laboratory-tested STI (CT or NG)c | 9.9 (35) | 10.5 (36) | 0.94 (0.58, 1.54) |
| Rapid repeat pregnancy | 22.9 (59) | 28.9 (68) | 0.74 (0.43, 1.28) |
Note. CI = confidence interval; CT = Chlamydia trachomatis; NICU = neonatal intensive care unit; NG = Neisseria gonorrheae; OR = odds ratio; STI = sexually transmitted infection.
% on the basis of those with outcomes data for each variable.
NICU, not registered as primary outcome; however, included as driver of cost.
Restricted to women with laboratory testing for CT and NG 1 year postpartum.
Small-for-gestational-age babies born to mothers at clinical sites that were randomized to group care were slightly less likely to be born preterm (8.3% vs 13.6%, respectively) and less likely to be admitted to the NICU (14.0% vs 18.7%, respectively); however, we are referring to only 148 infants born small for gestational age, and these differences were not statistically significant. There were no differences in other birth, neonatal, or reproductive health outcomes on the basis of intention-to-treat analyses (Table 2).
As-Treated Analysis
At sites randomized to group prenatal care, 22% of women (n = 127) attended no group visits; they received an average of 8.65 (SD = 4.25) individual visits. Among those who attended at least 1 group visit, the mean number of group visits was 5.29 (SD = 2.50; range = 1–10), indicating only modest adherence; these women also attended an average of 5.61 (SD = 3.55) individual prenatal care visits, for a total of 9.30 (SD = 3.82) visits. Women at sites randomized to individual care attended an average of 8.88 (SD = 4.19) individual prenatal care visits. There was no difference in the total number of prenatal care visits between study conditions (B [SE] = 1.06 [0.68]; P = .15).
The greater the number of group prenatal care visits that women attended, controlling for the number of individual prenatal care visits attended, the lower their odds of delivering a baby small for gestational age, preterm, or low birth weight (Table 3). Although there was no difference in admission to the NICU, attending more group prenatal care sessions was associated with having babies who spent fewer days in the NICU (B [SE] = −0.30 [0.02]; P < .001). Regarding reproductive health outcomes, attending more groups was associated with a lower likelihood of rapid repeat pregnancy, more condom use (B [SE] = 1.43 [0.62]; P = .02), and fewer acts of unprotected sexual intercourse (B [SE]= −0.03 [0.01]; P < .01). Results were unchanged when we included a restricted range of visits from 0 to 7, taking into account person-time of observation because women naturally entered prenatal care at different points during pregnancy and had varying lengths of gestation.21
TABLE 3—
As-Treated Results, Derived From Number of Group Prenatal Care Visits Attended: Group Prenatal Care Study; New York, NY; 2009–2012
| Outcome | AORa (95% CI) |
| Birth or neonatal | |
| Preterm birth at < 37 wk | 0.76 (0.69, 0.84) |
| Low birth weight, < 2500 g | 0.81 (0.73, 0.89) |
| Small for gestational age | 0.91 (0.85, 0.99) |
| Breastfeeding initiation | 1.03 (0.93, 1.14) |
| Admitted to NICUb | 0.93 (0.86, 1.01) |
| Reproductive health | |
| Laboratory-tested STI (CT or NG)c | 1.04 (0.94, 1.15) |
| Rapid repeat pregnancy | 0.88 (0.80, 0.97) |
Note. AOR = adjusted odds ratio; CI = confidence interval; CT = Chlamydia trachomatis; NICU = neonatal intensive care unit; NG = Neisseria gonorrheae; STI = sexually transmitted infection. Population size was n = 1148. Analyses were on the basis of the actual number of group prenatal care visits attended, regardless of study condition. All patients at clinical sites randomized to individual care received a “0” for number of group visits as did those who were at clinical sites randomized to group care but never attended (n = 127).
Analyses controlled for correlates of group visit attendance: born outside United States, living situation, nulliparous, gestational age at study entry, and individual care prenatal visits.
Not registered as primary outcome but included as driver of cost. Number of days in the NICU was associated with number of group visits: each increase in the number of group prenatal care visits attended reduced the number of days in the NICU by 0.30 (B [SE] = −0.30 [0.02]; P < .001).
Restricted to women with laboratory testing for CT and NG 1 year postpartum.
Finally, we conducted post hoc analyses to evaluate the impact of receiving at least a minimal dose: 50% of group prenatal visits scheduled (5 of 10). Five group prenatal care visits was the mean and modal number of visits for those at sites randomized to group care. The results of post hoc analyses generally replicated the results of the as-treated analyses (all P ≤ .05). Women who attended at least 50% of group sessions were significantly less likely than those who attended 4 or fewer sessions to have a preterm birth (4.1% vs 12.0%) or low birth weight baby (5.2% vs 10.7%) and had babies who spent fewer days in the NICU (mean ±SD = 0.81 ±2.44 days vs 1.99 ±9.51 days). Women who attended at least 50% of the group sessions also were less likely to experience rapid repeat pregnancy (16.9% vs 29.4%), used condoms more frequently (mean ±SD = 50.30% ±40.43% vs 39.84% ±39.83%), and engaged in fewer acts of unprotected sex (mean ±SD = 6.75 ±14.14 vs 7.55 ±13.00).
DISCUSSION
This cluster RCT demonstrated the effectiveness of group prenatal care bundled with a reproductive health promotion intervention in real-world clinical settings for decreasing the risk of delivering small-for-gestational-age infants. Although statistical significance was modest (P = .04), our effect size was moderate and in line with other intervention effects shown to prevent small for gestational age, such as antiplatelet therapy and supplementation.22 Being born small for gestational age has social and health implications across the developmental life span, including neurodevelopmental delays, obesity, and chronic diseases such as adult-onset diabetes and hypertension.23,24 Barker suggests that chronic disease originates during intrauterine development and that impaired fetal growth permanently changes the body’s structure, function, and metabolism.25 Previously having a small for gestational age infant also increases the risk of future poor reproductive outcomes.26
Although other birth, neonatal, and reproductive health outcomes were not significantly different using intention-to-treat analyses, in as-treated analyses a greater number of group prenatal care visits was associated with better health outcomes, such as a lower risk of preterm and low birth weight, fewer days in the NICU, and a lower risk of rapid repeat pregnancy—even after controlling for the number of traditional individual prenatal care visits. These findings are consistent with those of a previous RCT in which group prenatal care resulted in better reproductive and sexual health outcomes.10,11
This trial was designed to determine whether an evidence-based model of group prenatal care could be implemented in a broader range of urban clinical settings. We did not replicate the finding that demonstrated a reduction in rates of preterm delivery using intention-to-treat analyses. Instead, we found a slight but significant reduction in the risk for being small for gestational age. In this translational study, we observed substantial adherence challenges: 1 in 5 women at clinical sites randomized to group prenatal care never attended any group prenatal care, and the average number of group visits was 5 of 10.
Also, there was variation in terms of implementation fidelity. For example, clinical sites with fewer adolescents struggled to fill groups with women who met study inclusion for age. Consequently, some sites populated groups with patients with a broader range of gestational ages (i.e., across a 2-month period, resulting in some patients entering groups later and others delivering earlier). Other sites formed smaller groups and had to cancel sessions if too few women showed up for group care. This illustrates, in part, barriers faced by clinical sites when moving from individual patient care to scheduled group visits27 and likely reduced statistical power to detect intention-to-treat effects. Improving adherence to group care may result in positive outcomes, highlighting the need for patient support to attend prenatal care and implementation support at the practice and health system levels.
Limitations and Strengths
There are several limitations to our study. First, neither clusters nor participants could be blinded to study condition. However, bias was minimized by postrecruitment randomization (at the site level), and patients did not know whether their site was randomized to group or individual care until after consent. We have no information on content or emphasis of standard care. Low adherence could make it difficult to detect differences between conditions. As-treated analyses indicated the potential for more widespread effects to be observed with greater patient adherence, but these results should be interpreted with caution. Sites were urban health centers serving predominantly disadvantaged women of color; therefore, results may not be generalizable to other settings.
Furthermore, participants were limited to adolescents aged 14 to 21 years at higher risk for adverse maternal–child health outcomes28; thus, results may not be generalizable to older women. However, results could have implications for reducing persistent racial and ethnic disparities in birth and sexual health outcomes via the implementation of group prenatal care.29 Finally, as with any study—especially following participants for more than 1 year—other clinical, behavioral, and psychosocial factors could have confounded our results.
The cluster randomized design has many strengths, including evaluating effectiveness under conditions of actual use and the possibility of generalizing results to clinical practices similar to those in this trial.20 This design maintains the rigor and internal validity of a RCT while enhancing external validity through essential methodological features identified by Glasgow: (1) representative patients (i.e., urban settings, not homogenous nor least medically complex), (2) diverse ambulatory clinical practice settings (i.e., not just those with greatest expertise or the most resources), (3) a comparison condition that represents standard of care rather than no treatment, and (4) multiple outcomes.30 Results have implications for innovations in prenatal care, including clinical adoption, implementation, and sustainability.
Conclusions
Future research must replicate the effects of group prenatal care on maternal and child outcomes and identify potential mechanisms of effect: How does group care improve outcomes? Is it additional time afforded for education and skills building in groups, opportunity for social support, or inclusion of self-care that relates to outcomes observed? Adherence is another critical issue: What are patient and provider factors that influence adherence to prenatal care and how can we promote adherence (e.g., routine text messaging not widely available during study)? Cost and cost-effectiveness analyses are needed to evaluate potential savings and conditions under which cost savings may be incurred. Finally, future translational, dissemination, and implementation research should continue to identify factors that influence uptake, fidelity, sustainability, and the scale-up of innovations in patient-centered care that could improve health outcomes for mothers and babies.
Evidence of effectiveness is essential but not sufficient to ensure the adoption and sustainability of group prenatal care. Despite interest and investment in translational research, there remains an enormous gap between what we know can maximize the quality of health care delivery and what occurs in practice.31 Fuchs and Milstein argue that innovations do not diffuse quickly in health systems, owing in part to resistance from physicians, hospital administrators, insurers, and others.32 Group medical visits are challenging because they fundamentally transform traditional practice and require operational changes (e.g., scheduling, space, clinical workflow); however, group visits have been used in diverse settings and have resulted in benefits for patients, clinicians, and organizations.14
The successful translation of clinical innovations requires strategies that facilitate practice improvements and organizational change.30,33,34 It requires a commitment to implementation research and a willingness to change clinical practices to improve patient and population health. This may be no more important than during prenatal care, when interventions may lead to improved health trajectories across the life span for mothers and their children.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (NIH), National Institute of Mental Health (NIMH; grants R01 MH074399 and R01 MH074394, linked R01’s to J. R. I. and J. N. T.). V. Earnshaw’s work was additionally supported by a postdoctoral fellowship from the NIH, NIMH (grant T32-MH20031).
HUMAN PARTICIPANT PROTECTION
This study was approved by Yale University (26962), and Clinical Directors’ Network (004-06), Bronx Lebanon Hospital Center (05 08 08 02), Biomedical Research Alliance of New York (08-02-242(HHC)-202), Brookdale University Hospital and Medical Center (07-18), Brooklyn Hospital Center (624), Columbia University Medical Center (IRB-AAAD1707), Flushing Hospital (08/07-1), Lutheran Medical Center (53), and Public Health Solutions (032607). All participants provided written informed consent.
REFERENCES
- 1.Ickovics JR. “Bundling” HIV prevention: integrating services to promote synergistic gain. Prev Med. 2008;46(3):222–225. doi: 10.1016/j.ypmed.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schweizer M, Perencevich E, McDanel J et al. Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease Gram positive surgical site infections after cardiac or orthopedic surgery: systematic review and meta-analysis. BMJ. 2013;346:f2743. doi: 10.1136/bmj.f2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrman RE, Butler AS US Institute of Medicine, Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences and Prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 4.Braveman PA, Heck K, Egerter S et al. The role of socioeconomic factors in Black–White disparities in preterm birth. Am J Public Health. 2015;105:694–702. doi: 10.2105/AJPH.2014.302008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meade CS, Ickovics JR. Systematic review of sexual risk among pregnant and mothering teens in the USA: pregnancy as an opportunity for integrated prevention of STD and repeat pregnancy. Soc Sci Med. 2005;60(4):661–678. doi: 10.1016/j.socscimed.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Prochaska JJ, Prochaska JO. A review of multiple health behavior change interventions for primary prevention. Am J Lifestyle Med. 2011;5(3):208–221. doi: 10.1177/1559827610391883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conde-Agudelo A, Romero R, Nicolaides K et al. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2013;208(1):42.e1–42.e18. doi: 10.1016/j.ajog.2012.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371(9607):164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 9.Allen J, Gamble J, Stapleton H, Kildea S. Does the way maternity care is provided affect maternal and neonatal outcomes for young women? A review of the research literature. Women Birth. 2012;25(2):54–63. doi: 10.1016/j.wombi.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Ickovics JR, Kershaw T, Westdahl C et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007 doi: 10.1097/01.AOG.0000275284.24298.23. 110(2 pt 1):330–339. [Erratum in Obstet Gynecol. 2007;110(4):937] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kershaw TS, Magriples U, Westdahl C, Rising SS, Ickovics J. Pregnancy as a window of opportunity for HIV prevention: effects of an HIV intervention delivered within prenatal care. Am J Public Health. 2009;99(11):2079–2086. doi: 10.2105/AJPH.2008.154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299(2):211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 13.Feldman M. Cluster visits. Am J Nurs. 1974;74:1485–1488. [PubMed] [Google Scholar]
- 14.Noffsinger EB. Running Group Visits in Your Practice. New York, NY: Springer; 2009. [Google Scholar]
- 15.Eldridge S, Kerry SM. A Practical Guide to Cluster Randomised Trials in Health Services Research. Chichester, UK: John Wiley and Sons; 2012. [Google Scholar]
- 16.Campbell MK, Piaggio G, Elbourne DR, Altman DG CONSORT Group. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 17.Puffer S, Torgerson D, Watson J. Evidence for risk of bias in cluster randomised trials: review of recent trials published in three general medical journals. BMJ. 2003;327(7418):785–789. doi: 10.1136/bmj.327.7418.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massey Z, Rising SS, Ickovics J. CenteringPregnancy group prenatal care: promoting relationship-centered care. J Obstet Gynecol Neonatal Nurs. 2006;35(2):286–294. doi: 10.1111/j.1552-6909.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 19.Hernán MA, Hernandez-Diaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials. 2012;9(1):48–55. doi: 10.1177/1740774511420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh AW. Randomized controlled trials and clinical maternity care: moving on from intention-to-treat and other simplistic analyses of efficacy. BMC Pregnancy Childbirth. 2013;13:15. doi: 10.1186/1471-2393-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savitz DA, Hertz-Picciotto I, Poole C, Olshan AF. Epidemiologic measures of the course and outcome of pregnancy. Epidemiol Rev. 2002;24(2):91–101. doi: 10.1093/epirev/mxf006. [DOI] [PubMed] [Google Scholar]
- 22.Morris RK, Oliver EA, Malin G, Khan KS, Mead C. Effectiveness of interventions for the prevention of small-for-gestational age fetuses and perinatal mortality: a review of systematic reviews. Acta Obstet Gynecol Scand. 2013;92(2):143–151. doi: 10.1111/aogs.12029. [DOI] [PubMed] [Google Scholar]
- 23.Strauss RS. Adult functional outcome of those born small for gestational age: twenty-six-year follow-up of the 1970 British Birth Cohort. JAMA. 2000;283(5):625–632. doi: 10.1001/jama.283.5.625. [DOI] [PubMed] [Google Scholar]
- 24.Meas T, Deghmoun S, Armoogum P, Alberti C, Levy-Marchal C. Consequences of being born small for gestational age on body composition: an 8-year follow-up study. J Clin Endocrinol Metab. 2008;93(10):3804–3809. doi: 10.1210/jc.2008-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 26.Salihu HM, Salinas A, August EM, Mogos MF, Weldeselasse H, Whiteman VE. Small size for gestational age and the risk for infant mortality in the subsequent pregnancy. Ann Epidemiol. 2012;22(11):764–771. doi: 10.1016/j.annepidem.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novick G, Reid AE, Lewis J, Kershaw TS, Rising SS, Ickovics JR. Group prenatal care: model fidelity and outcomes. Am J Obstet Gynecol. 2013;209(2):112.e1–112.e6. doi: 10.1016/j.ajog.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeha D, Usta I, Ghulmiyyah L, Nassar A. A review of the risks and consequences of adolescent pregnancy. J Neonatal Perinatal Med. 2015 doi: 10.3233/NPM-15814038. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Chin MH, Goldmann D. Meaningful disparities reduction through research and translation programs. JAMA. 2011;305(4):404–405. doi: 10.1001/jama.2011.26. [DOI] [PubMed] [Google Scholar]
- 30.Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274–1281. doi: 10.2105/AJPH.2012.300755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green LW, Ottoson JM, García C, Hiatt RA. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annu Rev Public Health. 2009;30:151–174. doi: 10.1146/annurev.publhealth.031308.100049. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs VR, Milstein A. The $640 billion question—why does cost-effective care diffuse so slowly? N Engl J Med. 2011;364(21):1985–1987. doi: 10.1056/NEJMp1104675. [DOI] [PubMed] [Google Scholar]
- 33.Collins FS. Reengineering translational science: the time is right. Sci Transl Med. 2011;3(90) doi: 10.1126/scitranslmed.3002747. 90cm17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berwick DM. The science of improvement. JAMA. 2008;299(10):1182–1184. doi: 10.1001/jama.299.10.1182. [DOI] [PubMed] [Google Scholar]