Abstract
Purpose
To evaluate intraocular pressure (IOP) change after cataract surgery in non-glaucomatous eyes with narrow and open angles (OAs) and its relation to novel lens parameters measured by anterior segment optical coherence tomography (AS-OCT).
Setting
University affiliated hospital, Farabi Eye Hospital, Tehran, Iran.
Design
Prospective interventional case series.
Methods
In this prospective study, 85 non-glaucomatous eyes underwent phacoemulsification and lens implantation. Thirty-nine eyes had OAs and 46 eyes had narrow angles (NAs). IOP and biometric parameters were measured by AS-OCT preoperatively and 3 months after surgery. Change in IOP and its relation to biometric parameters, including lens vault (LV), anterior vault (AV), defined as the sum of the LV and the ACD, and relative LV (rLV), defined as the ratio of the LV to the AV, were evaluated. The main outcome measure was degree of IOP change after phacoemulsification.
Results
Of the 85 patients included in the analysis, 35 were male and 50 were female with an overall mean age of 62.2±8.9 years. The average IOP reduction was −4.95±2.26 mm Hg, from a preoperative mean of 17.12±2.47 mm Hg, at 3 months after cataract surgery. The amount of IOP reduction was significantly greater in the NA compared with the OA group. In multivariate linear regression analysis, preoperative IOP and AV were significantly associated with IOP decrease (all ≤0.03).
Conclusion
Cataract surgery results in IOP reduction in both OA and NA eyes. The amount of IOP reduction is related to AV.
Introduction
There is substantial data that cataract surgery has a lowering effect on intraocular pressure (IOP) in glaucomatous and non-glaucomatous eyes.1, 2, 3, 4, 5, 6, 7, 8, 9 This effect seems to be more pronounced in narrow angles (NAs) compared with open angles (OAs).2, 4, 5, 6, 10
Several studies have been performed to predict the amount of IOP change after cataract surgery on the basis of preoperative factors. These studies have shown that the higher the preoperative IOP and the shallower the anterior chamber depth (ACD), the more pronounced is the change in IOP after cataract surgery.5, 7, 11, 12, 13 Angle parameters such as angle opening distance (AOD) measured by anterior segment optical coherence tomography (AS-OCT) have also been reported to be associated with IOP reduction after phacoemulsification.9
However, few studies have examined the relationship between reduction in IOP and lens parameters.14 The crystalline lens has a pivotal role in narrowing the angle by pushing the peripheral iris anteriorly. Although many of the anatomical factors such as axial length (AL) and anterior chamber width cannot be changed, the lens remains one of the few modifiable factors that can secondarily influence the anterior chamber and angle parameters and thus the IOP. For example, Yang et al14 demonstrated that LT was significantly associated with a reduction in IOP after cataract surgery in normal eyes.
Other variables such as lens position (LP) and relative lens position (RLP), defined as LP=ACD−1/2 LT and RLP=LP/AL, were introduced to address the effect of the lens on the anterior chamber.15 However, there have been conflicting reports on the importance of LP and RLP, possibly because LT and ACD, which are the two constituents of these parameters, are interdependent and could compensate for each other.16, 17, 18
With the advent of AS-OCT, additional parameters related to LP have been assessed. Lens vault (LV) is a novel parameter introduced by Nongpuir et al,19 defined as the perpendicular distance between the line joining the two scleral spurs and the anterior pole of the lens. It has been shown that LV could be a preoperative predictor in angle widening and IOP reduction after cataract surgery in normal eyes.3 But ACD can compensate for LV; that is, if the ACD is deep enough, the LV, even when large, is less likely to increase the risk of angle closure or angle widening and IOP reduction after cataract surgery. Other new parameters, measured by AS-OCT such as anterior vault (AV) and relative LV (rLV), which are indicators of the combined effect of ACD and LV, may be more closely related to the degree of angle widening and IOP drop after cataract surgery than absolute values. These two novel parameters are defined as the sum of the LV and the ACD and the ratio of the LV to the AV, respectively.20 The purpose of the present study is to evaluate these novel lens parameters in non-glaucomatous eyes with NAs or OAs and their relation to IOP change after cataract surgery.
Materials and methods
In this prospective study, patients were consecutively recruited from the comprehensive and glaucoma clinics of Farabi Eye Hospital, Tehran, Iran from 1 October 2012 to 30 November 2013. Patients were enrolled if they met the inclusion and exclusion criteria. The study protocol followed the tenets of the Declaration of Helsinki and was approved by the Farabi Hospital Research Ethics Committee. Written informed consent was obtained from all patients prior to enrollment. Patients with visually significant cataracts and best-corrected visual acuity (BCVA) of worse than 20/40 were included.
Exclusion criteriaincluded: (1) previous penetrating or laser surgery, including peripheral iridotomy; (2) peripheral anterior synechiae (PAS); (3) IOP>30 mm Hg; (4) glaucomatous optic neuropathy determined by optic disc cupping or glaucomatous visual field loss; (5) use of glaucoma medication; (6) pseudoexfoliation; (7) AS-OCT images in which scleral spur could not be identified; and (8) major intraoperative or postoperative complications (eg, vitreous loss or endophthalmitis).
Demographic information was recorded. Examinations, including BCVA testing, IOP measurement, slit lamp and fundus examination, and gonioscopy, were performed. IOP was measured using Goldmannapplanation tonometry by a single trained ophthalmologist (FA). All the measurements were done during morning hours (0800–1200 hours). Three measurements were performed, and the average value was used for analysis.
Gonioscopy was performed using a Zeiss-style 4-mirror goniolens (Model G-4, Volk Optical, Mentor, OH, USA) in low light conditions and primary position by the same ophthalmologist (FA). The angle was graded using the Shaffer classification system. Eyes with Shaffer grades of 3 or 4 in 3 or all 4 quadrants were defined as OAs and those with Shaffer grades of 2 or less in 3 or all 4 quadrants as NAs. Indentation gonioscopy was carried out to rule out PAS.
A-scan biometry (Echoscan, model U3300, Nidek, Tokyo, Japan) was used to measure AL, lens thickness (LT), and ACD. Five readings were taken for each eye. After omitting the highest and lowest values, the mean of the other three readings was used for analysis.
Anterior segment optical coherence tomography
AS-OCT (Visante OCT; Carl Zeiss Meditec, Dublin, CA, USA) was performed for all patients in the dark (in a room without windows, with door closed and the only lighting from the AS-OCT screen). Scans were centered on the pupil and were obtained along the horizontal and vertical axes using the enhanced anterior segment single protocol. To ensure that the scans were optically aligned, which is important in assessing lens parameters, we captured images with a good corneal vertex reflex, and if it was not possible to include a robust vertex in the image, scans were positioned at the 3-O'clock or 9-O'clock corneal limbus according to the en face video camera display of the scan line relative to the eye.
The polarization of the scan, saturation, and noise of the images were adjusted to have the optimal image quality. At least three consecutive images were captured, and the image with the best quality regarding alignment and visibility of the scleral spurs was chosen for analysis.
Images were then processed using the customized software, the Zhongshan Angle Assessment Program (ZAAP) (Guangzhou, China).21 The only observer input needed was to determine the location of the two scleral spurs, which was done by a glaucoma specialist (SM). Because localization of the scleral spur was very important to obtain accurate measurements, images in which the scleral spurs were not clearly visible were eliminated. The algorithm of the ZAAP software automatically calculated several anterior segment and angle parameters.
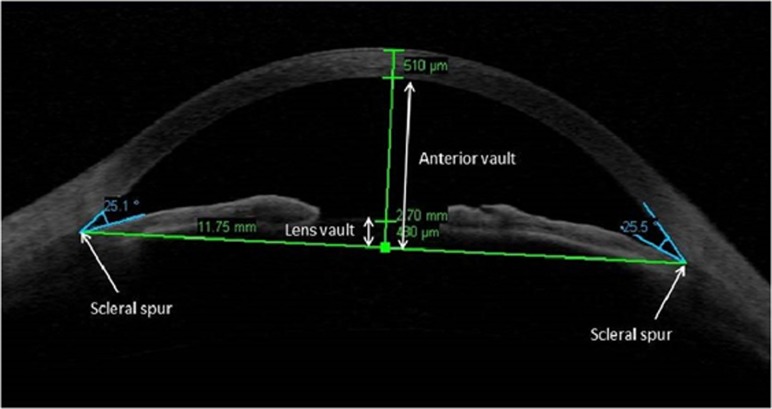
The measured AS-OCT parameters and their definitions are:
Anterior chamber depth (ACD): the distance from the endothelium at the center of the cornea to the anterior pole of the cataractous lens or IOL; anterior chamber width (ACW): the distance between the two scleral spurs; anterior chamber area (ACA): the cross-sectional area of the anterior chamber bordered by the posterior surface of the cornea, the anterior surface of the iris, and the anterior surface of the lens within the pupil; anterior chamber volume (ACV): the software calculated this value by plotting a vertical axis through the center of the ACA and rotating ACA 360 degrees around this vertical axis; lens vault (LV): the maximum perpendicular distance between the anterior lens surface to the horizontal line connecting the two scleral spurs on horizontal AS-OCT scans; anterior vault (AV): the maximum perpendicular distance between the posterior corneal surface to the horizontal line connecting the two scleral spurs on horizontal AS-OCT scans; relative AV (rAV): the AV divided by the AL; and relative LV (rLV): the LV divided by the AV.
Figure 1 also illustrates these parameters.
Figure 1.
Anterior segment optical coherence tomography image of anterior chamber demonstrating lens vault and anterior vault.
Surgical technique
Surgery was performed by a single surgeon (SM) in all subjects under topical anesthesia. Surgery consisted of routine phacoemulsification via a 3.2-mm temporal clear corneal incision, with an in-the-bag one-piece acrylic intraocular lens (AcrySof SA60AT, Alcon Laboratories, Inc., Fort Worth, TX, USA) implantation.
Postoperatively patients received topical antibiotics four times a day for 1 week, as well as topical 1% prednisolone acetate every 2 h, which was tapered gradually over 1 month. They returned for postoperative care on day 1, week 1, month 1, and month 3. In addition to standard of care procedures, IOP measurement and AS-OCT were repeated per protocol at month 3. The same ophthalmologist examined all patients postoperatively. The main outcome measure was degree of IOP change after phacoemulsification.
Statistical analysis
Statistical analysis was performed using the SPSS software, version 17 (SPSS, Inc., Chicago, IL, USA). The paired t-test was used to compare differences between preoperative and postoperative IOP change. Univariate analysis was used for assessing correlation between IOP change and lens variables. Linear regression analysis, adjusted for preoperative IOP, was performed to assess the effect of each variable on IOP drop. To determine the most important variable that was associated with IOP drop, we used a backward logistic regression model, including variables with P<0.15 in the linear regression model. Variance inflation factor was calculated to demonstrate multicollinearity for each parameter. P-value of <0.05 was considered statistically significant.
Results
The present study comprised 99 eyes of 99 consecutive patients who underwent cataract surgery, of which 14 eyes were excluded because of the inability to detect the scleral spur in AS-OCT images.
Of the 85 patients included in the analysis, 35 were male and 50 were female with an average age of 62.2±8.9 (37–81) years. Forty-six were NAs and 39 were OAs. The average BCVA before surgery was 0.58±0.14 Logarithm of the Minimum Angle of Resolution (logMAR), which improved to 0.14±0.07 (logMAR; P<0.001).
The average AL and LT before surgery was 23.36±1.84 and 4.67±0.59 mm, respectively. Table 1 summarizes preoperative ocular parameters measured by AS-OCT and A-Scan biometry. Preoperatively, the average IOP was 17.12±2.47 mm Hg, which dropped to 12.20±2.69 mm Hg at 3 months with an average change of −4.95±2.26 mm Hg (P<0.001).
Table 1. Preoperative ocular parameters measured by anterior segment optical coherence tomography or A-Scan biometry.
| Parameters | Mean±SD (range) |
|---|---|
| Axial length (mm) | 23.36±1.84 (19.5 to 30.23) |
| Anterior chamber depth (mm) | 2.50±0.51 (1.53 to 3.58) |
| Anterior chamber width (mm) | 11.49±0.54 (10.15 to 12.85) |
| Anterior chamber area (mm2) | 18.83±4.87 (8.96 to 32.86) |
| Angle opening distance 500 (AOD500) (mm) | 0.25±0.15 (0.01 to 0.70) |
| Angle opening distance 750 (AOD750) (mm) | 0.33±0.21 (0 to 1.07) |
| Trabecular iris space area 500 (TISA500) (mm2) | 0.10±0.06 (0.01 to 0.45) |
| Trabecular iris space area 750 (TISA750) (mm2) | 0.17±0.10 (0.03 to 0.45) |
| Lens thickness (mm) | 4.67±0.59 (3.42 to 5.83) |
| Lens vault (μm) | 524.37±422.43 (−417.70 to 1596.00) |
| Anterior vault (mm) | 3.05±0.20 (2.66 to 3.50) |
| Relative lens vault | 0.18±0.14 (−1.4 to 0.5) |
| Iris thickness 750 (mm) | 0.44±0.10 (0.26 to 0.69) |
| Iris thickness 2000 (mm) | 0.42±0.08 (0.20 to 0.62) |
| Iris area (mm2) | 1.50±0.24 (1.02 to 2.24) |
| Iris curvature (mm) | 0.29±0.13 (0.18 to 0.60) |
The results of univariate linear regression analysis of the association between IOP drop and various ocular parameters, including preoperative IOP, are shown in Table 2. Significant predictors of a reduction in IOP were preoperative IOP (P=0.001), AL (P<0.001), ACD (P=0.03), and AV (P=0.006). The univariate analysis demonstrated that, for a 1 mm Hg increase in preoperative IOP, the IOP decreased by 0.32 mm Hg. We also found a 0.59 mm Hg drop in IOP for each 1 mm decrease in AL.
Table 2. Univariate and multivariate analysis of the association between preoperative parameters and changes in intraocular pressure (IOP).
| Parameter |
Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| B±SD | CI (95%) | P-value | Adjusted P for preoperative IOP | B±SD | CI (95%) | P-value | VIF | |
| Age | 0.026±0.027 | −0.027 to 0.79 | 0.33 | 0.15 | 0.015±0.030 | −0.045 to 0.074 | 0.62 | 1.178 |
| Sex | 0.081±0.500 | −0913 to 1.076 | 0.87 | 0.66 | −0.383±0.509 | −1.401 to 0.634 | 0.45 | 1.175 |
| Preoperative IOP | −0.323±0.094 | −0.509 to 0.136 | 0.001 | — | −0.222±0.101 | −0.424 to −0.020 | 0.03 | 1.254 |
| Axial length | 0.594±0.130 | 0.334 to 0.853 | <0.001 | <0.001 | 0.661±0.205 | 0.252 to 1.071 | 0.002 | 2.495 |
| ACD | 1.032±0.480 | 0.077 to 1.988 | 0.03 | 0.25 | −1.252±0.853 | −2.957 to 0.453 | 0.14 | 3.111 |
| ACW | 0.924±0.437 | 0.055 to 1.794 | 0.03 | 0.02 | −0.966±0.744 | −2.454 to 0.522 | 0.19 | 2.704 |
| ACA | 0.117±0.05 | 0.019 to 0.216 | 0.02 | 0.01 | — | — | — | — |
| AOD500 | 5.140±1.483 | 2.190 to 8.090 | 0.001 | 0.001 | 1.953± 2.529 | −3.104 to 7.010 | 0.44 | 2.726 |
| TISA500 | 8.336±3.591 | 1.193 to 13.080 | 0.02 | 0.03 | — | — | — | — |
| Lens thickness | −0.370±0.627 | −1.636 to 0.897 | 0.55 | 0.83 | — | — | — | — |
| Lens vault | 0.703±0.587 | −1.872 to 0.465 | 0.23 | 0.26 | — | — | — | — |
| Anterior vault | 3.311±1.162 | 1.000 to 5.622 | 0.006 | 0.008 | 4.742±1.931 | 0.881 to 8.604 | 0.01 | 2.701 |
| rLV | −2.384±1.760 | −5.886 to 1.117 | 0.17 | 0.21 | — | — | — | — |
| lT750 | −2.651±2.845 | −8.350 to 3.049 | 0.35 | 0.91 | — | — | — | — |
| lT2000 | 2.565±3.263 | −3.971 to 9.100 | 0.43 | 0.46 | — | — | — | — |
| Iris area | 1.436±1.228 | −1.029 to 3.901 | 0.24 | 0.08 | — | — | — | — |
| Iris curvature | −0.560±2.016 | −4.598 to 3.479 | 0.78 | 0.93 | — | — | — | — |
Abbreviations: ACA, anterior chamber area; ACD, anterior chamber depth; ACW, anterior chamber width; AOD500, angle opening distance at 500 μm; IOP, intraocular pressure; IT750, iris thickness at 750 μm; IT2000, Iiis thickness at 2000 μm; rLV, relative lens vault; TISA500, trabecular iris space area at 500 μm; VIF, variance inflation factor.
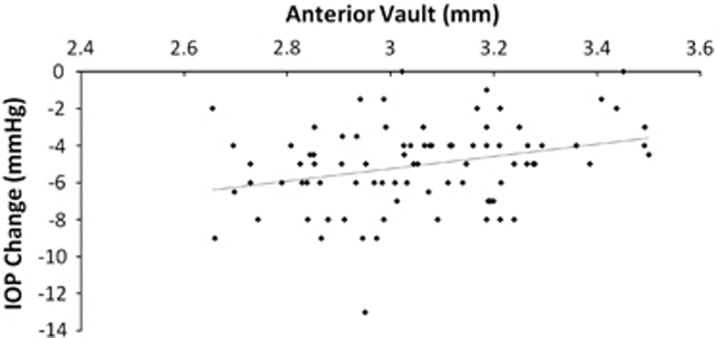
Greater IOP reduction after surgery was associated with smaller AV (Figure 2). There was no association between LT, LV, or rLV with IOP reduction in our cases.
Figure 2.
Correlation between intraocular pressure (IOP) reduction and anterior vault shows that greater IOP reduction after surgery is associated with smaller anterior vault (ß=3.311, P=0.008).
After adjusting for preoperative IOP, the significant predictors of an IOP reduction were AL and AV. In multivariate linear regression analysis, preoperative IOP (ß=−0.222, P=0.03) and AV (ß=4.742, P=0.02) were also significantly associated with a reduction in IOP after cataract surgery. (Table 2)
We found for every 0.1 mm decrease in preoperative AV the IOP decreased by 0.33 mm Hg after cataract surgery.
Table 3 shows different variables in OA and NA eyes. In NA eyes, LT, LV, and rLV were significantly greater than in OA eyes (P<0.005). ACD, AL, and AV were significantly less in NA eyes (P<0.001).
Table 3. Anterior segment parameters measured by anterior segment optical coherence tomography or A-scan biometry in the open and narrow angle eyes.
| Parameters | Open angle (n=39), mean±SD | Narrow angle (n=46), mean±SD | P-value |
|---|---|---|---|
| Change in IOP | −4.52±2.19 | −5.73±2.18 | 0.01 |
| Axial length (mm) | 24.04±1.94 | 22.41±1.23 | <0.001 |
| Anterior chamber depth (mm) | 2.85±0.40 | 2.15±0.33 | <0.001 |
| Anterior chamber width (mm) | 11.66±0.58 | 11.30±0.46 | 0.003 |
| Anterior chamber area (mm2) | 22.27±3.88 | 15.41±2.94 | <0.001 |
| Angle opening distance 500 (AOD500) (mm) | 0.358±0.130 | 0.140±0.085 | <0.001 |
| Angle opening distance 750 (AOD750) (mm) | 0.485±0.167 | 0.170±0.101 | <0.001 |
| Trabecular iris space area 500 (TISA500) (mm2) | 0.148±0.064 | 0.063±0.031 | <0.001 |
| Trabecular iris space area 750 (TISA750) (mm2) | 0.254±0.074 | 0.096±0.043 | <0.001 |
| Lens thickness (mm) | 4.41±0.57 | 4.91±0.50 | 0.005 |
| Lens vault (μm) | 288.3±331.2 | 810.1±337.4 | <0.001 |
| Anterior vault (mm) | 3.14±0.20 | 2.96±0.17 | <0.001 |
| Relative lens vault | 0.09±0.11 | 0.27±0.11 | <0.001 |
| Iris thickness 750 (mm) | 0.43±0.09 | 0.47±0.10 | 0.08 |
| Iris thickness 2000 (mm) | 0.43±0.09 | 0.45±0.09 | 0.95 |
| Iris area (mm2) | 1.49±0.27 | 1.50±0.24 | 0.94 |
| Iris curvature (mm) | 0.23±0.14 | 0.35±0.12 | 0.001 |
The mean IOP reduction in the NA group was −5.73±2.18 mm Hg, which was significantly more than that in the OA group after surgery (−4.52±2.19 mm Hg) (P=0.01). In OA eyes, preoperative IOP (ß=0.713, P<0.001) and AL (ß=−0.30, P=0.03) were the most important parameters that predicted IOP drop in multivariate linear regression. In NA eyes, the significant factors were preoperative IOP (ß=−0.405, P=0.02) and rLV (ß=6.396, P=0.05).
Discussion
The result of this study confirms previous reports of IOP reduction after cataract surgery.1, 2, 4, 13 In our series of non-glaucomatous patients with either OAs or NAs, the average IOP dropped −4.95±2.26 mm Hg from a preoperative value of 17.12±2.47 mm Hg, 3 months after cataract surgery. The amount of IOP reduction was significantly greater in NA eyes (−5.73±2.18 mm Hg) compared with the OA group (−4.52±2.19 mm Hg), and this reduction was related to AV.
Given the variability of the postoperative IOP response reported in the literature,1, 2, 4, 13 there has been significant effort made to find predictors of IOP reduction. In our study, we found that the preoperative IOP was a significant predictor of IOP drop. For every 1 mm Hg increase in preoperative IOP, the postoperative IOP decreased by 0.32 mm Hg. Consistent with our results, several studies have reported preoperative IOP as a significant predictive factor for postoperative IOP drop.4, 5, 11, 12
Recently, anterior chamber anatomic parameters measured by different anterior segment imaging modalities such as AS-OCT have become the center of focus to predict the amount of IOP reduction after cataract surgery. Most of these studies evaluated the anterior chamber angle and ACD as determinants of IOP drop.3, 9, 11, 12 However, studies on lens-related anterior segment parameters are quite rare. LT had been found to be a risk factor for angle-closure glaucoma.22, 23 It has been shown that not the entire LT but the portion of the lens located anterior to the scleral spur (LV) plays the most important anatomic part in crowding the angle.24
The role of LT as a predictor of IOP drop is controversial. In a study on non-glaucomatous subjects, Yang et al14 reported that LT was a significant predictor of IOP drop in univariate (P<0.05) and multivariate analysis (P<0.005). However, other studies did not find any significant relationship.1, 25
LV represents the portion of the lens that is located anterior to the scleral spur, might be more representative of the role of the lens in angle closure than LT,24 and has been shown to be an independent risk factor for angle-closure glaucoma.26 Huang et al3 also showed that, for visually significant cataract, preoperative LV was related to IOP reduction after surgery. There was no data on LT in their report. However, in a previous report, consistent with the result of the present study, we did not find any significant relation between LV and IOP reduction.1, 25
There are some novel lens and anterior segment parameters that have been recently introduced to evaluate anterior dimensions of the eye, including the lens. In the present study, based on AS-OCT scans, we investigated two novel biometric parameters: the AV, which represents the sum of the LV and the ACD, and the rLV, which represents the ratio of the LV to the AV.20 We found that AV was significantly associated with a reduction in IOP after cataract surgery in multivariate regression analysis. We did not find any significant association between LT, LV, or rLV and IOP reduction.
Although LV might be more representative of the role of the lens in angle closure as compared with LT,24, 26 the role of the ACD should not be forgotten; a very deep anterior chamber can compensate for the risk introduced by even large LVs and reduces the overall risk of angle closure and vice versa. In fact, if the ACD is deep enough, the LV, even when large, is less likely to increase the risk of angle closure. These facts suggest that other parameters such as AV and rLV, which are indicators of the combind effect of ACD and LV, may be more closely related to angle-closure risk than absolute values. In our study, a significant correlation was found between AV and IOP drop after cataract surgery but not between LV and IOP reduction.We also did not find any correlation between rLV and IOP drop. Although one reason that AV has significant correlation with IOP drop might be that it has ACD as a component in its measurement. But multivariate analysis demonstrated that AV is a better predictor of IOP change after phacoemulsification as it is an indicator of both LV and ACD. Smaller anterior segment might be a more important predictor of IOP drop than LP. In our study, rLV was a powerful predictor of IOP change in the NA group.
Different mechanisms for the IOP decrease after cataract surgery have been proposed, although the exact mechanism is still not fully understood. Trabecular endothelial remodeling in response to ultrasonic stress,27 prostaglandin release,28 and increase in aqueous outflow by expansion of the trabecular meshwork4 are some of the hypothetical mechanisms, but angle and ACD change induced by cataract removal has been shown to be the main mechanism of IOP change in OAs as well as NAs.14 Huang et al9 supported this hypothesis by showing that IOP reduction was associated with angle widening in both OA and NA eyes. It can be hypothesized that the main part of the IOP-lowering effect of cataract removal in normal eyes is implemented through the effect of the lens on angle anatomy in both OAs and NAs. In another study, Huang et al3 showed that the degree of angle widening and AC deepening after cataract surgery was correlated with the preoperative LV in normal eyes. Thus there is potential relevance between lens-imposed risk for angle closure and the role of the lens on IOP decrease after cataract surgery.
In our study, AL and ACD were associated with IOP drop in univariate analysis, but when considering other parameters such as AV and rLV in multivariate analysis, they were not significant factors. This is consistent with the results of Yang et al14 who found ACD and its change as significant predictors of IOP reduction only in univariate but not multivariate analysis. Huang et al9 also did not find any significant relationship between ACD deepening and IOP change after cataract surgery. Similarly, these studies did not show any correlations between IOP reduction and AL in multivariate analysis.9, 14 The reason may be that AL is a surrogate for the narrowness of the angle.
Cataract surgery has been shown to reduce IOP more in NAs compared with OAs.2 Huang et al9 reported an almost twofold greater IOP reduction in NA compared with OA eyes 6 months after surgery. Similarly, in our patients the mean IOP reduction was significantly more in the NA group compared with the OA group after surgery.
It should be noted that the present study also has some limitations. First, a 3-month follow-up period may not be sufficient for evaluating the long-term effect on IOP and the relationship with the ocular parameters. A longer follow-up duration would provide a more valuable assessment of the effects of phacoemulsification. However, it has been shown that the IOP decrease after cataract surgery did not show significant changes throughout a 6-month postoperative period.9 Second, all the patients enrolled in our study were Iranian, and it is not known whether these findings could be generalized to patients of other ethnicities, particularly those in Asia where ACG is prevalent. Finally, we had a relatively small sample size. Having a larger sample size in each subgroup might provide more valuable evaluation of factors influencing IOP reduction after cataract surgery.
In summary, in our study we found that cataract surgery results in significant IOP reduction in both OA and NA eyes. Furthermore, we have found that IOP reduction was associated with smaller AV, which could be used to help potentially predict IOP response after cataract surgery.
Acknowledgments
We thank Mrs Nasim Khatibi for her assistance in gathering data.
The authors declare no conflict of interest.
References
- Eslami Y, Latifi G, Moghimi S, Ghaffari R, Fakhraie G, Zarei R et al. Effect of adjunctive viscogonioplasty on drainage angle status in cataract surgery: a randomized clinical trial. Clin Experiment Ophthalmol 2013; 41: 368–378. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Hayashi H, Nakao F, Hayashi F. Effect of cataract surgery on intraocular pressure control in glaucoma patients. J Cataract Refract Surg 2001; 27: 1779–1786. [DOI] [PubMed] [Google Scholar]
- Huang G, Gonzalez E, Lee R, Chen YC, He M, Lin SC. Association of biometric factors with anterior chamber angle widening and intraocular pressure reduction after uneventful phacoemulsification for cataract. J Cataract Refract Surg 2012; 38: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poley BJ, Lindstrom RL, Samuelson TW, Schulze R Jr. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg 2009; 35: 1946–1955. [DOI] [PubMed] [Google Scholar]
- Shin HC, Subrayan V, Tajunisah I. Changes in anterior chamber depth and intraocular pressure after phacoemulsification in eyes with occludable angles. J Cataract Refract Surg 2010; 36: 1289–1295. [DOI] [PubMed] [Google Scholar]
- Shingleton BJ, Pasternack JJ, Hung JW, O'Donoghue MW. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma 2006; 15: 494–498. [DOI] [PubMed] [Google Scholar]
- Shrivastava A, Singh K. The effect of cataract extraction on intraocular pressure. Curr Opin Ophthalmol 2010; 21: 118–122. [DOI] [PubMed] [Google Scholar]
- Slabaugh MA, Chen PP. The effect of cataract extraction on intraocular pressure. Curr Opin Ophthalmol 2014; 25: 122–126. [DOI] [PubMed] [Google Scholar]
- Huang G, Gonzalez E, Peng PH, Lee R, Leeungurasatien T, He M et al. Anterior chamber depth, iridocorneal angle width, and intraocular pressure changes after phacoemulsification: narrow vs open iridocorneal angles. Arch Ophthalmol 2011; 129: 1283–1290. [DOI] [PubMed] [Google Scholar]
- Cimetta DJ, Cimetta AC. Intraocular pressure changes after clear corneal phacoemulsification in nonglaucomatous pseudoexfoliation syndrome. Eur J Ophthalmol 2008; 18: 77–81. [DOI] [PubMed] [Google Scholar]
- Issa SA, Pacheco J, Mahmood U, Nolan J, Beatty S. A novel index for predicting intraocular pressure reduction following cataract surgery. Br J Ophthalmol 2005; 89: 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K, Kashiwagi F, Tsukahara S. Effects of small-incision phacoemulsification and intraocular lens implantation on anterior chamber depth and intraocular pressure. J Glaucoma 2006; 15: 103–109. [DOI] [PubMed] [Google Scholar]
- Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg 2008; 34: 735–742. [DOI] [PubMed] [Google Scholar]
- Yang HS, Lee J, Choi S. Ocular biometric parameters associated with intraocular pressure reduction after cataract surgery in normal eyes. Am J Ophthalmol 2013; 156: 89–94 e1. [DOI] [PubMed] [Google Scholar]
- Saxena S, Agrawal PK, Pratap VB, Nath R, Saxena RC. The predictive value of the relative lens position in primary angle-closure glaucoma. Ann Ophthalmol 1993; 25: 453–456. [PubMed] [Google Scholar]
- Marchini G, Pagliarusco A, Toscano A, Tosi R, Brunelli C, Bonomi L. Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle-closure glaucoma. Ophthalmology 1998; 105: 2091–2098. [DOI] [PubMed] [Google Scholar]
- Salmon JF, Swanevelder SA, Donald MA. The dimensions of eyes with chronic angle-closure glaucoma. J Glaucoma 1994; 3: 237–243. [PubMed] [Google Scholar]
- Sihota R, Ghate D, Mohan S, Gupta V, Pandey RM, Dada T. Study of biometric parameters in family members of primary angle closure glaucoma patients. Eye 2008; 22: 521–527. [DOI] [PubMed] [Google Scholar]
- Nongpiur ME, He M, Amerasinghe N, Friedman DS, Tay WT, Baskaran M et al. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology 2011; 118: 474–479. [DOI] [PubMed] [Google Scholar]
- Kim YK, Yoo BW, Kim HC, Aung T, Park KH. Relative lens vault in subjects with angle closure. BMC Ophthalmol 2014; 14: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Console JW, Sakata LM, Aung T, Friedman DS, He M. Quantitative analysis of anterior segment optical coherence tomography images: the Zhongshan Angle Assessment Program. Br J Ophthalmol 2008; 92: 1612–1616. [DOI] [PubMed] [Google Scholar]
- Aung T, Nolan WP, Machin D, Seah SK, Baasanhu J, Khaw PT et al. Anterior chamber depth and the risk of primary angle closure in 2 East Asian populations. Arch Ophthalmol 2005; 123: 527–532. [DOI] [PubMed] [Google Scholar]
- Lavanya R, Wong TY, Friedman DS, Aung HT, Alfred T, Gao H et al. Determinants of angle closure in older Singaporeans. Arch Ophthalmol 2008; 126: 686–691. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Nongpiur ME, Aung T, He M, Mizoguchi T. Increased lens vault as a risk factor for angle closure: confirmation in a Japanese population. Graefes Arch Clin Exp Ophthalmol 2012; 250: 1863–1868. [DOI] [PubMed] [Google Scholar]
- Latifi G, Moghimi S, Eslami Y, Fakhraie G, Zarei R, Lin S. Effect of phacoemulsification on drainage angle status in angle closure eyes with or without extensive peripheral anterior synechiae. Eur J Ophthalmol 2012; e-pub ahead of print 3 August 2012; doi: 10.5301/ejo.5000191. [DOI] [PubMed]
- Tan GS, He M, Zhao W, Sakata LM, Li J, Nongpiur ME et al. Determinants of lens vault and association with narrow angles in patients from Singapore. Am J Ophthalmol 2012; 154: 39–46. [DOI] [PubMed] [Google Scholar]
- Wang N, Chintala SK, Fini ME, Schuman JS. Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response: a potential mechanism for intraocular pressure reduction after phacoemulsification. Invest Ophthalmol Vis Sci 2003; 44: 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalone N, Hyams M, Neiman S, Buckman G, Hod Y, Geyer O. Long-term intraocular pressure control after clear corneal phacoemulsification in glaucoma patients. J Cataract Refract Surg 2005; 31: 479–483. [DOI] [PubMed] [Google Scholar]