Abstract
Purpose
To report the outcomes of combined phacoemulsification and -deep sclerectomy (phaco-DS) from a single UK centre over a 10-year period.
Methods
Retrospective analysis of phaco-DS data extracted from an ongoing glaucoma surgery database within Calderdale and Huddersfield NHS Trust. Two hundred and ninety-six eyes of 282 patients were included. Data included patient demographics, pre- and postoperative intraocular pressure (IOP), use of mitomycin C (MMC), spacer device implantation, and follow-up details including surgical success rates. IOP success criteria were: (A) IOP <19 mm Hg and/or 20% decrease from baseline and (B) IOP <16 mm Hg and/or 30% drop from baseline.
Results
Mean follow-up was 63.5±35.3 months. MMC was applied in 145 eyes (49%). Kaplan–Meier success rates in all eyes for criteria A were 89.1% and 80% with glaucoma medications (qualified success) and 81.2% and 68.3% without medications (unqualified success) at 2 and 5 years, respectively. Qualified success for criteria B was 72.4 and 61.4% and unqualified rates were 67.2 and 55.2% for the same time periods. Repeated-measures ANOVA showed significantly lower IOP in the phaco-DS with MMC group up to 3 years postoperatively (P=0.002). Cox's proportional hazards for criteria B, however, showed no significant effect of MMC application in the long term (P=0.2). Increasing age and laser goniopuncture were positively associated with success, whereas the absence of spacer devices was negatively associated. At last follow-up, 20% of eyes were on glaucoma medications. Complication rates were low with hypotony rates of 0.68%.
Conclusions
This study confirms the long-term safety and efficacy of phaco-DS as a primary glaucoma procedure.
Introduction
Non-penetrating glaucoma surgery (NPGS) such as viscocanalostomy and deep sclerectomy (DS) were introduced in the 1990s as safer alternatives to trabeculectomy.1, 2 The essential difference between NPGS and trabeculectomy is that the procedure entails the creation of a filtration membrane, the trabeculo-Descemet's membrane, rather than a sclerostomy, with excision of the inner scleral flap creating a subscleral lake. Different outflow pathways have been proposed for NPGS including increased aqueous flow through Schlemm's canal, collection into an ‘intrascleral bleb', suprachoroidal drainage, and subconjunctival flow with bleb formation.3, 4, 5, 6, 7, 8, 9 Papers using modern technology such as ultrasound biomicroscopy and optical coherence tomography have now clearly demonstrated the presence of an 'intrascleral 'bleb' and 'subconjunctival flow'.10, 11
IOP lowering with DS and postoperative laser goniopuncture (LGP) has been shown to be comparable to trabeculectomy with a lower incidence of complications in the immediate postoperative period according to some reports.12, 13, 14, 15 In the presence of significant cataract with glaucoma, combined phacoemulsification with trabeculectomy is commonly performed.16 Phacoemulsification combined with DS (phaco-DS) has been shown to be as effective as phacoemulsification with trabeculectomy (phaco-trab) in lowering IOP.14, 17 In this large case series, we seek to report the outcomes of phaco-DS from a single UK centre over a 10-year period. To our knowledge, this is the largest cohort to describe this procedure with such long-term follow-up results.
Materials and methods
This study is a retrospective, comparative, non-randomised case series. Consecutive patients undergoing phaco-DS between August 2001 and March 2008 were identified from a correlational ongoing glaucoma surgery database (Microsoft Access 2010, Microsoft Corporation, Redmond, WA, USA). Data entry was completed at the time of surgery and contemporaneously at each postoperative visit. Three hundred and seventy-three eyes of 326 patients undergoing surgery were identified and 296 eyes of 282 patients were included in the study. Eyes with previous conjunctival or glaucoma surgery were excluded as were patients with a history of uveitis in either eye. Patients with <12 months follow-up were also excluded as were the fellow eyes of people undergoing eventual bilateral surgery. In the latter however, if one eye had antimetabolite enhancement and the other none, both eyes were included for analysis. The excluded eyes consisted of 32 fellow eyes, 23 eyes with follow-up <12 months, 15 eyes with prior trabeculectomy, one eye with previous DS, four uveitic eyes, one eye with ocular pemphigoid, and one with previous multiple Lucentis injections.
Data extracted from the database included patient demographics, Snellen visual acuity (VA), pre- and postoperative intraocular pressure (IOP), use of mitomycin C (MMC), spacer device implantation, postoperative complications, subsequent procedures including reoperation for glaucoma, and the use of supplemental medical therapy.
All procedures were performed or supervised closely by one consultant glaucoma surgeon (NA) using a standardised technique as described previously,18 with a few subsequent modifications that are detailed below. In brief, a standard DS was followed by a temporal clear corneal phacoemulsification. A 6-0 vicryl traction suture was used to infraduct the globe and a fornix-based conjunctival flap was fashioned. MMC was applied at a dose of 0.2 mg/ml for 2 min on four PVA sponge fragments placed under the conjunctival flap. A limbal-based 5 × 4 mm2 superficial scleral flap was created to ~1/3 scleral depth and reflected 1 mm into the clear cornea. Within the bed of the superficial flap, a 90% depth scleral flap was fashioned and the ends of Schlemm's canal cut. Dissection of the deep scleral flap was continued into clear cornea to expose a trabeculodescemetic membrane (TDM) of around 3–4 mm width. Juxtacanalicular trabecular meshwork was peeled using blunt-tipped capsulorhexis forceps. The deep scleral flap was then excised. Cases performed over the latter years involved a linear full-thickness scleral incision at the apex of the scleral lake and implantation of an HEMA spacer device (Esnoper V-2000; AJL Ophthalmics, Álava, Spain) into the suprachoroidal space, which was then anchored with one nylon 10-0 suture. Earlier cases involved the use of different spacer devices or viscoelastic to aid maintenance of the intrascleral lake (details are provided in Tables 1a and b).
Table 1a. Pre- and perioperative data.
| Pre- and perioperative data | Total | PDS | PDS-MMC | P-value |
|---|---|---|---|---|
| Number of eyes | 296 | 151 | 145 | |
| Age (years) | 80.3±6.6 | 81.05±6.1 | 79.0±6.8 | 0.0007 |
| Male/ Female | 128/168 | 52/99 | 79/66 | 0.0006 |
| Race | ||||
| Caucasian | 284 | 148 | 136 | |
| Indian | 06 | 02 | 04 | |
| African | 06 | 01 | 05 | |
| Preoperative glaucoma diagnoses | 0.32 | |||
| Primary open- angle glaucoma | 242 | 119 | 122 | |
| Normotensive glaucoma | 25 | 12 | 13 | |
| Pseudoexfoliation | 24 | 08 | 16 | |
| Pigmentary | 04 | 02 | 02 | |
| Primary angle closure glaucoma | 12 | 10 | 02 | |
| Mean follow-up (months) | 63.5±35.3 | 57.9±34.2 | 69.7±39.1 | 0.003 |
| Preoperative glaucoma medications | 2.1±1.6 | 1.9±1.0 | 2.4±2.0 | 0.13 |
| CD ratio | 0.84±0.1 | 0.8±0.1 | 0.85±0.1 | 0.13 |
| VF mean deviation (dB) | −13.4±8.0 | −11.9±7.7* (57 patients) | −14.9±8.0* (54 patients) | 0.04 |
| Intraoperative perforations | 38 | 19 | 19 | 1.0 |
| Spacer device | 0.0008 | |||
| High-molecular- weight viscoelastic | 77 | 52 | 25 | |
| SK Gel | 168 | 64 | 104 | |
| Aqua Flow | 37 | 23 | 14 | |
| Esnoper | 13 | 12 | 01 | |
| Healaflow | 01 | 00 | 01 | |
Table 1b. Snellen VA loss.
| Total | PDS | PDS-MMC | |
|---|---|---|---|
| VA loss >2 lines | 17 | 09 | 08 |
| ARMD | 09 | 05 | 04 |
| CRVO | 01 | 01 | 00 |
| Dementia (unable to reliably test refraction or VA) | 02 | 02 | 00 |
| Progressive retinitis pigmentosa | 01 | 00 | 01 |
| Advanced POAG | 02 | 00 | 02 |
| Cause unclear | 02 | 01 | 01 |
Abbreviations: CD, cup-disc; PDS, phaco-DS; PDS-MMC, phaco-DS+MMC.
The outer scleral flap and conjunctiva were then closed with nylon 10-0 interrupted sutures. If a microperforation occurred without iris prolapse, the procedure was continued as described above. If a macroperforation occurred, then a peripheral iridectomy was made in the presenting iris and the superficial scleral flap tied down more tightly. Postoperatively patients received prednisolone acetate 1% drops two hourly continued for a minimum of 8 weeks. Patients were initially followed up at weeks 1 and 5 or more frequently if required. Thereafter, follow-up was determined by clinical need. Where IOP exceeded the target IOP range, Nd:Yag LGP was performed with a Magna View contact gonioscopy lens (Ocular Instruments, Bellevue, WA, USA). Needle revision with 5-fluorouracil (5-FU) or MMC was performed if IOP was subsequently still elevated. Argon and Nd:YAG iridoplasty were performed either prophylactically to avoid iris prolapse into the goniopuncture or to remove incarcerated iris within it. These postoperative interventions were recorded contemporaneously as part of data collection. Detailed techniques for LGP and iridoplasty have already been described in a previous publication.19
Complete (unqualified) success criteria were defined as follows: (A) IOP <19 mm Hg and/or 20% decrease from baseline off any glaucoma medications. Failure in this group was defined as IOP=>19 mm Hg or IOP not reduced by 20% of preoperative IOP or IOP <6 mm Hg on two consecutive time points after 3 months; (B) IOP <16 mm Hg and/or 30% drop from baseline off any glaucoma medications. Failure in this group was defined as IOP=>16 mm Hg or IOP not reduced by 30% of preoperative IOP or IOP <6 mm Hg on two consecutive time points after 3 months. Partial (qualified) success was defined as any of the above but with at least one topical IOP-lowering medication. Surgical revision for high IOP and loss of light perception were considered a failure by all criteria. Needle revision and LGP were not considered as failures.The time to failure was defined either as the time from surgical treatment to reoperation for glaucoma or as the time from surgery to the first visit in which the patient had hypotony (IOP <6 mm Hg) or inadequately reduced IOP as per success criteria defined above. If a patient had an unsuccessful LGP or needle revision, failure was considered to have occurred on the visit when the decision to do these procedures was taken.
Reoperation for glaucoma or a complication was defined as additional surgery requiring a return to the operating theatre. Serious complications were defined as surgical complications associated with loss of two or more lines of Snellen VA for >6 months and/or reoperation to manage the complication. Eyes that tested Seidel positive within the first month of follow-up were classified as wound leaks, whereas those occurring after 1 month were categorised as bleb leaks. Patients who underwent additional surgery were censored from analysis of complications after the reoperation for glaucoma.
Statistical analysis
MedCalc (MedCalc Software, Ostend, Belgium) was used for statistical analysis. IOP changes over time and comparison of IOPs between groups was carried out by analysis of variance (ANOVA) (repeated-measures ANOVA). Non-parametric data such as VA and glaucoma medication changes were analysed by Friedmans ANOVA and Kruskal–Wallis test. Treatment comparisons of time to failure were assessed with Kaplan–Meier survival analysis, univariate log-rank test and Cox's regression analyses. All tests were two-tailed, and P-values <0.05 were taken to be significant. Categorical data was analysed by χ2 test with Yates correction and Fisher's exact test.
Results
Patient demographics and pre- and perioperative characteristics including use and type of spacer device and incidence of intraoperative perforations are shown in Table 1a. Two patients had both eyes included in the study as they had phaco-DS in one eye and phaco-DS with MMC in the other. The mean postoperative follow-up was 63.5±35.3 months. MMC was applied in 145 out of 296 eyes (49%) with no significant difference in mean age between those receiving or not receiving an antimetabolite (P=0.0007). There was a preponderance of female patients in the non-MMC group (P=0.0006). The vast majority of patients were of white Caucasian ethnicity and similarly most patients had a diagnosis of primary open-angle glaucoma (POAG). The MMC group had a higher number of mean preoperative glaucoma drops, but this was not statistically significant. The intraoperative perforation rate was 13% (38/296 eyes). Macroperforation with iris prolapse occurred in 14/38 cases. The most common spacer device used was a reticulated hyaluronic acid implant (SK Gel; Corneal Inc., Paris, France). High-molecular-weight viscoelastic (Healon GV) was used before the adoption of spacer utilisation.
Figure 1a summarises the VA changes over a 5-year period and shows that VA improved significantly after phaco-DS (P<0.001). Best-corrected VA was significantly better at year 1 (P=0.02) and year 5 (P=0.04) in the MMC group. However, a repeated-measures ANOVA with log transformation of decimalised Snellen VA showed no significant difference in completed cases until 2 years postoperatively (P=0.06). Table 1b describes any Snellen VA loss of more than two lines by cause and group.
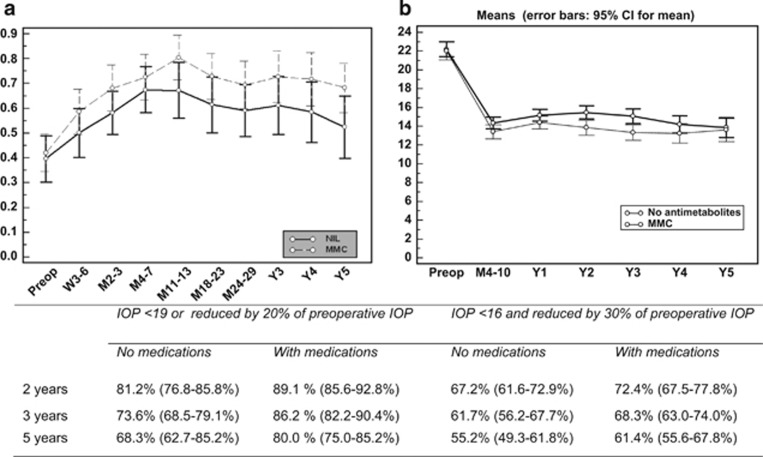
Figure 1.
(a) Mean decimalised VA changes (pre-op, preoperative VA; W, week; M, month; Y, year; error bars—95% confidence intervals). (b) Mean IOP (mm Hg) changes after phaco-DS with 95% confidence intervals over a 5-year follow-up period. Qualified and unqualified success rates for both sets of defined criteria at 2, 3, and 5 years are summarised in the table (bottom).
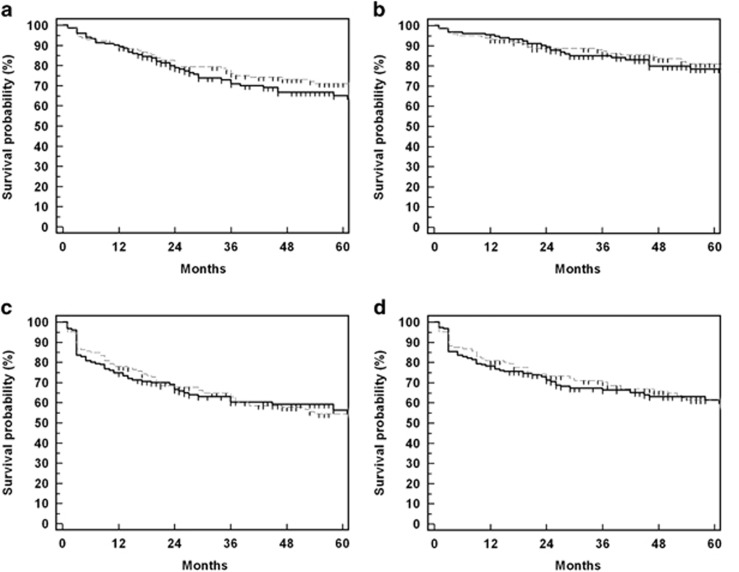
Figures 1b and 2 detail the success rates and survival curves for both augmented and non-augmented groups at different time intervals for the criteria defined above. Success rates in all eyes for criteria A were 89.1% and 80% with glaucoma medications (qualified success) and 81.2% and 68.3% without medications (unqualified success) at 2 and 5 years, respectively. Qualified success for criteria B was 72.4 and 61.4% and unqualified rates were 67.2 and 55.2% for the same time periods. IOP changes after surgery. Repeated-measures ANOVA for 217 eyes, which had completed 3 years of follow-up showed significantly lower IOP in the phaco-DS with MMC group (P=0.002). A follow-up of 5 years was completed in 117 eyes and the mean IOPs were significantly lower in the MMC group (P=0.05). Cox's proportional hazards for criteria B, however, showed no significant effect of MMC application in the long term (P=0.2). Increasing age and LGP were positively associated while the absence of spacer devices was negatively associated with success (Table 2).
Figure 2.
Kaplan–Meier curves for drainage procedure survival probability after phaco-DS (solid lines, phaco-DS with MMC; broken lines, phaco-DS without MMC). (a) IOP <19 mm Hg without medications; (b) IOP <19 mm Hg with medications; (c) IOP <16 mm Hg without medications; (d) IOP <16 mm Hg with medications.
Table 2. Cox proportional hazards model for covariate analysis.
| Covariate | Hazard ratio | 95% CI | Multivariate P-value | Univariate P-value log-rank test |
|---|---|---|---|---|
| Increasing age | 0.96 | 0.93–0.99 | 0.02 | |
| Male sex | 1.25 | 0.8448–1.84 | 0.26 | 0.14 |
| No spacer device | 1.67 | 1.07–2.58 | 0.02 | 0.15 |
| Laser goniopuncture | 0.28 | 0.17–0.45 | <0.0001 | 0.048 |
| Intraoperative perforation | 0.54 | 0.27–1.04 | 0.07 | 0.15 |
| Intraoperative MMC | 0.79 | 0.53–1.19 | 0.26 | 0.74 |
| Needle revision | 1.16 | 0.74–1.88 | 0.51 | 0.006 |
Deaths were checked from the hospital database (PASWEB). One hundred and twenty-nine patients (45.7%) died during the observation period, 65 from the non-augmented group and 62 from the MMC group. At the last follow-up visit, 29 eyes (20%) of the MMC group and 30 eyes (19.9%) of the non-augmented group were on medications to control IOP (P=1.0). Mean number of topical medications at last follow-up were 0.33±0.74 in the MMC and 0.33±0.73 in the no MMC group (P=0.9). The augmented phaco-DS group eyes had a significantly higher chance of undergoing LGP (75.7%) compared with the phaco-DS-only group (51.4%) within 5 years of surgery (P=0.05).
Table 3 describes surgical complications and subsequent additional procedures in detail. Complications were observed in 33 eyes (21.8%) of the phaco-DS and 48 eyes (33.1%) of the phaco-DS with MMC group (P=0.04). There was only one serious complication: delayed suprachoroidal haemorrhage was observed in an elderly male patient 1 week after phaco-DS MMC and intraoperative perforation. The haemorrhage was successfully drained through two inferior sclerotomies a month after phaco-DS. Choroidal detachments were observed in two eyes after phaco-DS with intraoperative perforation and in three eyes each following needle revision and LGP. Two patients developed macular folds with slight blurring of vision at 14 months and 4 years, respectively, after uncomplicated phaco-DS with MMC; resuturing of the scleral flap resulted in resolution of both hypotony and maculopathy. Hypotony was observed in six eyes (2.0%) and occurred after LGP or needle revision in four cases. No surgical intervention was required as the patients were asymptomatic and no maculopathy was noted. There were six wound leaks (2.0%), three in each group. Hyphema was uncommon (1.3%) and no delayed bleb leaks were observed in either group. No bleb-related infection or endophthalmitis was observed. The posterior capsule rupture rate was 3% (9/296 eyes).
Table 3. Surgical complications and subsequent procedures.
| Surgical complications | PDS–no MMC (total 151) | PDS-MMC (total 141) |
|---|---|---|
| Intraoperative | ||
| Conjunctival flap button-hole | 03 | 02 |
| Capsule zonular dialysis | 02 | 01 |
| Posterior capsular rupture | 05 | 04 |
| Nucleus fragments in vitreous | 02 | 00 |
| Postoperative | ||
| Shallow AC | 03 | 02 |
| Conjunctival wound edge leaks | 03 | 03 |
| Corneal oedema | 03 | 00 |
| Fibrin in anterior chamber | 02 | 00 |
| Hyphema | 01 | 03 |
| Nucleus fragments in anterior chamber | 01 | 00 |
| IOL haptic anterior to iris | 01 | 00 |
| Vitreous in anterior chamber | 00 | 01 |
| Delayed suprachoroidal haemorrhage | 00 | 01 |
| Hypotony | ||
| After surgery | 00 | 02 |
| After LGP | 00 | 03 |
| After needle revision | 01 | 00 |
| Iris synechiae at TDM window | 13 | 18 |
| Iris incarceration into goniopuncture | 00 | 01 |
| Choroidal detachment | 03 | 05 |
| After surgery | 02 | 00 |
| After needle revision | 01 | 02 |
| After laser goniopuncture | 00 | 03 |
| Acute IOP rise >30 mm Hg | 01 | 04 |
| Malignant glaucoma | 00 | 01 |
| Bleb dyaesthesia | 00 | 01 |
| IOL subluxation | 00 | 01 |
| Anterior capsular phimosis | 02 | 00 |
| Capsular block syndrome | 01 | 02 |
| Corneal decompensation | 00 | 01 |
| Subsequent procedures | ||
| Laser goniopuncture | 71 | 90 |
| Laser iridoplasty | 10 | 14 |
| Number of eyes undergoing needle revision | 25 | 31 |
| Total number of needle revisions | 32 | 44 |
| MMC | 17 | 36 |
| 5-FU | 11 | 07 |
| Bevacizumab | 04 | 01 |
| Success (20% IOP drop with same or less medications until last follow-up) | 12/25 eyes | 12/31 eyes |
| Laser capsulotomy | 05 | 01 |
| Selective laser trabeculoplasty | 01 | 00 |
| Scleral flap resuturing | 00 | 01 |
| Revision of DS | 00 | 01 |
| DS with bevacizumab | 00 | 01 |
| Trabeculectomy with MMC | 00 | 01 |
| Sclerotomy for choroidal drainage | 01 | 01 |
| Repositioning of IOL | 01 | 00 |
Discussion
There is a lack of consensus among glaucoma specialists as to the best surgical option for patients needing both cataract and glaucoma surgery. It is generally felt that phacoemulsification before drainage surgery is preferable to avoid the potential for increased scarring and failure caused by postoperative inflammation. For patients with significant cataract who need urgent drainage surgery, many glaucoma surgeons would advocate a combined procedure.20, 21 Traditionally phaco-trab has been shown to be effective albeit slightly less successful for such patients, with a higher incidence of bleb failure and complications in the long term.22 Several reports of NPGS describe combined cataract and glaucoma surgery with comparable or even better IOP-lowering outcomes.14, 17 To our knowledge, this case series represents the largest cohort with the longest follow-up of phaco-DS in the literature.
This study confirms the results of previous publications showing that phaco-DS is an effective glaucoma procedure both in terms of lowering IOP and in reducing glaucoma drop requirement.17, 18, 23 The 67.2 and 55.2% unqualified success rates for reducing IOP below 16 mm Hg at 2 and 5 years compares very favourably with the National Survey of Trabeculectomy results for trabeculectomy alone, which reported unqualified outcomes of 64% IOP <16 mm Hg as early as 1 year postoperatively.24 A much more recent multicentre audit of trabeculectomies performed by UK glaucoma surgeons reported an IOP of <19 and 16 mm Hg at 2 years without glaucoma medications in 74% and 68% of eyes, respectively.25 Our study success rates using the same criteria were highly comparable at 79% and 67%, respectively. In a comparative study, Gianoli et al17 reported success rates of 59% for phaco-DS and 52% for phaco-trab at 1 year, with no differences in the magnitude of IOP drop between the two procedures. In the same study, eyes with phaco-DS had significantly lower rates of hyphema and anterior chamber inflammation.17 However, our group has previously reported no differences in efficacy or complications between the two procedures.18 Wishart et al26 reported rates of 94% for phaco-DS at 3 years. The latter group, however, defined success as IOP of 21 mm Hg or less without medication. This level of IOP control may not be sufficient to control progression in eyes with advanced glaucoma.27
The slightly higher rate of complications in eyes receiving antimetabolite does not appear to be of clear clinical significance. For example, four eyes of the MMC group had an IOP >30 mm Hg on the first postoperative day. This was either related to viscoelastic left in the anterior chamber or inadequate drainage through the TDM rather than the specific use of MMC. The rate of LGP was higher in the MMC group and contributed to the increase in complications. One of the postulated advantages of NPGS is the lower risk of hypotony and infection related to the conjunctival bleb. However, success in DS has been correlated with the presence of a subconjunctival filtration bleb and a subscleral lake. MMC improves the efficacy of DS, as in trabeculectomy, by decreasing outflow resistance in the subconjunctival tissues and LGP may convert the DS to a full-thickness fistula, thus increasing the probability of hypotony in the long term.28 In the presence of an avascular bleb, LGP and needle revision may similarly increase the risk of bleb-related infections.28 Hypotony was observed in 3% of our cohort, which also compares favourably with the hypotonyrate of 7.2% reported by the aforementioned paper by Kirwan et al.25 Over a mean follow-up period of 63.5 months (in 296 eyes), we only observed three serious complications related to hypotony: two eyes required resuturing, whereas one resulted in delayed suprachoroidal haemorrhage. There were no bleb leaks and bleb-related infections.
LGP is considered an important adjunct to DS in achieving low target IOPs. Reported rates vary from 36 to 67%19, 28, 29, 30, 31 The overall rate of LGP in our cohort was 54.4%. Patients in the phaco-DS with MMC group had a significantly greater chance of undergoing LGP (75.7%) compared with the unaugmented phaco-DS group (51.4%) at 5 years after surgery (P=0.05). The difference was only marginally significant and perhaps could be attributed to the lower postoperative target IOPs for the MMC group. LGP, along with increasing age, was positively associated with success, whereas the absence of a spacer device was negatively associated. However, these results should be interpreted with caution as the hazards ratios for LGP and spacer devices had wide confidence intervals (as detailed in Table 2). The association of success with increasing age may possibly be related to a poorer healing response and to increased mortality reducing the length of follow-up.
The role of MMC in increasing IOP success rates after primary trabeculectomy is now well established.32, 33 There is also evidence for a similar role of MMC in primary DS.34, 35, 36, 37, 38, 39 The decision to use MMC during individual surgical cases was difficult to pinpoint from our database. Patients in the MMC group were slightly younger and probably had worse visual field (VF) mean deviation. Unfortunately, VF data was incomplete as it was not collected for earlier procedures (see Tables 1a and 1b). In some cases, the decision not to use MMC was taken during surgery if, for example, the subconjunctival tissues were thin and fragile. The mean postoperative IOP was significantly lower in the phaco-DS with MMC group up to 5 years postoperatively. However, univariate and multivariate actuarial analyses failed to show a significant effect of MMC application (P=0.2).This discrepancy may be because of the confounding effect of the large proportion of censored data related to patients passing away during the follow-up period.
As alluded to above, nearly half (45.7%) of patients in our series had died by their last follow-up period. This may reflect the increased population age of patients undergoing phaco-DS with a mean of 80.3±6.6 years. Similar death rates within comparable age groups undergoing NPGS have been highlighted before, particularly in combined surgery where the average age also appears to be higher compared with NPGS alone.26 The improvement in VA and the excellent 5-year IOP success rates make combined phaco-DS an attractive option for elderly patients who need glaucoma surgery. These patients also benefit from a significantly reduced need for drop application and a much less demanding postoperative follow-up regimen with decreased hospital visits. The higher proportion of female patients, with their increased life expectancy, may also reflect the advanced patient age noted in our study.
This study confirms the long-term safety and efficacy of phaco-DS as a primary procedure for coexisting cataract and glaucoma. It demonstrates significant IOP lowering outcomes, which are comparable to phaco-trab while also highlighting low complication rates. In the current glaucoma milieu, one must also consider the alternative of ‘microincisional' glaucoma surgery, such as trabecular microbypass stents (eg iStent; Glaukos Corporation, Laguna Hills, CA, USA) and ab interno trabeculectomy (Trabectome; NeoMedix Inc., Tustin, CA, USA), combined with cataract surgery. These procedures have the attraction of an excellent safety profile albeit less overall effective IOP reduction when compared with phaco-DS in our study.22, 40, 41, 42, 43, 44 Although the glaucoma community must watch and wait for the long-term results of these procedures, phaco-DS currently represents a safe and effective means of combining cataract and glaucoma surgery in the present, with a proven long-term track record.

The authors declare no conflict of interest.
Footnotes
The results of this study were presented at the UKEGS congress in Bristol, UK, November 2014.
References
- Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg 1999; 25: 316–322. [DOI] [PubMed] [Google Scholar]
- Sanchez E, Schnyder CC, Sickenberg M, Chiou AG, Hediguer SE, Mermoud A. Deep sclerectomy: results with and without collagen implant. Int Ophthalmol 1996; 20: 157–162. [DOI] [PubMed] [Google Scholar]
- Lei J, Sun N, Zhao X, Kang Q, Chen L, Fan X. Morphologic study of the drainage pathway using a tracer after a bypass filtering procedure in rabbit eyes. Ophthalmic Surg Lasers Imaging 2011; 42: 254–262. [DOI] [PubMed] [Google Scholar]
- Xu W, Yao K, Wu W, Li Z, Ye P. Change in outflow pathway of porcine eyes in vitro by nonpenetrating filtering surgery. Can J Ophthalmol 2010; 45: 632–636. [DOI] [PubMed] [Google Scholar]
- Aptel F, Dumas S, Denis P. Ultrasound biomicroscopy and optical coherence tomography imaging of filtering blebs after deep sclerectomy with new collagen implant. Eur J Ophthalmol 2009; 19: 223–230. [DOI] [PubMed] [Google Scholar]
- Delarive T, Rossier A, Rossier S, Ravinet E, Shaarawy T, Mermoud A. Aqueous dynamic and histological findings after deep sclerectomy with collagen implant in an animal model. Br J Ophthalmol 2003; 87: 1340–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DH, Johnson M. How does nonpenetrating glaucoma surgery work? Aqueous outflow resistance and glaucoma surgery. J Glaucoma 2001; 10: 55–67. [DOI] [PubMed] [Google Scholar]
- Roters S, Luke C, Jonescu-Cuypers CP, Engels BF, Jacobi PC, Konen W et al. Ultrasound biomicroscopy and its value in predicting the long term outcome of viscocanalostomy. Br J Ophthalmol 2002; 86: 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakova D, Roters S, Schnyder CC, Achache F, Jonescu-Cuypers C, Mermoud et al. Ultrasound biomicroscopy images: long-term results after deep sclerectomy with collagen implant. Graefes Arch Clin Exp Ophthalmol 2002; 240: 918–923. [DOI] [PubMed] [Google Scholar]
- Khairy HA, Atta HR, Green FD, van der Hoek J, Azuara-Blanco A. Ultrasound biomicroscopy in deep sclerectomy. Eye 2005; 19: 555–560. [DOI] [PubMed] [Google Scholar]
- Konstantopoulos A, Yadegarfar ME, Yadegarfar G, Stinghe A, Macleod A, Jacob et al. Deep sclerectomy versus trabeculectomy: a morphological study with anterior segment optical coherence tomography. Br J Ophthalmol 2013; 97: 708–714. [DOI] [PubMed] [Google Scholar]
- Ambresin A, Shaarawy T, Mermoud A. Deep sclerectomy with collagen implant in one eye compared with trabeculectomy in the other eye of the same patient. J Glaucoma 2002; 11: 214–220. [DOI] [PubMed] [Google Scholar]
- Mermoud A, Schnyder CC. Non-penetrating filtering surgery in glaucoma. Curr Opin Ophthalmol 2000; 11: 151–157. [DOI] [PubMed] [Google Scholar]
- Cillino S, Pace FD, Casuccio A, Calvaruso L, Morreale D, Vadalà M et al. Deep sclerectomy vs punch trabeculectomy with or without phacoemulsification: a randomized clinical trial. J Glaucoma 2004; 13: 500–506. [DOI] [PubMed] [Google Scholar]
- El Sayyad F, Helal M, El Kholify H, Khalil M, El-Maghraby A. Non-penetrating deep sclerectomy vs trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology 2000; 107: 1671–1674. [DOI] [PubMed] [Google Scholar]
- Friedman DS, Jampel HD, Lubomski LH, Kempen JH, Quigley H, Congdon N et al. Surgical strategies for coexisting glaucoma and cataract: anevidence-based update. Ophthalmology 2002; 109: 1902–1913. [DOI] [PubMed] [Google Scholar]
- Gianoli F, Schnyder CC, Bovey E, Mermoud A. Combined surgery forcataract and glaucoma: phacoemulsification and deepsclerectomy compared with phacoemulsification and trabeculectomy. J Cataract Refract Surg 1999; 25: 340–346. [DOI] [PubMed] [Google Scholar]
- Funnell CL, Clowes M, Anand N. Combined cataract andglaucoma surgery with mitomycin C: phacoemulsification-trabeculectomy compared to phacoemulsification-deep sclerectomy. Br J Ophthalmol 2005; 89: 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand N, Pilling R. Nd:YAG laser goniopuncture after deep sclerectomy: outcomes. Acta Ophthalmol 2010; 88: 110–115. [DOI] [PubMed] [Google Scholar]
- Vass C, Menapace R. Surgical strategies in patients with combined cataract and glaucoma. Curr Opin Ophthalmol 2004; 15: 61–66. [DOI] [PubMed] [Google Scholar]
- Friedman DS, Jampel HD, Lubomski LH, Kempen JH, Quigley H, Congdon N et al. Surgical strategies for coexisting glaucoma and cataract: an evidence-based update. Ophthalmology 2002; 109: 1902–1913. [DOI] [PubMed] [Google Scholar]
- Augustinus CJ, Zeyen T. The effect of phacoemulsification and combined phaco/glaucoma procedures on the intraocular pressure in open-angle glaucoma. A review of the literature. Bull Soc Belge Ophtalmol 2012; (320): 51–66. [PubMed]
- Anand S, Anand N. Combined phacoemulsification and deep sclerectomy (PDS) with intraoperative mitomycin C (MMC) augmentation. Eye 2008; 22(8): 1040–1049. [DOI] [PubMed] [Google Scholar]
- Edmunds B, Thompson JR, Salmon JF, Wormald RP. The National Survey of Trabeculectomy. III. Early and late complications. Eye 2002; 16: 297–303. [DOI] [PubMed] [Google Scholar]
- Kirwan JF, Lockwood AJ, Shah P, Macleod A, Broadway DC, King AJ et alTrabeculectomy Outcomes Group Audit Study Group. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology 2013; 120(12): 2532–2539. [DOI] [PubMed] [Google Scholar]
- Wishart PK, Wishart MS, Porooshani H. Viscocanalostomy and deep sclerectomy for the surgical treatment of glaucoma: a long term follow-up. Acta Ophthalmol Scand 2003; 81(4): 343–348. [DOI] [PubMed] [Google Scholar]
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol 2000; 130: 429–440. [DOI] [PubMed] [Google Scholar]
- Anand N, Kumar A, Gupta A. Primary phakic deep sclerectomy augmented with mitomycin C: long-term outcomes. J Glaucoma 2011; 20: 21–27. [DOI] [PubMed] [Google Scholar]
- Loscos J, Valldeperas X, Langhor K, Parera À, Romera P, Sabala A et al. Deep sclerectomy with supraciliaryhema implant (Esnoper® V-2000): results and complications. Int Ophthalmol 2015; 35: 693–699. [DOI] [PubMed] [Google Scholar]
- Alp MN, Yarangumeli A, Koz OG, Kural G. Nd:YAG laser goniopuncture in viscocanalostomy: penetration in non-penetrating glaucoma surgery. Int Ophthalmol 2010; 30(3): 245–252. [DOI] [PubMed] [Google Scholar]
- Mendrinos E, Mansouri K, Mermoud A, Shaarawy T. Long-term results of deep sclerectomy with collagen implant in exfoliative glaucoma. J Glaucoma 2009; 18(5): 361–367. [DOI] [PubMed] [Google Scholar]
- Wilkins M, Indar A, Wormald R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev 2001; (1): CD002897. [DOI] [PubMed]
- Cohen JS, Greff LJ, Novack GD, Wind BE. A placebo-controlled, double-masked evaluation of mitomycin C in combined glaucoma and cataract procedures. Ophthalmology 1996; 103: 1934–1942. [DOI] [PubMed] [Google Scholar]
- Kozobolis VP, Christodoulakis EV, Tzanakis N, Zacharopoulos I, Pallikaris IG. Primary deep sclerectomyvs primary deep sclerectomy with the use of mitomycin C in primary open-angle glaucoma. J Glaucoma 2002; 11: 287–293. [DOI] [PubMed] [Google Scholar]
- Neudorfer M, Sadetzki S, Anisimova S, Geyer O. Nonpenetrating deep sclerectomy with the use of adjunctive mitomycin C. Ophthalmic Surg Lasers Imaging 2004; 35: 6–12. [PubMed] [Google Scholar]
- Anand N, Atherley C. Deep sclerectomy augmented with mitomycin C. Eye 2005; 19: 442–450. [DOI] [PubMed] [Google Scholar]
- Cillino S, Di PF, Casuccio A, Lodato G. Deep sclerectomyvs punch trabeculectomy: effect of low-dosage mitomycin C. Ophthalmologica 2005; 219: 281–286. [DOI] [PubMed] [Google Scholar]
- Jampel HD, Friedman DS, Lubomski LH, Kempen JH, Quigley H, Congdon N et al. Effect of technique on intraocular pressure after combined cataract and glaucoma surgery: an evidence-based review. Ophthalmology 2002; 109: 2215–2224. [DOI] [PubMed] [Google Scholar]
- Shin DH, Kim YY, Sheth N, Ren J, Shah M, Kim C et al. The role of adjunctive mitomycin C in secondary glaucoma triple procedure as compared to primary glaucoma triple procedure. Ophthalmology 1998; 105: 740–745. [DOI] [PubMed] [Google Scholar]
- Belovay GW, Naqi A, Chan BJ, Rateb M. Ahmed II. Using multiple trabecular micro bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg 2012; 38(11): 1911–1917. [DOI] [PubMed] [Google Scholar]
- Craven ER, Katz LJ, Wells JM, Giamporcaro JE, iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg 2012; 38(8): 1339–1345. [DOI] [PubMed] [Google Scholar]
- Saheb H. Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol 2012; 23(2): 96–104. [DOI] [PubMed] [Google Scholar]
- Jordan JF, Wecker T, van Oterendorp C, Anton A, Reinhard T, Boehringer D et al. Trabectome surgery for primary and secondary open angle glaucomas. Graefes Arch Clin Exp Ophthalmol 2013; 251(12): 2753–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja Y, Ma KhinPyi S, Malihi M, Hodge DO, Sit AJ. Clinical results of ab interno trabeculotomy using the trabectome for open-angle glaucoma: the Mayo Clinic series in Rochester, Minnesota. Am J Ophthalmol 2013; 156(5): 927–935. [DOI] [PubMed] [Google Scholar]