Abstract
There is increasing evidence in the literature regarding translaminar pressure difference's (TPD) role in the pathophysiology of glaucoma. The optic nerve is exposed not only to intraocular pressure in the eye, but also to intracranial pressure (ICP), as it is surrounded by cerebrospinal fluid in the subarachnoid space. Although pilot studies have identified the potential importance of TPD in glaucoma, limited available data currently prevent a comprehensive description of the role that TPD may have in glaucomatous pathophysiology. In this review, we present all available qualified data from a systematic review of the literature of the role of TPD in open-angle glaucoma (OAG). PubMed (Medline), OVID Medline, ScienceDirect, SpringerLink, and all available library databases were reviewed and subsequent meta-analysis of pooled mean differences are presented where appropriate. Five papers including 396 patients met criteria for inclusion to the analysis. Importantly, we included all observational studies despite differences in ICP measurement methods, as there is no consensus regarding best-practice ICP measurements in glaucoma. Our results show that not only TPD is higher in glaucoma patients compared with healthy subjects, it is related to structural glaucomatous changes of the optic disc. Our analysis suggests further longitudinal prospective studies are needed to investigate the influence of TPD in OAG, with a goal of overcoming methodological weaknesses of previous studies.
Introduction
Glaucoma is the second leading cause of blindness worldwide.1 Quigley et al reported that there are 60.5 million people suffering from glaucoma in the world and it is predicted that in 2020 this number will increase to 79.6 million, with 74% having open-angle glaucoma (OAG).2 The prevalence of glaucoma, which increases with age, is increasing primarily as the population ages. According to the World Health Organization (WHO) there is about 2.65% of the global population over 40 years of age who has glaucoma.3 The global disability adjusted life years of glaucoma has risen for the past 20 years: from 443 000 years in 1990 to 943 000 years in 2010.4, 5 Glaucoma is characterized by structural optic nerve head (ONH) and visual field changes that may occur at any intraocular pressure (IOP) level, depending on each person's individual susceptibility. Although lowering IOP helps to decelerate or stabilize the disease, vast numbers of patients still develop and progress in glaucoma, despite an IOP within normal range.6 It has been shown that in addition to high IOP there are many additional risk factors including: lower ocular perfusion pressure; reduced ocular blood flow; low blood pressure (BP); myopia; and several others.7, 8, 9, 10 Evidence confirms that these non-IOP factors lead to apoptotic processes associated with glaucoma.11 Recently, researchers have began to focus on intracranial pressure (ICP) and translaminar pressure difference (TPD) as having a potential role in glaucomatous optic neuropathy.12, 13 The optic nerve is exposed not only to IOP in the eye, but also to ICP, as it is surrounded by cerebrospinal fluid (CSF) in the subarachnoid space (SAS). The lamina cribrosa demarcates these two pressurized zones and the pressure difference between them is called TPD (TPD=IOP – ICP).14 Physiologically, the difference between IOP (14.3(2.6) mm Hg) and ICP (12.9(1.9) mm Hg, in the supine position) is small.15 A higher TPD may lead to abnormal function and damage of the optic nerve due to changes in axonal transportation, deformation of the lamina cribrosa, altered blood flow or a combination thereof leading to glaucomatous damage.16 Furthermore, it is considered that the TPD may be a primary pressure related factor for glaucoma, as the ONH is located at the junction between the intraocular and retrobulbar spaces.17 However, the role of TPD in glaucoma pathogenesis and its progression remains unclear as the gold standard for ICP evaluation is an invasive measurement of the pressure in the CSF via lumbar puncture or via implantation of a pressure sensor into a cerebral ventricle.18, 19, 20 Importantly, this invasiveness includes the potential risk for intracranial hemorrhages and infection.21 To overcome these invasive limitations, several approaches have been proposed to estimate ICP noninvasively including: transcranial Doppler ultrasonography; tympanic membrane displacement; ophthalmodynamometry; and measurement of optic nerve sheath diameter.22 For instance, Xie et al estimated mathematical ICP formula based on three parameters: diastolic BP; age and body mass index (ICP=0.44 × body mass index (kg/m2)+0.16 × diastolic BP (mm Hg)−0.18 × age (years)−1.9); and Bland–Altman analysis revealed that 40 of 42 measurements were within the 95% limits of agreement.23 All of these approaches are based on correlation of anatomical or physiological parameters of the human head and brain with ICP. Unfortunately, correlation-based approaches are not accurate for real-quantitative ICP value measurement. To the best of our knowledge, this is the first review and meta-analysis to present all available qualified data from a systematic review of the literature of the role of TPD in OAG.
Materials and methods
A comprehensive literature search was performed via electronic databases of PubMed (Medline), OVID Medline, ScienceDirect, SpringerLink, and all available library databases with reference cross-matching to identify all observational studies evaluating TPD in patients with OAG. In our literature search we included a combination of keywords, such as ‘translaminar pressure difference', ‘translaminar pressure gradient', or ‘trans-lamina cribrosa pressure difference' and ‘glaucoma'. Search strategy was carried out on articles published over the past 10 years (from November 2004 to November 2014). The search was performed by two independent researchers (AD and LB) until all relevant articles were identified. The completeness of searches was validated by the primary author (LS) using all available library databases. We included all observational studies despite differences in ICP measurement methods (invasive or noninvasive) as there is no consensus regarding best-practice measurements of ICP in glaucoma.
Study quality assessment was based on the following criteria:
The study type and number of subjects.
Information on the characteristics of the studied population.
Information on the inclusion criteria.
Data processing quality.
Approval of the Ethics Committee.
Data including age, IOP, ICP, and TPD were collected and statistical analysis was performed using the statistical analysis program (SPSS version 22, ‘Insight Solutions', Vilnius, Lithuania). The analysis of the quantitative variables included calculation of the weighted averages mean and SD (× (SD)). Two articles subdivided data into categories based on the form of primary open-angle glaucoma (POAG), thus weighted averages of all data points and SD were calculated.15, 24 One article presented data of IOP and ICP, while TPD was used just in correlations; the data were converted to numerical values using formula TPD=IOP−ICP.25 The hypothesis of equality among groups was analyzed using t-test. The level of significance P<0.05 was considered significant.
Results
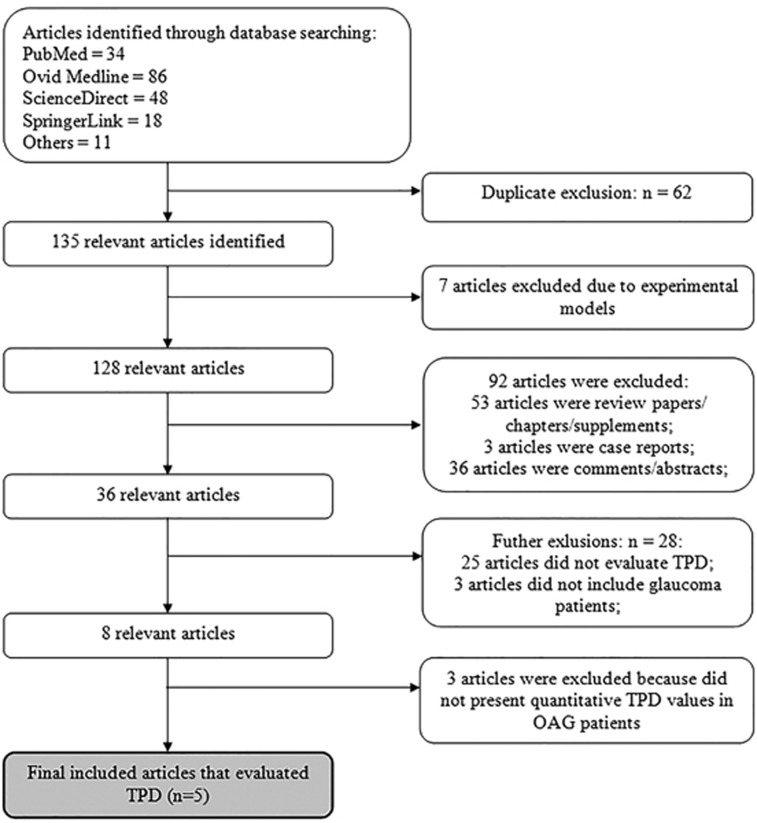
A total of 135 articles were identified from the search strategy and five studies that reported quantitative TPD parameters directly were deemed appropriate for inclusion.12, 15, 24, 25, 26 Figure 1 shows the article selection process for studies included in the final meta-analysis. Ninety-two papers including case reports, comments, letters, editorials, abstracts, and review papers/chapters were excluded, as this systematic review was sought observational studies. For clinical applicability, only data from human subjects were included, thus eliminating articles27, 28, 29, 30, 31, 32, 33 that used experimental models. Twenty-five papers were excluded because there was no evaluation of TPD.34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 Three papers were eliminated because glaucoma patients were not analyzed in these studies.59, 60, 61, 62 Three articles which did not present quantitative TPD values in OAG group were also excluded62, 63, 64 Table 1 shows the characteristics of papers (including 181 POAG patients and 215 healthy subjects) included to systematic review.12, 15, 24, 25, 26
Figure 1.
Summary of article selection process. TPD, translaminar pressure difference; OAG, open-angle glaucoma.
Table 1. Characteristics of the studies.
| Authors | Ren et al12 | Ren et al15 | Siaudvytyte et al24 | Berdahl et al25 | Berdahl et al26 |
|---|---|---|---|---|---|
| Study type | Observational, prospective | Observational, prospective | Observational, prospective | Observational, retrospective | Observational, retrospective |
| Included groups | POAG (NTG+HTG), OH | NTG, HTG, healthy | NTG, HTG, healthy | POAG and healthy | POAG (NTG+HTG), NTG, OH, healthy |
| ICP measurement method | Invasive | Invasive | Noninvasive | Invasive | Invasive |
| Other data | |||||
| Day of IOP and ICP measurements | Same day | Same day | Same day | Maximum IOP before lumbar puncture | Maximum IOP before lumbar puncture |
| Wash-out period | 1 month for NTG, HTG – did not include | 1 month for NTG, HTG – did not include | Did not include | Did not include | Did not include |
| Neurologist consultation | + | + | — | + | + |
| Approval of the Ethics Committee | + | + | + | + | + |
Abbreviations: HTG, high-tension glaucoma; ICP, intracranial pressure; IOP, intraocular pressure; NTG, normal tension glaucoma; OH, ocular hypertension; POAG, primary open-angle glaucoma.
Two retrospective studies25, 26 and three prospective studies12, 15, 24 matched all search criteria. One study evaluated ICP noninvasively using a two-depth transcranial Doppler (TCD) device (Vittamed UAB, Kaunas, Lithuania),24 based on simultaneous measurements of blood-flow parameters in intracranial and extracranial segments of the ophthalmic artery (OA). The value of external pressure, when OA blood flow in both segments was equal, was fixed and expressed automatically in absolute units mm Hg. The sensitivity, specificity, and diagnostic value of this device was proven in the previous studies with neurological patients.65, 66
Table 2 shows the results of the included studies. A meta-analysis of 396 patients was followed out and it was found that IOP, ICP, and TPD statistically significantly differed between POAG and healthy subjects (P<0.001).12, 15, 24, 25, 26 Three articles15, 24, 26 subdivided patients into normal-tension glaucoma (NTG) and high-tension glaucoma (HTG) categories; thus, meta-analysis of these data was also performed (Table 3). It was observed that IOP was significantly higher in HTG group than in healthy subjects or NTG groups (P<0.001). ICP was significantly lower in NTG group compared with HTG or healthy subjects (P<0.001). Evaluation of TPD revealed statistically significant differences between all groups: between HTG and NTG or healthy subjects, and NTG with healthy subjects (P<0.001). Importantly, HTG group was significantly younger than NTG group or healthy subjects (P<0.05). Some of these studies analyzed relationship between TPD and structural/functional glaucomatous changes12, 15, 24, 25 and found that higher TPD was associated with glaucomatous visual field loss or bigger structural glaucomatous changes. Summary of these studies is shown in Table 4.
Table 2. Results of meta-analysis between primary open-angle glaucoma and healthy subjects.
| Group | Parameters | Ren et al12 | Ren et al15 | Siaudvytyte et al24 | Berdahl et al25 | Berdahl et al26 | Total |
|---|---|---|---|---|---|---|---|
| POAG group | Number of patients (N (%)) | 35 (19.3%) | 43 (23.8%) | 18 (9.9%) | 28 (15.5%) | 57 (31.5%) | 181 (100%) |
| Age (years) | 45.0 (16.0) | 43.4 (4.4) | 55.7 (1.0) | 71.5 | 70.5 (12.9) | 57.8 (12.7) | |
| IOP (mm Hg) | 21.0 (5.0) | 21.6 (3.9) | 19.2 (5.7) | 24.3 (6.1) | 22.2 (6.4) | 21.9 (1.4)* | |
| ICP (mm Hg) | 11.0 (3.0) | 11.0 (1.0) | 8.2 (0.8) | 9.2 (2.9) | 9.3 (3.2) | 9.9 (1.0)** | |
| TPD (mm Hg) | 11.0 (5.0) | 10.6 (2.8) | 11.0 (4.8) | 15,1 | 11.6 (11.0) | 11.7 (1.5)*** | |
| Control group | Number of patients (N (%)) | — | 71 (33.0%) | 9 (4.2%) | 49 (22.8%) | 86 (40.0%) | 215 (100%) |
| Age (years) | — | 45.7 (11.3) | 51.9 (6.6) | 68.9 | 68.2 (8.6) | 60.2 (10.8) | |
| IOP (mm Hg) | — | 14.3 (2.6) | 15.9 (2.1) | 16.4 (2.8) | 16.1 (2.7) | 15.6 (0.9)* | |
| ICP (mm Hg) | — | 12.9 (1.9) | 10.5 (3.0) | 13.0 (4.2) | 11.8 (0.71) | 12.7 (0.5)** | |
| TPD (mm Hg) | — | 1.4 (1.7) | 5.4 (3.3) | 3.4 | 3.3 (4.0) | 2.8 (1.1)*** |
Abbreviations: ICP, intracranial pressure; IOP, intraocular pressure; POAG, primary open-angle glaucoma; TPD, translaminar pressure difference.
*P, **P, and ***P are <0.05, significant difference between two groups (t-test).
Table 3. Results of meta-analysis between normal tension glaucoma, high tension glaucoma and healthy subjects.
| Group | Parameters | Ren et al15 | Siaudvytyte et al24 | Berdahl et al26 | Total |
|---|---|---|---|---|---|
| NTG group | Number of patients (N (%)) | 14 (41.2%) | 9 (26.5%) | 11 (32.3%) | 34 (100%) |
| Age (years) | 49.6 (12.1) | 56.6 (10.4) | 68.0 (17.1) | 57.4 (8.0)* | |
| IOP (mm Hg) | 16.1 (1.9) | 13.7 (1.6) | 17.3 (2.7) | 15.9 (1.4)** | |
| ICP (mm Hg) | 9.5 (2.2) | 7.4 (2.7) | 9.3 (3.2) | 8.7 (0.9)*** | |
| TPD (mm Hg) | 6.6 (3.6) | 6.3 (3.1) | 7.4 (4.8) | 6.8 (0.5)**** | |
| HTG group | Number of patients (N (%)) | 29 (76.3%) | 9 (23.7%) | — | 38 (100%) |
| Age (years) | 40.4 (16.1) | 54.7 (15.6) | — | 43.8 (6.2)* | |
| IOP (mm Hg) | 24.3 (3.2) | 24.7 (6.8) | — | 24.4 (0.2)** | |
| ICP (mm Hg) | 11.7 (2.7) | 8.9 (1.9) | — | 11.0 (1.2)*** | |
| TPD (mm Hg) | 12.5 (4.1) | 15.7 (7.7) | — | 13.3 (1.4)**** | |
| Control group | Number of patients (N (%)) | 71 (42.8%) | 9 (5.4%) | 86 (51.8%) | 166 (100%) |
| Age (years) | 45.7 (11.3) | 51.9 (6.6) | 68.2 (8.6) | 57.7 (11.0)* | |
| IOP (mm Hg) | 14.3 (2.6) | 15.9 (2.1) | 16.1 (2.7) | 15.3 (0.9)** | |
| ICP (mm Hg) | 12.9 (1.9) | 10.5 (3.0) | 12.7 (3.9) | 12.2 (0.7)*** | |
| TPD (mm Hg) | 1.4 (1.7) | 5.4 (3.3) | 3.3 (4.0) | 2.6 (1.1)**** |
Abbreviations: HTG, high-tension glaucoma; ICP, intracranial pressure; IOP, intraocular pressure; NTG, normal tension glaucoma; TPD, translaminar pressure difference.
*P, **P, ***P and ****P are <0.05, significant difference between three groups (t-test).
Table 4. Summary of the correlations between translaminar pressure difference and structural/functional glaucomatous changes.
| Authors | Number of patients | Subjects | Analyzed parameter | Technology | Correlation | P-value |
|---|---|---|---|---|---|---|
| Ren et al12 | 52 | POAG and OH | NRA | HRT | r=−0.38 | 0.006 |
| Mean defect | HFA | r=0.38 | 0.007 | |||
| Ren et al15 | 114 | NTG, HTG, healthy | Mean defect | HFA | r=0.69 | 0.005 |
| Siaudvytyte et al24 | 9 | NTG | NRA | HRT | r=−0.83 | 0.01 |
| Berdahl et al25 | 77 | POAG and healthy | Cup-to-disc ratio | Medical records | r2=0.399 | <0.0001 |
Abbreviations: HFA, Humphrey Field Analyzer; HRT, Heidelberg Retina Tomograph; HTG, high-tension glaucoma; Mean defect, Mean glaucomatous visual field defect (dB); NRA, neuroretinal rim area; NTG, normal tension glaucoma; OH, ocular hypertension; POAG, primary open-angle glaucoma.
Discussion
Although the importance of ICP and TPD in glaucomatous pathophysiology is beginning to emerge, scarce available data have greatly limited our understanding of this possible contributory mechanism. Therefore, we performed a first of its kind comprehensive meta-analysis to investigate the current state of knowledge of TPD in OAG.
Our meta-analysis found that ICP was significantly lower in patients with POAG, particularly in NTG, than in healthy subjects.12, 15, 24, 25, 26 TPD was almost two times higher in patients with NTG, and nearly five times higher in patients with HTG, compared with healthy controls.15, 24, 26 As the ONH is exposed both to IOP and ICP, the TPD is an important parameter, and its reduction might assist in halting the progression of glaucoma. Jonas et al analyzed TPD and found statistically significant difference between glaucomatous and nonglaucomatous eyes (P<0.001); however, glaucomatous group included not only OAG but also angle-closure glaucoma patients,62, 64 therefore, these studies were excluded from meta-analysis.
Siaudvytyte et al found that higher TPD was associated with lower neuroretinal rim area (NRA) in NTG,24 whereas no association was found in HTG or healthy subjects. This data suggests that NTG patients are more susceptible to TPD differences. Berdahl et al25 found similar results in POAG and healthy subjects. It is important to note that this study has a limitation as measurements were obtained inconsistently within 1-year period of lumbar puncture performance. Whereas Siaudvytyte et al did not include neurological examination to exclude neurological disorders.24 Finally, Ren et al12, 15 found the relation between NRA, mean visual field defects and TPD, analyzing all study group.
There are several limitations of this meta-analysis to acknowledge. First, the limited uniformity of the available data from differing methodological approaches in the various studies makes direct comparisons difficult and the number of patients in experimental and control groups were often limited. Also, a majority of the selected studies24, 25, 26 did not include a washout period, which may account for differing study results. Specifically, Ren et al12, 15 included a washout period just for NTG patients, limiting accountability for possible effects of hypotensive agents on ICP. This is especially important in subjects who may have been using carbonic anhydrase inhibitors, as they are known to have systemic effects, which may influence of CSF production and result in ICP reduction.67
In addition, it should be mentioned that ICP-measuring methodologies were different between studies. Four studies used golden standard invasive ICP measuring method via lumbar puncture,12, 15, 25, 26 whereas Siaudvytyte et al in their study used noninvasive two-depth TCD device, which is currently the only available method for absolute ICP value numerical and automatic measurement that does not need an individual patient-specific calibration.24 A prospective study with 108 neurological patients showed that diagnostic sensitivity, specificity and the area under the ROC curve of this noninvasive absolute ICP method were 68.0%, 84.3%, and 0.87, respectively.66 However, the measuring error still remains, as we can see from the results—ICP values were higher in studies that used invasive ICP measuring methods.12, 15, 25, 26
Furthermore, it remains unclear whether the CSF pressure, measured by lumbar puncture, corresponds to the CSF pressure in the orbit around the optic nerve. The CSF dynamics at this area is different, as there are numerous septae present that could limit free flow of CSF. 68 In addition, unlike in other areas, the dura of optic nerve sheath contains atypical meningeal tissue with lymphoid characteristics.69 Killer and colleagues found that patients with NTG have decreased CSF flow between the basal cisterns and the SAS surrounding the optic nerve. Such a difference has not been established in healthy subjects. This could explain why patients with NTG have lower ICP.53 However, experimental studies on dogs showed that CSF pressure in optic nerve SAS is equal to CSF pressure in the lateral ventricle of the brain at the level of eye.70 Moreover, it was established that the CSF pressure measured by lumbar puncture corresponds to ICP in the lateral decubitus position.18 However, the method depends on the optic nerve path at SAS between the orbital and intracranial parts. It is not known what happens when the optic nerve canal is blocked (for example, in cases of suprasellar meningioma, tuberculous meningitis, or intracanalicular OA aneurysm).
One important consideration is that IOP, ICP, and TPD are dynamic parameters and changes in posture or individual activities might affect these measurements, respectively. In studies included in meta-analysis, the IOP was measured in the sitting position, whereas ICP was assessed in the supine or lateral decubitus positions.12, 15, 25, 26 Humans evolved with gravity, and gravity affects human physiology—CSF pools in the caudal spinal canal and CSF pressure at eye level is much lower than CSF pressure in the caudal spinal column in the upright position.18, 71 However in microgravity environment, CSF is distributed throughout the SAS tending to equalize pressure in all compartments and negate any posture-induced flow, resulting in higher than normal CSF pressure at eye level.72 Several studies have shown that changes in posture cause pressure changes in all body fluid spaces, cardiac output, peripheral resistance, and blood flow to various vascular beds.73, 74 IOP changes by 2.9 mm Hg in healthy subjects and by 3.9 mm Hg in patients with glaucoma while changing body position from sitting to supine.75 Furthermore, head elevation decreases ICP by displacing CSF into the spinal canal and by improving cerebral venous drainage by opening alternative venous channels in the posterior circulation that remain closed while patients remain recumbent. Experimental studies showed that CSF pressure in the sitting position at the level of the occipital prominence, equivalent to eye level, ranged between 0 and −10 mm Hg.71 One of the ways to interpret ICP values in the sitting position is mathematical modeling, whereas standard body position for ICP measuring is lateral decubitus/supine.76
Another important aspect to consider is that authors analyzed simplified TPD, calculated by the following formula (TPD=IOP−ICP), and did not clarify the fact that TPD is actually the difference between IOP and the retrolaminar tissue pressure, which is largely determined by the optic nerve SAS pressure and pia mater characteristics. Furthermore, the optic nerve SAS pressure is influenced by orbital pressure from the sides.77 Morgan et al, analyzed correlation between CSF pressure and retrolaminar tissue pressure in dogs and found that retrolaminar tissue pressure was basically identical to the CSF pressure in the ventricles, when CSF pressure was above 2 mm Hg. Below 2 mm Hg, the tissue pressure was approximately constant, perhaps reflecting the orbital tissue pressing against the nerve when CSF pressure is very low.14 It would be interesting to analyze translaminar pressure gradient (defined as pressure distribution across the lamina cribrosa, altered by lamina cribrosa thickness) as the most obvious changes in glaucoma occur in the lamina cribrosa. It is estimated that lamina cribrosa is thinner and more bowed in glaucomatous eyes (201 microns) compared with normal eyes (457 microns).78
To answer the question whether TPD is important in glaucoma, we need longitudinal studies in addition to the retrospective and prospective studies performed thus far. Therefore, noninvasive techniques for ICP measurements would be helpful in the studies with human patients. Moreover, along with clinical studies, suitable animal models will also be helpful in understanding the role of ICP in glaucoma. Chowdhury et al published the study on comprehensive animal model where ICP can be manually reduced or increased over an extended period of time, that is valuable to study the balance between IOP and ICP and its role in glaucomatous optic neuropathy.79 Moreover, there are a lot of points that need explication to further elucidate ICP role in glaucoma. As CSF dynamics are poorly ascertained, there is no clear answer whether normal cardiac variations in ICP exist or how body position or activity of the person affect it. Although it is accepted that IOP diurnal fluctuations are greater in eyes with glaucoma,80 question is whether there are similar variations in ICP and single measurement of ICP can represent short or long-term pressure variations that could have a role in glaucoma. Furthermore, IOP fluctuations have been proposed as an independent risk factor for glaucomatous damage.81 Wostyn et al arose hypothesis that ICP fluctuations may result in significant fluctuations of the TPD and could exert repetitive shear stress on the lamina cribrosa and ganglion cell axons, leading to glaucomatous damage.82 Interestingly, other researchers found that Valsalva maneuver led to reduce or reverse of the TPD, which was associated with decreased optic cup-related parameters and enlarged neuroretinal rim-related parameters.61 Therefore, it would be interesting to estimate if alternative treatment strategies aiming to increase ICP could be beneficial for glaucoma patients.
Conclusion
Our meta-analysis of all qualified data shows that patients with NTG and HTG have higher TPD than healthy subjects. Importantly, higher TPD is associated with larger optic disc structural changes in patients with OAG. However, our conclusions are based on the current literature data, which are limited in scope and execution and have significant differences and weaknesses in the methodologies they utilized. With acknowledgement of these weaknesses, the available data suggest that there is a need for further longitudinal prospective clinical and experimental studies investigating the influence of TPD in glaucoma.
Acknowledgments
This research is funded by the European Social Fund under the Global Grant measure.
AR is an inventor of noninvasive ICP measurement technology, which is patented in the US and EU. The remaining authors declare no conflict of interest.
References
- Leske MC. Open-angle glaucoma: an epidemiological overview. Ophthalmic Epidemiol 2007; 14(4): 166–172. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006; 90(3): 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol 2011; 152(4): 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380(9859): 2197–2223. [DOI] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380(9859): 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske CM, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007; 114(11): 1965–1972. [DOI] [PubMed] [Google Scholar]
- Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol 2013; 13(1): 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B, BESs Study Group. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology 2008; 115(1): 85–93. [DOI] [PubMed] [Google Scholar]
- Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt study. Ophthalmology 2000; 107(7): 1287–1293. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Blood flow in the optic nerve head and factors that may influence it. Prog Retin Eye Res 2001; 20(5): 595–624. [DOI] [PubMed] [Google Scholar]
- Flammer J, Haefliger IO, Orgul S, Resink T. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma 1999; 8(3): 212–219. [PubMed] [Google Scholar]
- Ren R, Wang N, Zhang X, Cui T, Jonas JB. Trans-lamina cribrosa pressure difference correlated with neuroretinal rim area in glaucoma. Graefes Arch Clin Exp Ophthalmol 2011; 249(7): 1057–1063. [DOI] [PubMed] [Google Scholar]
- Jonas JB. Role of cerebrospinal fluid pressure in the pathogenesis of glaucoma. Acta Ophthalmol 2011; 89(6): 505–514. [DOI] [PubMed] [Google Scholar]
- Morgan WH, Yu DY, Alder VA, Cringle SJ, Cooper RL, House PH et al. The correlation between cerebrospinal fluid pressure and retrolaminar tissue pressure. Invest Ophthalmol Vis Sci 1998; 39(8): 1419–1428. [PubMed] [Google Scholar]
- Ren R, Jonas JB, Tian G, Zhen Y, Ma K, Li S et al. Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology 2010; 117(2): 259–266. [DOI] [PubMed] [Google Scholar]
- Morgan WH, Chauhan BC, Yu DY, Cringle SJ, Alder VA, House PH. Optic disc movement with variations in intraocular and cerebrospinal fluid pressure. Invest Ophthalmol Vis Sci 2002; 43(10): 3236–3242. [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 2005; 24(1): 39–73. [DOI] [PubMed] [Google Scholar]
- Lenfeldt N, Koskinen L, Bergenhein A, Malm J, Eklund A. CSF pressure assessed by lumbar puncture agrees with intracranial pressure. Neurology 2007; 68(2): 155–158. [DOI] [PubMed] [Google Scholar]
- Andrews P, Citerio G, Longhi L. NICEM consensus on neurological monitoring in acute neurological disease. Intensive Care Med 2008; 34(8): 1362–1370. [DOI] [PubMed] [Google Scholar]
- Digre KB, Corbett JJ. Idiopathic intracranial hypertension (pseudotumor cerebri): a reappraisal. Neurologist 2001; 7: 62–67. [Google Scholar]
- Zeng T, Gao L. Management of patients with severe traumatic brain injury guided by intraventricular intracranial pressure monitoring: a report of 136 cases. Chin J Traumatol 2010; 13(3): 146–151. [PubMed] [Google Scholar]
- Siaudvytyte L, Januleviciene J, Ragauskas A, Bartusis L, Siesky B, Harris A. Update in intracranial pressure evaluation methods and translaminar pressure gradient role in glaucoma. Acta Ophthalmol 2014; 93(1): 9–15. [DOI] [PubMed] [Google Scholar]
- Xie X, Zhang X, Fu J, Wang H, Jonas JB, Peng X et al. Noninvasive intracranial pressure estimation by orbital subarachnoid space measurement: the Beijing Intracranial and Intraocular Pressure (iCOP) study. Crit Care 2013; 17(4): R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siaudvytyte L, Januleviciene I, Ragauskas A, Bartusis L, Meiliuniene I, Siesky B et al. The difference in translaminar pressure gradient and neuroretinal rim area in glaucoma and healthy subjects. J Ophthalmol 2014; 2014: 937360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdahl JP, Allingham RR, Johnson DH. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 2008; 115(5): 763–768. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR. Intracranial Pressure in Primary Open-Angle Glaucoma, Normal Tension Glaucoma, and Ocular Hypertension: A Case-Control Study. Invest Ophthalmol Vis Sci 2008; 49(12): 5412–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BC, Hammes NM, Johnson PL, Shekhar A, McKinnon SJ, Allingham RR. Dorsomedial/Perifornical hypothalamic stimulation increases intraocular pressure, intracranial pressure, and the translaminar pressure gradient. Invest Ophthalmol Vis Sci 2012; 53(11): 7328–7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Berenshtein E, Holbach L. Lamina cribrosa thickness ans spatial relationship between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest Ophthalmol Vis Sci 2004; 45(8): 2660–2665. [DOI] [PubMed] [Google Scholar]
- Yang D, Fu J, Hou R, Liu K, Jonas JB, Wang H et al. Optic neuropathy induced by experimentally reduced cerebrospinal fluid pressure in monkeys. Invest Ophthalmol Vis Sci 2014; 55(5): 3067–3073. [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, Morgan WH, Bass L, Ye L, McKnight C, Cringle SJ et al. Elevated pressure induced astrocyte damage in the optic nerve. Brain Res 2008; 1244: 142–154. [DOI] [PubMed] [Google Scholar]
- Janssen SF, Gorgels TG, Ten Brink JB, Jansonius NM, Bergen AA. Gene expression-based comparison of the human secretory neuroepithelia of the brain choroid plexus and the ocular ciliary body: potential implications for glaucoma. Fluids Barriers CNS 2014; 11(1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grytz R, Meschike G, Jonas JB. The collagen fibril architecture in the lamina cribrosa and peripapillary sclera predicted by a computational remodeling approach. Biomech Model Mechanobiol 2011; 10(3): 371–382. [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, Kang MH, Yu P, Chan G, Morgan WH, Cringle SJ et al. Comparative quantitative study of astrocytes and capillary in optic nerve laminar regions. Exp Eye Res 2014; 121: 11–22. [DOI] [PubMed] [Google Scholar]
- Pinto LA, Vandewalle E, Pronk A, Stalmans I. Intraocular pressure correlates with optic nerve sheath diameter in patients with normal tension glaucoma. Graefes Arch Clin Exp Ophthalmol 2012; 250(7): 1075–1080. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Kim TW, Kim M, Kim H. Influence of lamina cribrosa thickness and depth on the rate of progressive retinal nerve fiber layer thinning. Ophthalmology 2014; 122(4): 721–729. [DOI] [PubMed] [Google Scholar]
- Wright TM, Gohrarian I, Gardiner SK, Sehi M, Greenfield DS. Short-term enhancement of visual field sensitivity in glaucomatous eyes following surgical intraocular pressure reduction. Am J Ophthalmol 2014; 159(2): 378–385. [DOI] [PubMed] [Google Scholar]
- Lee YA, Shih YF, Lin LL, Huang JY, Wang TH. Association between high myopia and progression of visual field loss in primary open-angle glaucoma. J Formos Med Assoc 2008; 107(12): 952–957. [DOI] [PubMed] [Google Scholar]
- Park HY, Jeon SH, Park CK. Enhanced depth imaging detects lamina cribrosa thickness differences in normal tension glaucoma and primary open-angle glaucoma. Ophthalmology 2012; 119(1): 10–20. [DOI] [PubMed] [Google Scholar]
- Agoumi Y, Sharpe GP, Hutchison DM, Nicolela MT, Artes PH, Chauhan BC. Laminar and prelaminar tissue displacement during intraocular pressure elevation in glaucoma patients and healthy controls. Ophthalmology 2011; 118(1): 52–59. [DOI] [PubMed] [Google Scholar]
- Buys YM, Alasbali T, Jin YP, Smith M, Gouws P, Geffen N et al. Effect of sleeping in a head-up position on intraocular pressure in patients with glaucoma. Ophthalmology 2010; 117(7): 1348–1351. [DOI] [PubMed] [Google Scholar]
- Sehi M, Goharian I, Konduru R, Tan O, Srinivas S, Sadda SR et al. Retinal blood flow in glaucomatous eyes with single-hemifield damage. Ophthalmology 2014; 121(3): 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Xie X, Yang D, Xian J, Li Y, Ren R et al. Orbital cerebrospinal fluid space in glaucoma: the Beijing intracranial and intraocular pressure (iCOP) study. Ophthalmology 2012; 119(10): 2065–2073. [DOI] [PubMed] [Google Scholar]
- Willekens K, Pinto LA, Vandewalle E, Marques-Neves C, Stalmans I. Higher optic nerve sheath diameters are associated with lower ocular blood flow velocities in glaucoma patients. Graefes Arch Clin Exp Ophthalmol 2014; 252(3): 477–483. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Kim TW, Weinreb RN, Suh MH, Kim H. Lamina cribrosa thickness is not correlated with central corneal thickness or axial length in healthy eyes: central corneal thickness, axial length, and lamina cribrosa thickness. Graefes Arch Clin Exp Ophthalmol 2013; 251(3): 847–854. [DOI] [PubMed] [Google Scholar]
- Golzan SM, Kim MO, Seddighi AS, Avolio A, Graham SL. Non-invasive estimation of cerebrospinal fluid pressure waveforms by means of retinal venous pulsatility and central aortic blood pressure. Ann Biomed Eng 2012; 40(9): 1940–1948. [DOI] [PubMed] [Google Scholar]
- Oku Y, Oku H, Park M, Hayashi K, Takahashi H, Shouji T et al. Long axial length as risk factor for normal tension glaucoma. Graefes Arch Clin Exp Ophthalmol 2009; 247(6): 781–787. [DOI] [PubMed] [Google Scholar]
- Mojtaba Golzan S, Leaney J, Cordina R, Avolio A, Celermajer DS, Graham SL. Spontaneous retinal venous pulsatility in patients with cyanotic congenital heart disease. Heart Vessels 2012; 27(6): 618–623. [DOI] [PubMed] [Google Scholar]
- Kurna SA, Akar G, Altun A, Agirman Y, Gozke E, Sengor T. Confocal scanning laser tomography of the optic nerve head on the patients with Alzheimer's disease compared to glaucoma and control. Int Ophthalmol 2014; 34(6): 1203–1211. [DOI] [PubMed] [Google Scholar]
- Barrancos C, Rebolleda G, Oblanca N, Cabarga C, Muñoz-Negrete FJ. Changes in lamina cribrosa and prelaminar tissue after deep sclerectomy. Eye (Lond) 2014; 28(1): 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G, Killer HE, Forrer A, Huber AR, Jaggi GP. Lipocalin-like prostaglandin D synthase (L-PGDS) concentration in aqueous humour in patients with open-angle glaucoma. J Glaucoma 2014; 23(3): 164–168. [DOI] [PubMed] [Google Scholar]
- Mader TH, Gibson CR, Pass AF, Lee AG, Killer HE, Hansen HC et al. Optic disc edema in an astronaut after repeat long-duration space flight. J Neuroophthalmol 2013; 33(3): 249–255. [DOI] [PubMed] [Google Scholar]
- Chang PY, Chang SW. Corneal biomechanics, optic disc morphology, and macular ganglion cell complex in myopia. J Glaucoma 2013; 22(5): 358–362. [DOI] [PubMed] [Google Scholar]
- Killer HE, Miller NR, Flammer J, Meyer P, Weinreb RN, Remonda L et al. Cerebrospinal fluid exchange in the optic nerve in normal-tension glaucoma. Br J Ophthalmol 2012; 96(4): 544–548. [DOI] [PubMed] [Google Scholar]
- Oliveira C, Harizman N, Girkin CA, Xie A, Tello C, Liebmann JM et al. Axial length and optic disc size in normal eyes. Br J Ophthalmol 2007; 91(1): 37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi GP, Miller NR, Flammer J, Weinreb RN, Remonda L, Killer HE. Optic nerve sheath diameter in normal-tension glaucoma patients. Br J Ophthalmol 2012; 96(1): 53–56. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Wang N, Wang S, Wang YX, You QS, Yang D et al. Retinal vessel diameter and estimated cerebrospinal fluid pressure in arterial hypertension: The Beijing Eye Study. Am J Hypertens 2014; 27(9): 1170–1178. [DOI] [PubMed] [Google Scholar]
- Ekstrom C, Kilander L. Pseudoexfoliation and Alzhaimer's disease: a population-based 30-year follow-up study. Acta Ophthalmol 2014; 92(4): 355–358. [DOI] [PubMed] [Google Scholar]
- Helmer C, Malet F, Rougier MB, Schweitzer C, Colin J, Delyfer MN et al. Is there a link between open-angle glaucoma and dementia? The Three-City-Alienor cohort. Ann Neurol 2013; 74(2): 171–179. [DOI] [PubMed] [Google Scholar]
- Fleischman D, Berdahl JP, Zaydlarova J, Stinnett S, Fautsch MP, Allingham RR. Cerebrospinal fluid pressure decreases with older age. PLoS One 2012; 7(12): e52664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Zhang X, Wang N, Li B, Tian G, Jonas JB. Cerebrospinal fluid pressure in ocular hypertension. Acta Ophthalmol 2011; 89(2): e142–e148. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang X, Jonas JB, Wang H, Zhang X, Peng X et al. Valsalva manoeuver, intra-ocular pressure, cerebrospinal fluid pressure, optic disc topography: Beijing intracranial and intra-ocular pressure study. Acta Ophthalmol 2013; 92(6): e475–e480. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Wang NL, Wang YX, You QS, Xie X, Yang D et al. Estimated trans-lamina cribrosa pressure difference versus intraocular pressure as biomarker for open-angle glaucoma. The Beijing Eye Study 2011. Acta Ophthalmol 2014; 93(1): e7–e13. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Wang N, Wang YX, You QS, Xie X, Yang D et al. Body height, estimated cerebrospinal fluid pressure and open-angle glaucoma. The Beijing Eye Study 2011. PLoS One 2014; 9(1): e86678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Nangia V, Wang N, Bhate K, Nangia P, Nangia P et al. Trans-lamina cribrosa pressure difference and open-angle glaucoma. The central India eye and medical study. PLoS One 2013; 8(12): e82284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragauskas A, Matijosaitis V, Zakelis R, Petrikonis K, Rastenyte D, Piper I et al. Clinical assessment of noninvasive intracranial pressure absolute value measurement method. Neurology 2012; 78(21): 1684–1691. [DOI] [PubMed] [Google Scholar]
- Ragauskas A, Bartusis L, Piper I, Zakelis R, Matijosaitis V, Petrikonis K et al. Improved diagnostic value of a TCD-based non-invasive ICP measurement method compared with the sonographic ONSD method for detecting elevated intracranial pressure. Neurol Res 2014; 36(7): 607–614. [DOI] [PubMed] [Google Scholar]
- Samuels MA. Disturbances of cerebrospinal fluid and its circulation, including hydrocephalus, pseudotumor cerebri, and low-pressure syndromes. In Samuels MA ed. Adams and Victor's Principles Of Neurology. 9th edn. McGraw-Hill: NY, USA, 2009. [Google Scholar]
- Jaggi GP, Mironov A, Huber AR, Killer HE. Optic nerve compartment syndrome in a patient with optic nerve sheath meningioma. Eur J Ophthalmol 2007; 17(3): 454–458. [DOI] [PubMed] [Google Scholar]
- Killer HE, Jaggi G, Miller NR, Flammer J, Meyer P. Does immunohistochemistry allow easy detection of lymphatics in the optic nerve sheath? J Histochem Cytochem 2008; 56(12): 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WH, Yu DY, Cooper RL, Alder VA, Cringle SJ, Constable IJ. The influence of cerebrospinal fluid pressure on the lamina cribrosa tissue pressure gradient. Invest Ophthalmol Vis Sci 1995; 36(6): 1163–1172. [PubMed] [Google Scholar]
- Magnaes B. Body position and cerebrospinal fluid pressure. Part 2: Clinical studies on orthostatic pressure and the hydrostatic indifferent point. J Neurosurg 1976; 44(6): 698–705. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, Yu DY, Morgan WH. The translaminar pressure gradient in sustained zero gravity, idiopathic intracranial hypertension, and glaucoma. Med Hypotheses 2012; 79: 719–724. [DOI] [PubMed] [Google Scholar]
- Durward QJ, Amacher AL, Del Maestro RF, Sibbald WJ. Cerebral and cardiovascular responses to changes in head elevation in patients with intracranial hypertension. J Neurosurg 1983; 59(6): 938–944. [DOI] [PubMed] [Google Scholar]
- Magnaes B. Body position and cerebral fluid pressure. Part 1: Clinical studies on the effect of rapid postural changes. J Neurosurg 1976; 44(6): 687–697. [DOI] [PubMed] [Google Scholar]
- Jain MR, Marmion VJ. Rapid pneumatic and Machey-Marg applanation tonometry to evaluate the postural effect on intraocular pressure. Br J Ophthalmol 1976; 60(10): 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med 2014; 40(9): 1189–1209. [DOI] [PubMed] [Google Scholar]
- Morgan WH, Yu DY, Balaratnasingam C. The role of cerebrospinal fluid pressure in glaucoma pathophysiology: the dark side of the optic disc. J Glaucoma 2008; 17(5): 408–413. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Berenshtein E, Holbach L. Anatomic relationship between lamina cribrosa, intraocular space and cerebrospinal fluid space. Invest Ophthalmol Vis Sci 2003; 44(12): 5189–5195. [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury U, Holman BH, Fautsch MP. A novel rat model to study the role of intracranial pressure modulation on optic neuropathies. PLoS One 2013; 8(12): e82151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky JT. Diurnal variations in intraocular pressure. Trans Am Ophthalmol Soc 1991; 89: 757–790. [PMC free article] [PubMed] [Google Scholar]
- Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000; 9: 134–142. [DOI] [PubMed] [Google Scholar]
- Wostyn P, De Groot V, Audenaert K, De Deyn PP. Are intracranial pressure fluctuations important in glaucoma? Med Hypotheses 2011; 77: 598–600. [DOI] [PubMed] [Google Scholar]