Abstract
L-asparaginase is an integral component of therapy for acute lymphoblastic leukemia. However, asparaginase-related complications, including the development of hypersensitivity reactions, can limit its use in individual patients. Of considerable concern in the setting of clinical allergy is the development of neutralizing antibodies and associated asparaginase inactivity. Also problematic in the use of asparaginase is the potential for the development of silent inactivation, with the formation of neutralizing antibodies and reduced asparaginase activity in the absence of a clinically evident allergic reaction. Here we present guidelines for the identification and management of clinical hypersensitivity and silent inactivation with Escherichia coli- and Erwinia chrysanthemi- derived asparaginase preparations. These guidelines were developed by a consensus panel of experts following a review of the available published data. We provide a consensus of expert opinions on the role of serum asparaginase level assessment, indications for switching asparaginase preparation, and monitoring after change in asparaginase preparation.
Introduction
L-asparaginase is an integral component of therapy for acute lymphoblastic leukemia (ALL). However, asparaginase-related complications, including the development of hypersensitivity reactions, pose clinical challenges. Children and adolescents with ALL who receive an inadequate course of planned asparaginase therapy due to either intolerable side effects or silent inactivation have been shown to have inferior outcomes compared with those who receive the majority of intended doses of asparaginase, highlighting the importance of maximizing the delivery of planned asparaginase therapy.1–3
L-asparaginase preparations are bacterial enzymes derived from either Escherichia coli (E. coli) or Erwinia chrysanthemi (Erwinia). E. coli-derived preparations include native E. coli-asparaginase, no longer available in the United States, and pegylated formulations (pegaspargase), in which the E. coli-derived enzyme is modified by the covalent attachment of polyethylene glycol. Erwinia asparaginase is an alternative asparaginase preparation, antigenically distinct from E. coli-derived asparaginase forms. The clinical effectiveness of asparaginase is thought to be based upon the adequate depletion of asparagine. Lymphoblasts, dependent on extracellular sources of asparagine, are considered to be selectively vulnerable to asparagine depletion.4
Exposure to asparaginase, a foreign protein, has the capacity to trigger the development of anti-asparaginase antibodies. Prior studies have demonstrated the association of anti-asparaginase antibodies, which can neutralize enzymatic activity, with clinical hypersensitivity.3,5,6 Indeed, a major limitation in the capacity to deliver the intended up-front asparaginase therapy is the high rate of occurrence of hypersensitivity reactions, frequently reported in as many as 30% of patients receiving E. coli-derived asparaginase, but with reported rates as high as 70%.1,3,7–9 Allergic reaction symptoms range from local reactions at the site of intramuscular injection to severe systemic reactions including anaphylaxis, which can occur with intramuscular or intravenous administration. Also demonstrated has been the phenomenon of “silent inactivation,” with the formation of neutralizing antibodies and reduced asparaginase activity in the absence of a clinically evident allergic reaction.2,3,10–12 The risk of development of clinical allergy and silent inactivation may be influenced by several factors including the formulation preparation of asparaginase, the route of administration, the schedule of administration (such as in schedules with intermittent dosing with gaps in asparaginase exposure followed by reintroduction of asparaginase), the line of treatment (i.e. relapse protocols) and the concurrent use of other chemotherapeutic agents including corticosteroids.13
An important clinical concern in the setting of both overt allergy to asparaginase and silent inactivation is that continued asparaginase therapy with the same formulation will be clinically harmful and therapeutically ineffective, ultimately contributing to poorer outcomes. Here we propose recommendations for the identification and management of clinical hypersensitivity and silent inactivation with E. coli- and Erwinia chrysanthemi-derived asparaginase preparations, with the goal of maximizing the delivery of the planned asparaginase therapy. We highlight the potential clinical role of therapeutic drug monitoring (TDM) through serum asparaginase level assessment, indications for switching asparaginase preparations, and recommendations for monitoring after changes in asparaginase preparation.
Serum Asparaginase Activity Assessment
The ability to identify patients with inadequate asparaginase activity is of great value in clinical decision making and has the potential to improve clinical outcomes. There are, however, multiple considerations to take into account in the implementation of pharmacokinetic monitoring of asparaginase for clinical use.
What should be measured?
Given that the aim of asparaginase therapy is asparagine depletion, the measurement of asparagine from the blood would appear to be the most direct assessment of asparaginase effectiveness. However, there are several limitations to directly measuring asparagine levels that make such a strategy impractical and unreliable for clinical use. The measurement of asparagine is technically difficult due to the rapid ex vivo metabolism of asparagine in the presence of asparaginase. Reliable sample acquisition entails collection on ice water and centrifugation, extraction of serum, and de-proteinization/acidification to inhibit the reaction, in a very limited time-frame (less than 5–15 minutes). Because of these logistical challenges, the assessment of serum asparagine levels is not realistically achievable for broad clinical application.14–16 Furthermore, data from studies measuring asparagine levels are often difficult to interpret because different cut-off values have been used for the definition of complete asparagine depletion.
The measurement of anti-asparaginase antibodies could also be considered, and are frequently measured in the context of clinical research investigations. However, there are no commercially clinically validated tests available at the present time. Moreover, the specificity of anti-asparaginase antibodies to predict inactivation has been found to be low compared with measuring asparaginase activity itself; many patients appear to develop anti-asparaginase antibodies without any signs of clinical allergy or inactivation of asparaginase, and antibody levels in patients with and without hypersensitivity overlap.12 Antibody assessment itself is therefore not well suited for current clinical use.
The measurement of asparaginase activity levels is technically feasible, reproducible, and reliable, and is considered to best correlate with clinical effectiveness. Previously, asparaginase activity levels were only measured in the research setting, but a growing number of providers now have access to real-time, validated asparaginase activity measurements. Several European treatment protocols already recommend the monitoring of asparaginase activity in the context of routine clinical care. Currently, the assessment of asparaginase activity is often performed by the use of a reaction with indooxine.17 In North America, an FDA-approved asparaginase activity assay is currently commercially available (AIBio Tech, Richmond, VA, USA.)
What defines optimal asparaginase activity?
The pharmacodynamic goal of asparaginase therapy is complete asparagine depletion. Nonetheless, the level of asparaginase activity necessary for complete asparagine depletion is unclear. A threshold of 0.1 IU/mL has been used in many research and treatment protocols to define therapeutic asparaginase activity, as levels above this threshold have been found to result in complete asparagine depletion.18–21 In 1981, Riccardi et al. administered E. coli and Erwinia asparaginase to rhesus monkeys and patients and found that plasma asparaginase activity levels above 0.1 IU/mL resulted in complete asparagine depletion in CSF and plasma.18 This cut-off of ≥ 0.1 IU/mL has been confirmed and used in many clinical trials.9,19,22–24 The question arises whether a lower threshold, for example of 0.05 IU/mL, also leads to complete asparagine depletion. Rizzari and colleagues showed that trough asparaginase activity levels of < 0.05 IU/mL, obtained either with native E. Coli or Erwinia asparaginase, resulted in serum and CSF asparagine depletion in children with ALL.25 In some studies activity levels as low as 0.02 IU/mL26,27 or 0.03 IU/mL21,28 resulted in sufficient depletion. In contrast, the only study indicating that higher activity levels are needed is a recent COG study of two pegylated E. coli asparaginase preparations, calaspargase pegol and pegaspargase, in which the plasma asparagine level began to rebound once plasma asparaginase activity declined below 0.4 IU/mL.29 However, based on the literature to date, we consider that a nadir serum asparaginase activity level of ≥ 0.1 IU/mL appears to be an appropriate and safe target level, because complete depletion is observed less consistently with asparaginase activity levels below this cut-off. In addition, in the absence of further data, we consider a desirable level of activity for patients receiving pegaspargase to be defined as ≥ 0.1 IU/mL at 14 days post-administration. For patients receiving multiple doses per week of native E. coli or Erwinia asparaginase, we consider a desirable level of activity to be ≥ 0.1 IU/mL prior to each administered dose.
When should asparaginase activity be assessed?
The timing of serum asparaginase assessment is another important aspect in the implementation of TDM for asparaginase therapy. The majority of childhood ALL trials now utilize pegaspargase, which has a plasma half-life notably longer than native E. coli asparaginase (5.73 ± 3.24 days, compared with 1.28 ± 0.3 days, respectively).30 Most reports use the trough level at day 14 to define the efficacy of the pegaspargase treatment. Information on the desirable target levels of asparaginase activity at time points prior to day 14 (that would ensure a level ≥ 0.1 IU/mL at day 14) is lacking. Still, assessments at earlier time points can be informative, as levels < 0.1 IU/mL prior to day 14 would indicate that the day 14 trough level will be too low.
In summary:
Serum asparaginase activity levels are the best and most reliable indicators of asparaginase efficacy.
Trough asparaginase activity levels ≥ 0.1 IU/mL appears to be a safe target level to ensure therapeutic benefit.
Anti-asparaginase antibodies and asparagine measurements are not indicated for clinical decision making outside the context of a clinical trial.
Recommendations in the setting of clinical allergy
The development of clinical hypersensitivity is considered a strong indicator that an individual patient has developed anti-asparaginase antibodies and will have reduced asparaginase activity. The ultimate concern is that continued use of asparaginase of the same formulation will be ineffective in the treatment of leukemia and may lead to poorer outcomes. Continuing the drug should be discouraged, even when it is clinically possible to administer the same preparation using premedication such as steroids and antihistamines or decreasing the infusion rate, as these measures reduce the symptoms of the allergy but do not prevent the inactivation of asparaginase by the antibodies.
Clinical hypersensitivity reactions are characterized by a range of symptoms, from mild localized reactions at the site of intramuscular injection to severe systemic reactions with features such as urticaria, bronchospasm, angioedema, and anaphylaxis following either intramuscular or intravenous administration. When grading, the severity of reactions is based on the Common Terminology Criteria for Adverse Events v4.03 (CTCAE) classification (Table 1), features of Grade 1 allergic reactions include transient flushing or rash without need for intervention, Grade 2 reactions include indication for intervention or interruption of infusion, Grade 3 are prolonged/recurrent reactions with the need for hospitalization for clinical sequelae, and Grade 4 reactions are life threatening. CTCAE v4.03 has a separate grading for anaphylaxis.31 However, in reviewing criteria such as these and in clinical practice, identifying a clinical allergy is not always straightforward. For example, it can be difficult to determine whether a Grade 1 reaction, with transient flushing, truly represents an allergic reaction. A concern in the setting of a questionable reaction would be the failure to positively identify and act upon true hypersensitivity. Indeed, some studies suggest that even a Grade 1 reaction can be associated with inactivation.12 With intravenously administered asparaginase, infusion reaction may occur, often late during an infusion, which can be confused with an allergic reaction.32 While there are signs and symptoms that mimic clinical allergy, these are not truly allergic reactions and are not associated with inactivation. In these cases asparaginase activity levels may be informative as to whether or not to continue the drug.
Table 1.
Common Terminology Criteria for Adverse Events v4.03 (CTCAE) classification for allergic reactions and anaphylaxis.
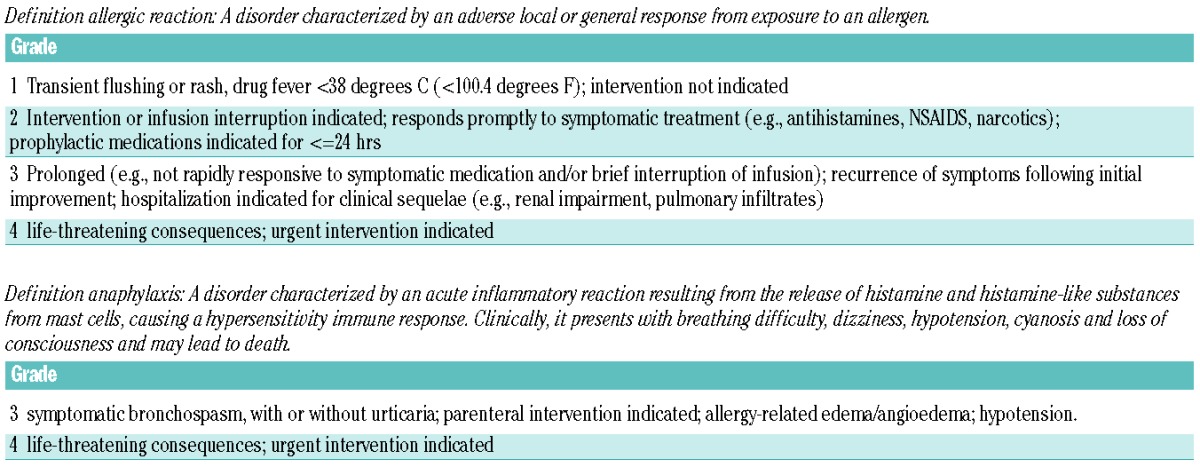
Therefore, we propose the following recommendations based upon the severity of clinically evident allergic reaction and route of administration. In the setting of CTCAE Grade 2 or higher reactions, we recommend that switching asparaginase preparation is indicated, with no definite need for testing of asparaginase levels. In the setting of Grade 1 reactions, or when a reaction has occurred of questionable significance, we recommend real-time monitoring of serum asparaginase activity levels. Presuming the use of intravenous pegaspargase, we recommend checking a level within one week of dose administration. When the previous dose is truncated because of an allergic reaction it is hard to interpret an activity level following this dose. The main goal of this level recheck is to determine whether activity is present. If the level is non-detectable, then no further E. coli-derived asparaginase should be utilized and the patient should be switched to an Erwinia-derived preparation. If the level is detectable, we recommend rechecking a 14-day trough level, and a subsequent dose of pegaspargase may be carefully administered. Premedication with agents such as antihistamines or corticosteroids should not be used in the absence of checking asparaginase activity levels. Activity levels should be checked after 7 and 14 days and should be above 0.1 IU/mL. When the activity level is less than the desired threshold of 0.1 IU/mL, the asparaginase preparation should be switched.
With intramuscular asparaginase, with any questionable reaction, we recommend checking a serum asparaginase level because the complete dose will have been administered and the level would therefore be informative (as there can be clinical confusion with regard to local irritation vs. clinical allergic reaction.)
In summary:
Grade 1 reactions and questionable reaction following intravenous administration: Monitor serum asparaginase level in real-time within 7 days to identify inactivation.
Grade 2–4 reactions following intravenous or intramuscular administration: Switch asparaginase preparation, without definite need to check asparaginase levels.
Any questionable reaction with intramuscular administration requires checking asparaginase activity level.
Recommendations in the setting of silent inactivation
“Silent inactivation” is the development of asparaginase antibodies and asparaginase inactivity without the development of overt or recognized allergy symptoms. From a clinical perspective, the concern is that with continued asparaginase administration in the setting of silent inactivation, the patient will not receive the benefit of effective asparaginase therapy which might otherwise be mitigated by switching to an alternative asparaginase preparation. Recent data suggest that the development of silent inactivation is clinically important in the context of leukemia-directed therapy, and that acting upon silent inactivation may improve outcome.2,3 The results of the DFCI ALL Consortium Protocol 00–01 suggested that the evaluation of nadir serum asparaginase levels in patients receiving native E. coli asparaginase, and changing asparaginase preparation in the setting of silent inactivation (as defined by persistently low nadir serum asparaginase activity, with or without antibody positivity), was associated with improved outcome in children with ALL.2 These results highlight the importance of switching asparaginase preparation in the setting of silent inactivation.
What is the definition of silent inactivation?
Silent Inactivation is caused by neutralizing anti-drug antibodies (to asparaginase or PEG) resulting in asparaginase inactivity without the development of overt allergy symptoms. It can be identified with (trough) asparaginase activity levels below the lower limit of quantification (LLQ) occurring in patients without clinical allergy, preferably measured in 2 independent samples to minimize the number of patients tested false positive. Specifically, with the use of pegaspargase (given every two weeks), a day 7 asparaginase activity level below 0.1 IU/mL and/or a day 14 level below the LLQ would be consistent with silent inactivation. Although less frequently used in contemporary pediatric ALL trials, silent inactivation is demonstrated for native E. coli asparaginase if the 72 hours post-dose level is below the LLQ (in a two times a week schedule), or if the level 7 days post-dosing is below the LLQ (in a weekly administration schedule). With the use of Erwinia asparaginase (three times a week schedule), a 48 hours post-dose level below the LLQ would raise concern for silent inactivation (please see more detailed discussion of monitoring with the use of Erwinia asparaginase below). In addition, low activity levels should always be interpreted in the context of the dosing and frequency of the administration of asparaginase. For example, with the use of Erwinia asparaginase, based on large inter-individual differences in clearance, low trough levels at 72 hours and beyond could reflect a need for more frequent dosing, rather than silent inactivation itself.
Who should be screened for silent inactivation?
As noted above, the concern that patients could continue to be exposed to an agent that has been rendered ineffective when alternative agents exist is compelling. Our conclusion is that screening for silent inactivation should be considered in all patients undergoing therapy for ALL with asparaginase. This may be particularly important following gaps in asparaginase therapy or in the setting of the treatment of relapsed leukemia.
When should patients be screened for silent inactivation?
Silent inactivation of E. coli-derived asparaginase has been reported in induction and intensification phases.2,12 Some patients may have developed antibodies to PEG before the start of pegaspargase treatment, presumably due to previous exposure to PEG (for example in creams).33 We recommend the testing of serum asparaginase activity level after the first dose of E. coli-derived asparaginase. With the use of pegaspargase, this should be done within 7 days of the dose. If the level is detectable but less than 0.1 IU/mL, activity should be rechecked at day 14. The recommended frequency of screening after the first dose of asparaginase depends upon the dosing schedule. For patients due to receive multiple asparaginase doses without any prolonged gap between doses (e.g., pegylated asparaginase given every 14 days), it would be reasonable to confirm a low or undetectable level after a subsequent dose, and to change to a different preparation (eg, Erwinia) if two consecutive levels are undetectable. When there is a gap between asparaginase doses, we recommend checking a level after the first dose of asparaginase administered after the gap, with a gap defined as a period in which asparaginase activity level will have decreased to < LLQ between doses. In practice, this is usually the case when there is an interval of at least 4 weeks between pegylated asparaginase doses. With native E. coli asparaginase, we recommend measuring a trough level after the first dose and after every reintroduction of asparaginase. Levels could be checked more frequently based on clinician discretion. If there are a limited number of asparaginase doses in the treatment plan and/or intermittent dosing (prolonged gaps between doses), we would recommend screening for silent inactivation after every asparaginase dose, and one could consider switching preparation based upon a single undetectable or low level rather than confirming it with two separate measurements. This monitoring plan would foster the maximization of asparaginase exposure.
In summary:
All patients should undergo therapeutic drug monitoring for silent inactivation.
Silent Inactivation can be identified by the assessment of serum asparaginase activity, preferably measured in 2 independent samples.
Measure serum asparaginase activity level within 7 days of the first dose of pegaspargase in induction and following every reintroduction after a gap in asparaginase. With native E. coli asparaginase, consider measuring a trough level after the first dose and after every reintroduction.
Silent inactivation of pegaspargase (administered every other week) is defined as a day 7 level below 0.1 IU/mL and/or day 14 level below the LLQ.
Silent inactivation of native E. coli or Erwinia asparaginase is defined as a trough level below the LLQ. Trough levels should always be interpreted in the context of dosing and frequency e.g.:
Native E coli: asparaginase activity below the LLQ 72 hours post-dosing in a two times a week administration schedule, or below the LLQ 7 days post-dosing in a weekly administration schedule.
Erwinia: a 48 hour post-dose activity level below the LLQ in a three times a week schedule.
Consider confirmation of a low or undetectable level based on the planned schedule of asparaginase administration.
Switching preparations
When changing asparaginase preparation, those receiving native E. coli asparaginase who develop clinical allergy or silent inactivation should switch to either pegaspargase or to Erwinia asparaginase, with the choice of a second-line agent dependent upon protocol specifications and preparation availability. Patients initially receiving pegaspargase may only be switched to Erwinia asparaginase; switching to native E. coli asparaginase should not be considered an option.
Erwinia asparaginase is antigenically distinct from E. coli-derived asparaginase. The half-life of Erwinia asparaginase is shorter than other forms of asparaginase, and the shorter half-life of Erwinia asparaginase has demonstrated clinical implications, with the need for more frequent dosing (every 2–3 days).10,34 When utilized in patients with a history of E. coli asparaginase hypersensitivity reactions, the majority of patients receiving Erwinia asparaginase have been shown to achieve goal nadir asparaginase activity levels. A subsequent allergy to Erwinia asparaginase (in patients who previously developed an allergy to E. coli derived-preparations) has been reported at rates of 3–33%.12,35,36 Tong and colleagues reported that 97% of patients who switched to Erwinia asparaginase after hypersensitivity to pegaspargase were able to complete their full planned course of asparaginase in a continuous dosing schedule.12
While Erwinia asparaginase has been utilized in the treatment of childhood ALL for several decades, there remains variability in practice with regard to optimal dosing, dosing interval, and route of administration. Intravenous administration has been routinely utilized in Europe in a dose of 20,000 IU/m2 three times a week. FDA-approved IM dosing of Erwinia asparaginase is 25,000 IU/m2 three times per week (Monday/Wednesday/Friday), for six total doses for each planned dose of pegaspargase. Twice weekly IM or IV dosing has also been utilized with apparent efficacy.12,35 Although IM administration has largely been utilized in the United States, Erwinia asparaginase is now approved for IV use as well.37 The optimal dose, schedule, and route of Erwinia asparaginase, however, remain unclear and are worthy of further investigation. Large inter-individual differences in clearance underscore the importance of individualized dosing schedules based on asparaginase activity.
Asparaginase activity monitoring after change in preparation to Erwinia asparaginase
After switching to Erwinia asparaginase, the monitoring of serum asparaginase levels is of continued importance, however with a slightly different underlying rationale. After a switch to Erwinia asparaginase due to allergy to pegaspargase, there are no additional alternative preparations available. The emphasis on asparaginase activity monitoring with the use of Erwinia asparaginase is to inform the individualization of dosing rather than the switching of asparaginase preparation. Based on large inter-individual differences in clearance, low nadir levels at 72 hours or 96 hours after a dose of Erwinia may represent the need for more frequent dosing rather than silent inactivation, and levels should be interpreted in this context. In theory, persistently low 48 hour trough levels with appropriate dosing of Erwinia asparaginase could allow clinicians the possibility of considering alternative treatments to asparaginase, although the implementation of such alterations would be dependent on individual treatment protocols, and the impact of such alterations are unknown.
We recommend checking trough serum asparaginase levels every 2 weeks in continuous schedules (and after each 2-week block of Erwinia asparaginase in intermittent dosing schedules). Specifically, levels after Erwinia asparaginase should be performed 48 hours after dosing, with an aim of asparaginase activity levels greater than 0.1 IU/mL. Because Erwinia asparaginase is frequently dosed on a Monday/Wednesday/Friday schedule, there may also be clinical value in checking a level 72 hours after dosing (after the Friday dose and before the following Monday dose). It may be reasonable to adjust dosing or interval to maintain a level greater than 0.1 IU/mL. In addition, there may be differences in the pharmacokinetics of IM compared with IV administered Erwinia asparaginase, making the assessment of levels of particular importance with IV administration.
Future directions and opportunities
The ability to identify patients with inadequate asparaginase activity has the potential to inform clinical decision making. Previously, asparaginase activity levels were only measured in the context of research, but a growing number of providers now have access to real-time validated asparaginase activity measurements. Several European treatment protocols already recommend the monitoring of asparaginase activity. Since therapeutic drug monitoring has started to play a role in daily practice, guidelines are needed for identifying and managing hypersensitivity to asparaginase.
The goal of asparaginase therapy is to achieve complete asparagine depletion. As noted above, due to the difficulties in directly measuring serum asparagine, it is much easier and more reliable to measure asparaginase activity levels to guarantee effective treatment. To date, an asparaginase activity level ≥ 0.1 IU/mL appears to be a reasonable target level to ensure therapeutic benefit. Ultimately, in interpreting asparaginase activity levels and in considering the most appropriate target threshold, it is the association with clinical outcome that matters the most. More data are needed to more precisely determine the optimal target level and to more robustly characterize the relationship between asparaginase activity levels and long-term outcomes. Yet even without further data, monitoring drug levels to detect silent inactivation may potentially improve outcome and could prevent continued administration of a drug that is ineffective.
In addition, more detailed pharmacokinetic analyses will be of value to improve individualized dosing strategies. Currently, most reports use asparaginase activity trough levels at day 14 to determine the efficacy of the pegaspargase treatment. Information on the optimal target levels of asparaginase prior to day 14 (for example, at day 3 and day 7) to ensure an adequate level at day 14 is lacking.
Due to hospital budget restrictions and the increasing costs of treatment of childhood ALL, more insight into the costs of treatment with the various asparaginase preparations would be very useful. Tong and colleagues demonstrated that administration of pegaspargase in first-line therapy was cost effective compared to native E.coli asparaginase, as it leads to a lower frequency of hypersensitivity and consequently less use of Erwinia asparaginase.38 Switching to Erwinia asparaginase in case of clinical allergy or silent inactivation has considerable impact on the total ALL treatment costs.38 Erwinia asparaginase is more expensive and patients require more visits due to the more frequent administration schedule. This change in frequency of hospital visits may negatively affect the quality of life of the child and parents. However, the change in preparation may also have a favorable impact on outcome. Health economic analyses, taking into account all the costs of switching to Erwinia asparaginase, gained life years or quality-adjusted life years (QALY) and downstream costs of treatment failure are complicated but necessary in order to inform discussion on whether the change in preparation in case of allergy or silent inactivation is cost-effective. This will depend on the intensity of asparaginase therapy, the costs of Erwinia asparaginase and other treatment costs, and detailed cost-effectiveness considerations will differ by country and by treatment plan.
In addition to the detection of silent inactivation, therapeutic drug monitoring can be used to individualize dosing by adapting the dose based on activity levels. With the use of pegaspargase, activity levels are generally much higher than with native E. coli asparaginase. Therapeutic drug monitoring to inform dosing has the potential to reduce the pegaspargase dose and thereby to reduce medication costs. Future investigations could explore optimal maximum nadir serum asparaginase levels with regard to clinical efficacy, toxicity, and cost, considering also the additional costs of the therapeutic drug monitoring program itself. Further characterization of long-term outcomes and comprehensive cost analyses would further inform clinical practice.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/100/3/279
References
- 1.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211–1218. [DOI] [PubMed] [Google Scholar]
- 2.Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study–Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31(9):1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panosyan EH, Seibel NL, Martin-Aragon S, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 2004;26(4):217–226. [DOI] [PubMed] [Google Scholar]
- 4.Muller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998;28(2):97–113. [DOI] [PubMed] [Google Scholar]
- 5.Woo MH, Hak LJ, Storm MC, et al. Anti-asparaginase antibodies following E. coli asparaginase therapy in pediatric acute lymphoblastic leukemia. Leukemia. 1998;12(10): 1527–1533. [DOI] [PubMed] [Google Scholar]
- 6.Zalewska-Szewczyk B, Andrzejewski W, Mlynarski W, Jedrychowska-Danska K, Witas H, Bodalski J. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2007;48(5):931–936. [DOI] [PubMed] [Google Scholar]
- 7.Muller HJ, Beier R, Loning L, et al. Pharmacokinetics of native Escherichia coli asparaginase (Asparaginase medac) and hypersensitivity reactions in ALL-BFM 95 reinduction treatment. Br J Haematol. 2001;114(4):794–799. [DOI] [PubMed] [Google Scholar]
- 8.Woo MH, Hak LJ, Storm MC, et al. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2000;18(7):1525–1532. [DOI] [PubMed] [Google Scholar]
- 9.Appel IM, Kazemier KM, Boos J, et al. Pharmacokinetic, pharmacodynamic and intracellular effects of PEG-asparaginase in newly diagnosed childhood acute lymphoblastic leukemia: results from a single agent window study. Leukemia. 2008;22(9):1665–1679. [DOI] [PubMed] [Google Scholar]
- 10.Asselin BL. The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol. 1999;457:621–629. [PubMed] [Google Scholar]
- 11.Vieira Pinheiro JP, Ahlke E, Nowak-Gottl U, et al. Pharmacokinetic dose adjustment of Erwinia asparaginase in protocol II of the paediatric ALL/NHL-BFM treatment protocols. Br J Haematol. 1999;104(2):313–320. [DOI] [PubMed] [Google Scholar]
- 12.Tong WH, Pieters R, Kaspers GJ, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123(13):2026–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieters R, Hunger SP, Boos J, et al. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117(2):238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentili D, Zucchetti M, Conter V, Masera G, D’Incalci M. Determination of L-asparagine in biological samples in the presence of L-asparaginase. J Chromatogr B Biomed Appl. 1994;657(1):47–52. [DOI] [PubMed] [Google Scholar]
- 15.Asselin BL, Lorenson MY, Whitin JC, et al. Measurement of serum L-asparagine in the presence of L-asparaginase requires the presence of an L-asparaginase inhibitor. Cancer Res. 1991;51(24):6568–6573. [PubMed] [Google Scholar]
- 16.Lanvers-Kaminsky C, Westhoff PS, D’Incalci M, Zucchetti M, Boos J. Immediate cooling does not prevent the ex vivo hydrolysis of L-asparagine by asparaginase. Ther Drug Monit. 2014;36(4):549–552. [DOI] [PubMed] [Google Scholar]
- 17.Lanvers C, Vieira Pinheiro JP, Hempel G, Wuerthwein G, Boos J. Analytical validation of a microplate reader-based method for the therapeutic drug monitoring of L-asparaginase in human serum. Anal Biochem. 2002;309(1):117–126. [DOI] [PubMed] [Google Scholar]
- 18.Riccardi R, Holcenberg JS, Glaubiger DL, Wood JH, Poplack DG. L-asparaginase pharmacokinetics and asparagine levels in cerebrospinal fluid of rhesus monkeys and humans. Cancer Res. 1981;41(11 Pt 1):4554–4558. [PubMed] [Google Scholar]
- 19.Avramis VI, Sencer S, Periclou AP, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2002;99(6):1986–1994. [DOI] [PubMed] [Google Scholar]
- 20.Boos J, Werber G, Ahlke E, et al. Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur J Cancer. 1996;32A(9): 1544–1550. [DOI] [PubMed] [Google Scholar]
- 21.Ahlke E, Nowak-Gottl U, Schulze-Westhoff P, et al. Dose reduction of asparaginase under pharmacokinetic and pharmacodynamic control during induction therapy in children with acute lymphoblastic leukaemia. Br J Haematol. 1997;96(4):675–681. [DOI] [PubMed] [Google Scholar]
- 22.Strullu M, Corradini N, Audrain M, et al. Silent hypersensitivity to Escherichia coli asparaginase in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2010;51(8):1464–1472. [DOI] [PubMed] [Google Scholar]
- 23.Avramis VI, Martin-Aragon S, Avramis EV, Asselin BL. Pharmacoanalytical assays of Erwinia asparaginase (erwinase) and pharmacokinetic results in high-risk acute lymphoblastic leukemia (HR ALL) patients: simulations of erwinase population PK-PD models. Anticancer Res. 2007;27(4C):2561–2572. [PubMed] [Google Scholar]
- 24.Albertsen BK, Schroder H, Ingerslev J, et al. Comparison of intramuscular therapy with Erwinia asparaginase and asparaginase Medac: pharmacokinetics, pharmacodynamics, formation of antibodies and influence on the coagulation system. Br J Haematol. 2001;115(4):983–990. [DOI] [PubMed] [Google Scholar]
- 25.Rizzari C, Zucchetti M, Conter V, et al. L-asparagine depletion and L-asparaginase activity in children with acute lymphoblastic leukemia receiving i.m. or i.v. Erwinia C. or E. coli L-asparaginase as first exposure. Ann Oncol. 2000;11(2):189–193. [DOI] [PubMed] [Google Scholar]
- 26.Pieters R, Appel I, Kuehnel HJ, et al. Pharmacokinetics, pharmacodynamics, efficacy, and safety of a new recombinant asparaginase preparation in children with previously untreated acute lymphoblastic leukemia: a randomized phase 2 clinical trial. Blood. 2008;112(13):4832–4838. [DOI] [PubMed] [Google Scholar]
- 27.Tsurusawa M, Shimomura Y, Asami K, et al. Long-term results of the Japanese Childhood Cancer and Leukemia Study Group studies 811, 841, 874 and 911 on childhood acute lymphoblastic leukemia. Leukemia. 2010; 24(2):335–344. [DOI] [PubMed] [Google Scholar]
- 28.Rizzari C, Citterio M, Zucchetti M, et al. A pharmacological study on pegylated asparaginase used in front-line treatment of children with acute lymphoblastic leukemia. Haematologica. 2006;91(1):24–31. [PubMed] [Google Scholar]
- 29.Angiolillo AL, Schore RJ, Devidas M, et al. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from Children’s Oncology Group Study AALL07P4. J Clin Oncol. 2014;32(34): 3874–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol. 1993;11(9):1780–1786. [DOI] [PubMed] [Google Scholar]
- 31.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 Published: 2009 (v4.03: June 14, 2010). U.S.DEPARTMENT OF HEALTH AND HUMAN SERVICES, National Institutes of Health National Cancer Institute; Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf. [Google Scholar]
- 32.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12(5):601–609. [DOI] [PubMed] [Google Scholar]
- 33.Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv. 2012;9(11): 1319–1323. [DOI] [PubMed] [Google Scholar]
- 34.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109(3):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vrooman LM, Supko JG, Neuberg DS, et al. Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billett AL, Carls A, Gelber RD, Sallan SE. Allergic reactions to Erwinia asparaginase in children with acute lymphoblastic leukemia who had previous allergic reactions to Escherichia coli asparaginase. Cancer. 1992;70(1):201–206. [DOI] [PubMed] [Google Scholar]
- 37.Vrooman LM, Kirov II, Dreyer ZE, et al. Activity and Toxicity of Intravenous Erwinia Asparaginase Following Allergy to E. coli-Derived Asparaginase in Children and Adolescents With Acute Lymphoblastic Leukemia. Pediatr Blood Cancer. 2015. September 16 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong WH, van der Sluis IM, Alleman CJ, et al. Cost-analysis of treatment of childhood acute lymphoblastic leukemia with asparaginase preparations: the impact of expensive chemotherapy. Haematologica. 2013;98(5): 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]


