Abstract
Response criteria for multiple myeloma are based upon changes in monoclonal protein levels quantified using serum and/or urine protein electrophoresis. The latter lacks sensitivity at low monoclonal protein levels and since 2001, the serum free light chain test has been available and its clinical utility proven, yet guidelines have not recommended it as a replacement for urine assessment. Herein we evaluated responses using serum free light chain measurements and serum and urine electrophoresis after 2 and 4 cycles of therapy and after stem cell transplantation in 25 light chain and 157 intact immunoglobulin myeloma patients enrolled in the IFM 2007-02 MM trial. All 25 light chain patients had measurable disease by serum free light chain and urine methods at presentation. By contrast 98 out of 157 intact immunoglobulin patients had measurable disease by serum free light chain compared to 55 out of 157 by urine electrophoresis. In all patients there was substantial agreement between predicate (serum/urine protein electrophoresis) and test (serum protein electrophoresis and serum free light chain) methods for response assessment (Weighted Kappa=0.83). Urine immunofixation became negative in 47% light chain and 43% intact immunoglobulin patients after 2 cycles of therapy. At this time the serum free light chain ratio normalised in only 11% and 27% patients, respectively. In summary we found good agreement between methods for response assessment, but the serum free light chain test provided greater sensitivity than urine electrophoresis for monitoring. To our knowledge this is the first report comparing both methods for response assignment based on the International Myeloma Working Group guidelines.
Introduction
Plasma cell dyscrasias are a disparate group of premalignant and malignant disorders. These conditions are commonly characterized by the production of monoclonal proteins (M-protein) which may be intact immunoglobulins (M-Ig), free light chains (FLC) or, less frequently, free heavy chains. Rarely do the disorders present without the production of any M-protein. The monoclonal components are usually identified and quantified by electrophoresis and immunofixation of serum (SPE + sIFE) and urine (UPE + uIFE) proteins; such approaches are required for the diagnosis and monitoring of patients with multiple myeloma (MM).1 Whilst these techniques are adequate for the majority of MM patients, those with light chain only MM (LCMM) and oligosecretory MM can be challenging to monitor.2 In these patients, 24h UPE is recommended for monitoring Bence Jones protein (BJP) changes during follow-up; however, (i) BJP levels in urine are influenced by renal function, particularly when produced at low concentrations; (ii) there can be significant fluctuations in BJP levels measured by UPE during monitoring of individual patients; and (iii) up to 19% of urine samples contain monoclonal intact immunoglobulin that may interfere with BJP measurements.3–5 In addition, the provision of urine at the time of diagnosis and during monitoring can be an issue due to incomplete urine collection and variable compliance of between 5%–52%.6–9
The introduction of the polyclonal antibody based Freelite® assays in 2001 was an important addition to the laboratory and physicians’ armamentarium for the diagnosis,2,10,11 monitoring12–15 and prognosis16–18 of patients with monoclonal gammopathies (MG). The largest screening study to date comparing the utility of SPE, sIFE, UPE, uIFE and serum free light chain (sFLC) for screening for MG disorders included 1877 patients and concluded that SPE and sFLC provide a simple first-line methodology for screening for high tumour burden MG; and urine tests and sIFE can be ordered more selectively.2 These results were independently confirmed in another study of 923 patients.19 Subsequently, international guidelines recommended the use of sFLC in combination with SPE and sIFE for the diagnosis of MG, negating the need for urine analysis other than when AL amyloidosis is suspected.20
Monitoring sFLC concentrations for response assignment is currently only recommended for patients with non-measurable disease by electrophoretic methods and for determining stringent complete response (sCR); since FLC concentrations in the serum and urine of individual patients do not correlate and response assessment may differ between methods, guidelines do not recommend the use of the sFLC assay as a replacement for 24h urine collections for monitoring MM patients.20 However, Bradwell et al. studied 82 LCMM patients and indicated that urine analysis may overestimate the response to therapy by becoming negative in 32% patients, compared to only 11% patients whose sFLC ratio normalized.4 The discrepancy is clinically relevant since normalisation of serum FLC levels and ratio has been associated with improved outcomes in both LCMM21 and IIMM22 patients.
The aim of this study was to compare the performance of sFLC as a replacement for urine tests for quantifying monoclonal protein expression at presentation and for response assignment during the monitoring of LCMM and IIMM patients.
Methods
Patients and serum samples
We selected 182 patients (25 LCMM, 157 IIMM) from the InterGroupe Francophone du Myélome (IFM) 2007-02 MM trial (Clinical Trials Register.eu identifier: 2007-005204-40) who had serum and 24h urine samples collected at presentation and at least one follow-up sample at the end of any second or fourth cycle of induction therapy or post ASCT (median 4 samples, range 2–5). In accordance with the trial protocol patients had been centrally randomised equally into two treatment arms to receive 4 cycles of bortezomib and dexamethasone (VD) or bortezomib and thalidomide plus dexamethasone (VtD), followed by 1 cycle of high-dose melphalan plus ASCT.23 Serum samples were retrospectively analysed for FLC concentrations and the results compared with those obtained by 24h urine measurements at the time of collection.
Laboratory methods
Serum FLCκ and FLCλ concentrations were measured by Freelite® (The Binding Site Group Ltd, UK) on a Dade Behring BNTMII nephelometer (Siemens GmbH, Germany). Reference ranges for sFLC have been previously published (normal range: sFLCκ 3.3–19.4 mg/L, sFLCλ 5.7–26.3 mg/L, sFLCκ/λ ratio 0.26–1.65).24 The sFLC results were compared with serum and urine electrophoresis and immunofixation data collected at the time of the original clinical trial.25
Response assessment
We applied the International Myeloma Working Group (IMWG) criteria for measurable disease and response assessment.20,26,27 Briefly, measurable disease by SPE refers to M-Ig >10 g/L, by UPE to BJP concentrations ≥200 mg/24h, and by sFLC as an abnormal sFLC ratio with involved (tumour) FLC (iFLC) concentrations ≥100 mg/L. sFLC responses were scored based on changes in dFLC (difference between the involved and uninvolved FLC concentrations). In accordance with IMWG guidelines, partial response (PR) was defined by urine tests as a decrease in BJP by ≥90% or to <200 mg/24h and by sFLC as >50% decrease in dFLC; very good partial response (VGPR) was defined as detectable BJP by uIFE but not by UPE, or BJP levels <100 mg/24h by UPE, and by sFLC as >90% decrease in dFLC. Bone marrow information was lacking, thus preventing the assignment of complete response (CR); instead negative serum IFE, negative uIFE and normalisation of sFLC ratio were used as best possible response. Progressive disease (PD) by urine tests was assigned by a 25% increase in BJP with ≥200 mg/24h increase in absolute values, and by sFLC by 25% increase in dFLC with >100 mg/L increase in absolute values. Stable disease (SD) was assigned to patients not fitting any of the above response criteria. For IIMM patients each level of response was assigned based on serum and urine changes as specified above; where methods disagreed the lowest response was assigned.
Statistical analyses
Correlations between sFLC and UPE measurements were carried out using Pearson’s correlation analyses and calculation of the correlation coefficient (r) using Analyse-it (v. 2.25) software. Concordance in response assignment by either method was studied using quadratic Weighted Kappa analyses. Weighted Kappa values >0.81 correspond to near perfect agreement; values >0.61, >0.41, and >0.21 represent substantial, moderate and fair agreement, respectively.28 Percentage agreement corresponds to the number of samples with concordant responses.
Ethical considerations
The study was approved by the local ethical committee and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent from participating patients was required.
Results
Diagnostic sensitivity and measurable disease
All 25 LCMM patients had abnormal sFLC ratios and positive UPE at presentation and monoclonal protein levels consistent with measurable disease (Figure 1 and Table 1), although correlation between sFLC and urine assays for monoclonal serum free light chain concentrations was poor (r=0.27, data not shown). A number of patients appeared to have very high levels of monoclonal protein according to one test but only modest levels according to the other: 4 out of 25 patients had iFLC levels >10000 mg/L (median 12500 (10400–22000)) but BJP levels by UPE of 3800 (1200–7200) mg/24h; by contrast 3 out of 25 patients had BJP >10000 mg/24h (median 12500 (10000–42000)) but median iFLC levels were 6200 (6500–8600) mg/L.
Figure 1.
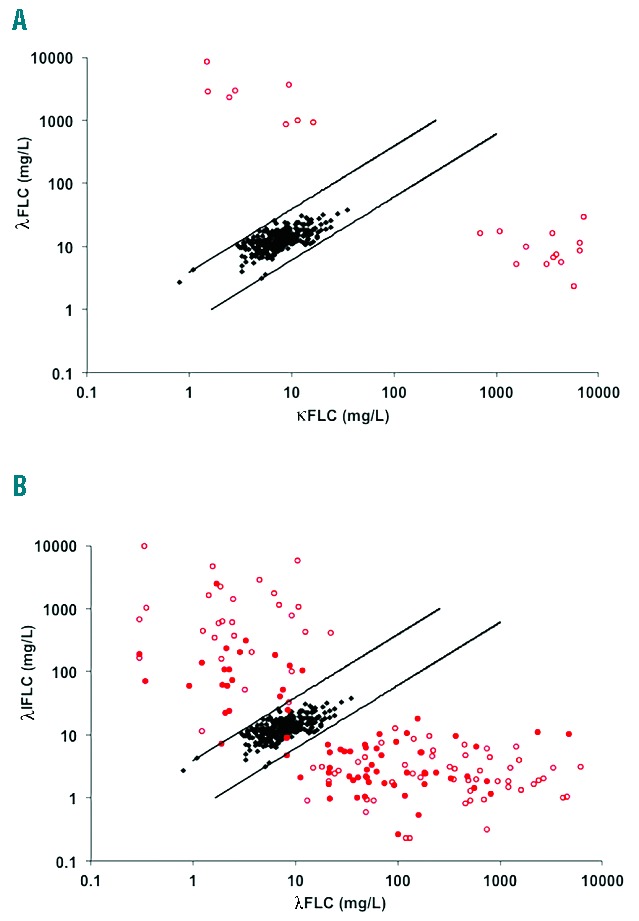
Scatter charts of serum κFLC and λFLC. (A) All 25 LCMM patients had an abnormal sFLC ratio and were positive by uIFE. (B) 154/157 IIMM patients presented with an abnormal sFLC ratio. uIFE was positive in 85/157 patients (positive uIFE open red circles, negative uIFE solid red circles, black diamonds: normal blood donor sera (n=282), parallel lines: 100 percentile normal range for sFLC (ratio, 0.26–1.65).
Table 1.
Baseline characteristics.
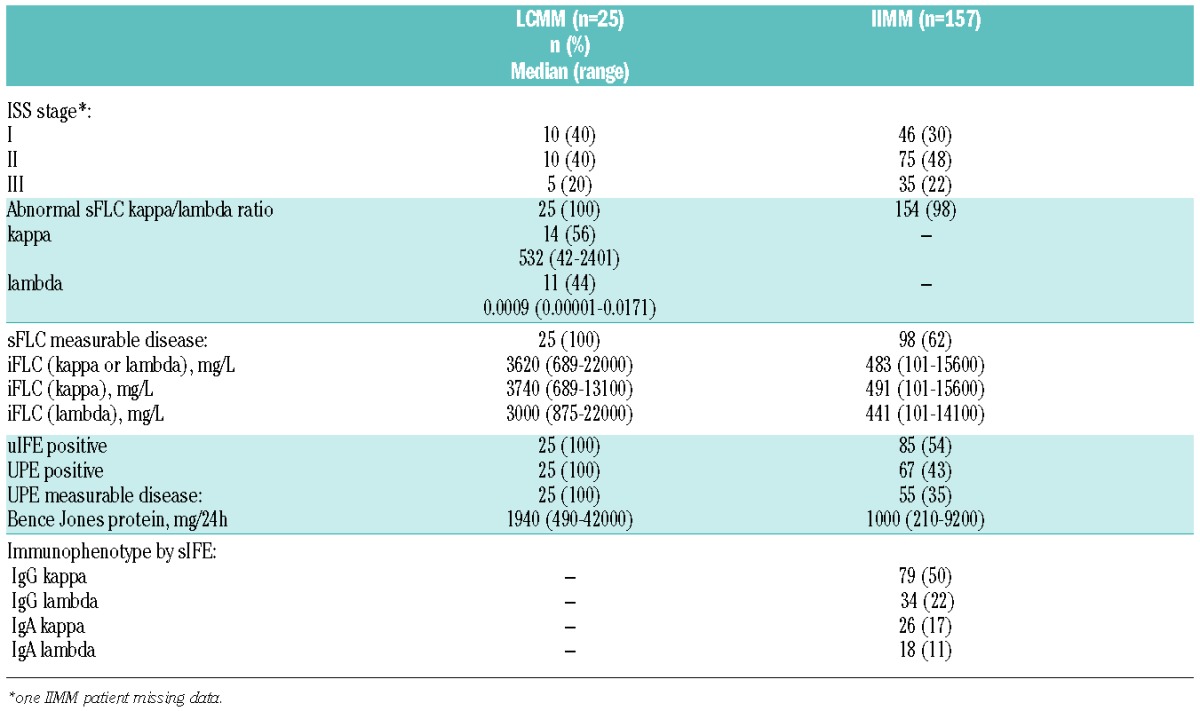
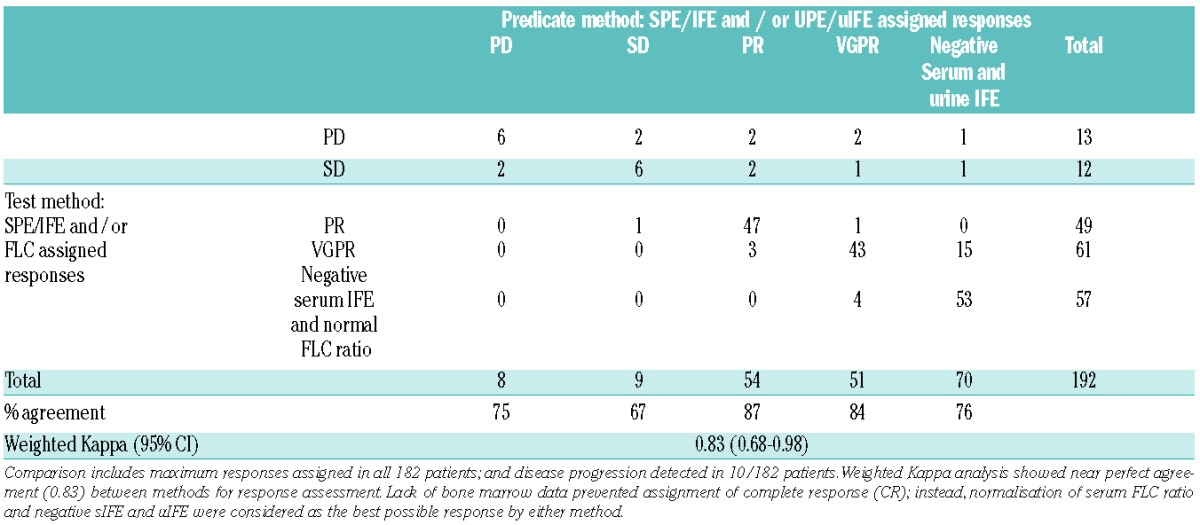
Table 2.
Weighted Kappa analysis comparing IMWG responses as determined by predicate (serum and/or urine electrophoresis) and test (serum electrophoresis and/or serum FLC) methods in 25 LCMM and 157 IIMM patients.
In IIMM patients, by contrast, sFLC ratios were abnormal in 154 out of 157 (98%) IIMM patients whereas 85 out of 157 (54%) were positive by uIFE and 67 out of 157 (43%) by UPE (Table 1 and Figure 1B). 98 out of 157 (62%) patients had measurable disease by sFLC and 55 out of 157 (35%) by UPE. Correlation between sFLC and UPE measurements was poor (r=0.36), as was the correlation between intact immunoglobulin by SPE and sFLC (r=−0.06) or UPE (r=−0.26) (data not shown).
Response assessment
In all patients (25 LCMM and 157 IIMM; in 10 patients two responses were scored: at maximum response and at progression as determined by at least one test), comparison in response evaluation between predicate methods (serum and/or urine electrophoresis) using IMWG criteria and test assessment (serum electrophoresis and serum FLC) demonstrated concordance in 155 out of 192 (81%) cases (6 PD, 6 SD, 47 PR, 43 VGPR and 53 patients in whom all serum and urine tests normalised). Weighted Kappa analysis indicated near perfect agreement between methods for response assignment (WK (95%CI): 0.85 (0.68–0.98)). The most significant difference among the 37 out of 192 discrepant responses was for 1 LCMM patient who progressed at cycle 4 by serum FLC assessment (iFLC=200mg/L) whilst urine IFE was negative. Conversely, UPE identified progressive disease in 2 LCMM patients in whom sFLC assessment indicated stable disease. In addition, relapse involving an apparent “light chain escape” was seen in 3 IIMM patients: in 2 of these 3 patients light chain escape was identified by both sFLC and UPE, while in the third patient it was detected by sFLC analysis alone.
A direct comparison between urine and serum FLC assessment during follow-up showed that the sFLC ratio had normalised in only 2 out of 19 (11%) LCMM patients with serum and urine measurements available at the end of cycle 2, compared to 9 out of 19 (47%) patients in whom uIFE had become negative. Similarly, the sFLC ratio had normalised in 3 out of 21 (14%) and 8 out of 21 (38%) patients at cycles 4 and post-ASCT, respectively, compared to 14 out of 21 (67%) patients with negative uIFE at either time point (Figure 2A). Likewise in IIMM patients with measurable disease and complete data by serum and urine IFE and serum FLC for each time point analysed, 19 out of 44 (43%) patients had a negative uIFE at the end of cycle 2 compared to 12 out of 44 (27%) patients in whom the FLC ratio had normalised and 4 out of 44 (9%) patients for whom serum IFE had become negative. Similar discrepant results were found at the end of cycle 4 and post-ASCT (Figure 2B).
Figure 2.
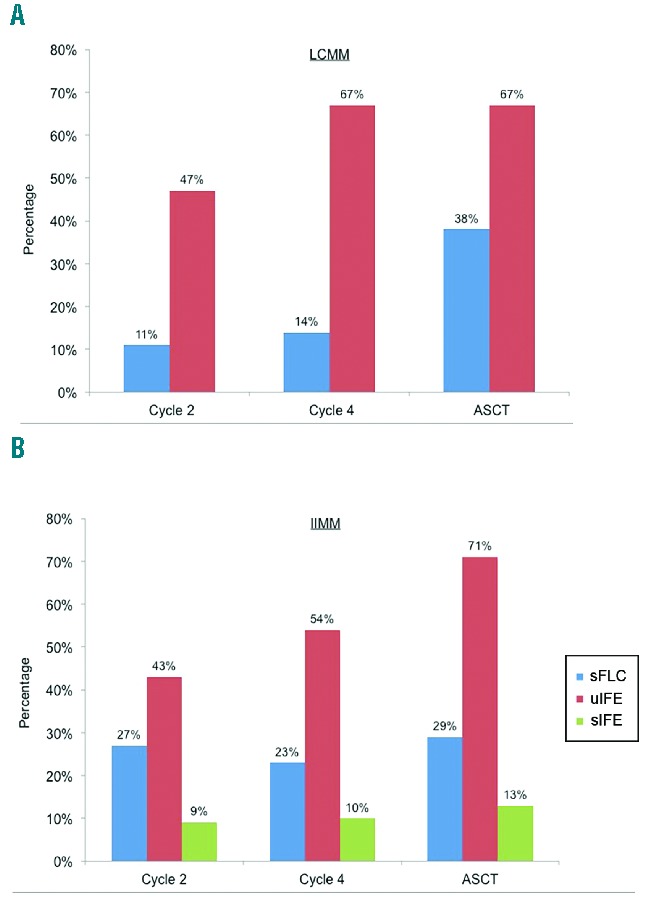
Normalisation of serum and urine tests during follow-up. A) Bar chart shows percentage of LCMM patients whose sFLC ratio (blue bar) or uIFE (red bar) had normalised at the end of cycle 2 (n=19), cycle 4 (n=21) and post autologous stem cell transplant (ASCT; n=21). B) Bar chart shows percentage of IIMM patients whose sFLC ratio (blue bar), uIFE (red bar) or serum IFE (green bar) had normalised at the end of cycle 2 (n=44), cycle 4 (n=48) and post-ASCT (n=38). Analyses include patients with measurable levels of disease by each method at presentation as per IMWG guidelines, and complete data for all tests at each time point.
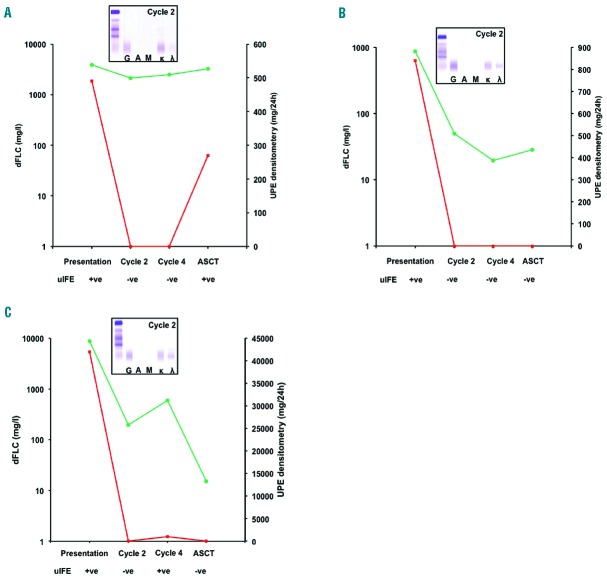
Three case studies are presented of LCMM patients in whom sFLC remained abnormal while urine tests normalised during monitoring. In all three cases urine assessment became negative for the presence of BJP at cycle 2, whereas sFLC remained abnormal and serum immunofixation was positive (Figure 3). Finally, 5 out of 157 IIMM patients had M-Ig <10g/L by SPE but measurable levels of disease by both UPE and sFLC. During monitoring UPE became negative in all 5 patients by cycle 2, whereas an abnormal sFLC ratio and positive serum immunofixation indicated persistent disease (data not shown).
Figure 3.
Case studies of 3 LCMM patients monitored during the course of therapy. At presentation all three patients were UPE and uIFE positive and had abnormal sFLC ratios. In all three patients the sFLC ratio remained abnormal throughout monitoring. (A) dFLC indicated no response to therapy in this κFLC patient. UPE and uIFE became negative at cycle 2; monoclonal bands reappeared post-transplantation, indicating disease progression. (B) and (C) depict two λFLC patients; in both cases dFLC indicated VGPR at the end of cycle 2 whereas UPE and uIFE became negative. In all cases sIFE confirmed the presence of residual disease. dFLC: green lines; UPE: red lines; positive uIFE: +ve; negative uIFE: −ve; inserted gels show sIFE results at the end of cycle 2.
Discussion
24h urine collection can help distinguish between glomerular and tubular proteinuria.29,30 However, the collection of samples is cumbersome and inconvenient for the patient, leading to poor compliance,7–9 and the laboratory treatment of the samples varies and can be laborious.31,32 In addition, the excretion of BJP is affected by kidney function, and electrophoretic methods for quantifying the monoclonal protein in urine from patients with plasma cell dyscrasias can be insensitive.4 Therefore there is a need for alternative tests that overcome the limitations imposed by urine testing. In recent years the use of sFLC analysis for screening and monitoring patients with monoclonal gammopathies has gained importance and has been proposed as a potential substitute for 24h urine assessment, as it may resolve some of the difficulties associated with this approach.
In keeping with previous publications33 we found poor correlation between sFLC and BJP urine excretion levels in both LCMM (r=0.27) and IIMM (r=0.36) patients. In 4 out of 25 LCMM patients, sFLC reported disproportionately high concentrations of the monoclonal protein relative to UPE; these values may be influenced by the aggregation state of the FLC as previously reported.34–36 Conversely, in 3 out of 25 patients it was urine measurements that reported improbably high BJP levels by UPE densitometry; aggregation is unlikely to have caused these results, and an analytical error or the presence of intact immunoglobulin fragments cannot be discounted.5
Acknowledging the quantitative differences between the assays, sFLC tests were more sensitive than UPE and uIFE during follow-up in LCMM patients, with sIFE data confirming clonality and corroborating the sFLC results in some patients. For instance, UPE became negative in 2 LCMM patients but reappeared at the next assessment, indicating relapse; whereas FLC indicated stable disease. In both of these patients positive sIFE confirmed the presence of clonal light chains, indicating that, in some instances, treatment responses may be overestimated by urine analysis. More clinically relevant was the identification of one LCMM patient with progressive disease by an increase in iFLC >200mg/L at the end of cycle 4, which would have qualified the patient for receiving treatment even in the absence of clinical symptoms, according to IMWG;37 urine tests at this time point were negative.
We also found that sFLC had greater sensitivity than urine analysis for the detection of monoclonal FLC in patients expressing intact monoclonal immunoglobulins. This result may not be surprising as FLC production in IIMM is generally lower than that in LCMM,12 and it is probable that the lack of FLC in the urine was due to reabsorption in the kidneys. In support of this we observed the rapid disappearance of BJP in urine in a disproportionately high percentage of patients after only 2 cycles of therapy, compared to serum assessment. This suggests that sFLC quantification may reflect the tumour’s response to therapy better than BJP measurements. This might be particularly relevant for monitoring IIMM patients with non-measurable disease by SPE (M-Ig <10g/L). Our data supports a role for sFLC measurement for monitoring these patients, as UPE overestimated the response in all 5 oligosecretory patients in our cohort by becoming negative by cycle 2, whereas an abnormal sFLC ratio and elevated iFLC indicated the presence of disease at this stage, which was supported by a positive IFE. Importantly, these discrepancies may have clinical impact as normalisation of the sFLC ratio to achieve stringent CR,26 and indeed at any level of response,22,38,39 associates with improved survival outcomes.
We further report on 3 patients undergoing clonal changes consistent with light chain escape, one of whom was identified by sFLC analysis only. Changes in the production of monoclonal protein by MM tumour cells (“light chain escape”) were described over 50 years ago via urine analysis40,41 and are readily identified by sFLC analysis.42–44 The MRCIX trial has provided the largest and most comprehensive study to date into this phenomenon, and reported that nearly 50% of patients with light chain escape as identified by sFLC monitoring were missed by urine assessment.44 Importantly the study also demonstrated an association of light chain escape with poorer prognosis. Recent research has shown that the use of novel, more intensive therapies has brought about an increase in the frequency of light chain escape at relapse;45 in this context it would appear that the superior sensitivity of sFLC over urine measurements provides additional clinical information, making a case for the use of serum over urine assessments for monitoring MM patients.
The sensitivity of the tests is likely to be influenced by renal function.3,4 A previous study suggested that serum concentrations of 133 mg/L kappa and 278 mg/L lambda light chains are required to overwhelm the reabsorption capacity of the kidney and allow detection of FLCs in urine;3 the impact of charge46 and blood pressure47 may influence the presence of FLC in urine, adding to the subjective nature of the electrophoretic assessment and making these cut-offs somewhat subjective. sFLC assessment can also be affected by renal function, with a proportional increase of kappa over lambda FLC concentrations as renal function deteriorates that may result in a shift in the sFLCκ/λ ratio. Hence a renal reference range has been proposed that corrects for renal function in patients with kidney disease.48,49 It is improbable that renal function affected our findings since the study protocol excluded all patients with renal impairment (median creatininemia = 88 (71–103) μM);23 furthermore, when tested, the use of the renal reference range during monitoring in our population had no impact on the results (data not shown).
A limitation of our study is the lack of bone marrow data, which prevented us from assigning complete responses (CR) as defined by IMWG guidelines. Instead, we reported patients in which BJP was no longer detected in urine electrophoresis and those whose sFLC ratio and SPE had normalised, as a surrogate for CR. It must be pointed out though that about 86% of these patients would have achieved a conventional CR should bone marrow information have been available.50 Future studies must formally address the degree of response indicated by the various serum and urine assessments together with bone marrow infiltration and immunohistology/flow cytometry data, with the ultimate goal of determining which method provides the most relevant clinical information in terms of progression and survival outcome.
In summary, we have shown that replacing urine for sFLC measurements does not significantly affect response evaluation in a combined population of LCMM and IIMM patients. However sFLC assessment provides a more sensitive measure of tumour FLC production than urine analysis, and has a greater concordance with serum monoclonal immunoglobulin markers for the presence of monoclonal protein during follow-up. On this basis, we feel our results add to previous reports suggesting that sFLC analysis could be considered as a suitable alternative to urine electrophoresis when monitoring myeloma patients.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/3/356
References
- 1.Kyle R, Child JA, Anderson K, et al. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5)749–757. [PubMed] [Google Scholar]
- 2.Katzmann JA, Kyle RA, Benson J, et al. Screening panels for detection of monoclonal gammopathies. Clin Chem. 2009;55(8): 1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowrousian MR, Brandhorst D, Sammet C, et al. Serum free light chain analysis and urine immunofixation electrophoresis in patients with multiple myeloma. Clin Cancer Res. 2005;11(24 Pt 1):8706–8714. [DOI] [PubMed] [Google Scholar]
- 4.Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361(9356):489–491. [DOI] [PubMed] [Google Scholar]
- 5.Siegel DS, McBride L, Bilotti E, et al. Inaccuracies in 24-hour urine testing for monoclonal gammopathies. Lab Med. 2009;40(6):341–344. [Google Scholar]
- 6.Fidler CJ, Hussein AKA, Gandhi N, et al. Evaluating trends in diagnostic and prognostic testing for multiple myeloma. Blood. 2011;118(21):2067 Abstr. [Google Scholar]
- 7.Robson EJD, Taylor J, Beardsmore C, Basu S, Mead G, Lovatt T. Utility of serum free light chain analysis when screening for lympho-proliferative disorders. Lab Med. 2009;40(6): 325–329. [Google Scholar]
- 8.Beetham R, Wassell J, Wallage MJ, Whiteway AJ, James JA. Can serum free light chains replace urine electrophoresis in the detection of monoclonal gammopathies? Ann Clin Biochem. 2007;44(Pt 6):516–522. [DOI] [PubMed] [Google Scholar]
- 9.Holding S, Spradbery D, Hoole R, et al. Use of serum free light chain analysis and urine protein electrophoresis for detection of monoclonal gammopathies. Clin Chem Lab Med. 2011;49(1):83–88. [DOI] [PubMed] [Google Scholar]
- 10.Bradwell AR, Carr-Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47(4): 673–680. [PubMed] [Google Scholar]
- 11.Katzmann JA, Dispenzieri A, Kyle RA, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc. 2006;81(12):1575–1578. [DOI] [PubMed] [Google Scholar]
- 12.Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol. 2004;126(3): 348–354. [DOI] [PubMed] [Google Scholar]
- 13.Abraham RS, Clark RJ, Bryant SC, et al. Correlation of serum immunoglobulin free light chain quantification with urinary Bence Jones protein in light chain myeloma. Clin Chem. 2002;48(4):655–657. [PubMed] [Google Scholar]
- 14.Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood. 2001;97(9):2900–2902. [DOI] [PubMed] [Google Scholar]
- 15.Lachmann HJ, Gallimore R, Gillmore JD, et al. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 2003;122(1):78–84. [DOI] [PubMed] [Google Scholar]
- 16.Dispenzieri A, Lacy MQ, Katzmann JA, et al. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2006;107(8):3378–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111(2):785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106(3):812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill PG, Forsyth JM, Rai B, Mayne S. Serum free light chains: an alternative to the urine Bence Jones proteins screening test for monoclonal gammopathies. Clin Chem. 2006; 52(9):1743–1748. [DOI] [PubMed] [Google Scholar]
- 20.Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215–224. [DOI] [PubMed] [Google Scholar]
- 21.Boyle E, Brioli A, Leleu X, et al. The value of serum free light chain monitoring compared to urinary Bence-Jones measurement in light chain only myeloma. Blood. 2013;122(21): 1895a [Google Scholar]
- 22.Kapoor P, Kumar SK, Dispenzieri A, et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2013;31(36):4529–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118(22):5752–5758. [DOI] [PubMed] [Google Scholar]
- 24.Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437–1444. [PubMed] [Google Scholar]
- 25.Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118(22):5752–5758. [DOI] [PubMed] [Google Scholar]
- 26.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9): 1467–1473. [DOI] [PubMed] [Google Scholar]
- 27.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18): 4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 29.Bottini PV, Ribeiro Alves MA, Garlipp CR. Electrophoretic pattern of concentrated urine: comparison between 24-hour collection and random samples. Am J Kidney Dis. 2002;39(1):E2. [DOI] [PubMed] [Google Scholar]
- 30.Levinson SS. Polyclonal free light chain of Ig may interfere with interpretation of monoclonal free light chain / ratio. Ann Clin Lab Sci. 2010;40(4):348–353. [PubMed] [Google Scholar]
- 31.Levinson SS, Keren DF. Free light chains of immunoglobulins: clinical laboratory analysis. Clin Chem. 1994;40(10):1869–1878. [PubMed] [Google Scholar]
- 32.Kaplan JS, Horowitz GL. Twenty-four-hour Bence-Jones protein determinations: can we ensure accuracy? Arch Pathol Lab Med. 2011;135(8):1048–1051. [DOI] [PubMed] [Google Scholar]
- 33.Dispenzieri A, Zhang L, Katzmann JA, et al. Appraisal of immunoglobulin free light chain as a marker of response. Blood. 2008;111(10):4908–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abraham RS, Charlesworth MC, Owen BA, et al. Trimolecular complexes of lambda light chain dimers in serum of a patient with multiple myeloma. Clin Chem. 2002;48(10): 1805–1811. [PubMed] [Google Scholar]
- 35.Harding SJ, Sharp K, Steiner A, et al. Quantification of polymerising serum free light chains. Clin Lymphoma Myeloma. 2009;9(s1):S101–S102. [Google Scholar]
- 36.Harding S, Provot F, Beuscart JB, et al. Aggregated serum free light chains may prevent adequate removal by high cut-off haemodialysis. Nephrol Dial Transplant. 2011;26(4):1438–1440. [DOI] [PubMed] [Google Scholar]
- 37.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18): 4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alhaj MM, Rajkumar SV, Dispenzieri A, et al. Utility of serum free light chain measurements in multiple myeloma patients not achieving complete response to therapy. Leukemia. 2015;29(10):2033–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drayson MT, Berlanga O, Plant T, Newnham NJ, Young P, Harding S. Immunoglobulin heavy/light chain measurements during monitoring provide prognostic information of relapse after therapy in myeloma patients. Blood. 2012;120(21): 3964 Abstr. [Google Scholar]
- 40.Hobbs JR. Growth rates and responses to treatment in human myelomatosis. Br J Haematol. 1969;16(6):607–617. [DOI] [PubMed] [Google Scholar]
- 41.Hobbs JR. Monitoring myelomatosis. Arch Intern Med. 1975;135(1):125–130. [PubMed] [Google Scholar]
- 42.Dawson MA, Patil S, Spencer A. Extramedullary relapse of multiple myeloma associated with a shift in secretion from intact immunoglobulin to light chains. Haematologica. 2007;92(1):143–144. [DOI] [PubMed] [Google Scholar]
- 43.Hobbs JA, Drayson MT, Sharp K, Harding S, Bradwell AR, Mead GP. Frequency of altered monoclonal protein production at relapse of multiple myeloma. Br J Haematol. 2009;148(4):659–661. [DOI] [PubMed] [Google Scholar]
- 44.Brioli A, Giles H, Pawlyn C, et al. Serum free immunoglobulin light chain evaluation as a marker of impact from intraclonal heterogeneity on myeloma outcome. Blood. 2014;123(22):3414–3419. [DOI] [PubMed] [Google Scholar]
- 45.Kuhnemund A, Liebisch P, Bauchmuller K, et al. ‘Light-chain escape-multiple myeloma’-an escape phenomenon from plateau phase: report of the largest patient series using LC-monitoring. J Cancer Res Clin Oncol. 2009;135(3):477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klassen RB, Allen PL, Batuman V, Crenshaw K, Hammond TG. Light chains are a ligand for megalin. J Appl Physiol. 2005;98(1):257–263. [DOI] [PubMed] [Google Scholar]
- 47.Ritz E. Nephrology beyond JASN: Plasma exchange for acute renal failure of myeloma - logical, yet ineffective. J Am Soc Nephrol. 2006;17:914–916. [DOI] [PubMed] [Google Scholar]
- 48.Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(6):1684–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement aids the diagnosis of myeloma in patients with severe renal failure. BMC Nephrol. 2008;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chee CE, Kumar S, Larson DR, et al. The importance of bone marrow examination in determining complete response to therapy in patients with multiple myeloma. Blood. 2009;114(13):2617–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]