Abstract
We analyzed the impact of human cytomegalovirus infection on the development of natural killer cells in 27 pediatric patients affected by hematological malignancies, who had received a HLA-haploidentical hematopoietic stem cell transplantation, depleted of both α/β+ T cells and B cells. In line with previous studies in adult recipients of umbilical cord blood transplantation, we found that human cytomegalovirus reactivation accelerated the emergence of mature natural killer cells. Thus, most children displayed a progressive expansion of a memory-like natural killer cell subset expressing NKG2C, a putative receptor for human cytomegalovirus, and CD57, a marker of terminal natural killer cell differentiation. NKG2C+CD57+ natural killer cells were detectable by month 3 following hematopoietic stem cell transplantation and expanded until at least month 12. These cells were characterized by high killer Ig-like receptors (KIRs) and leukocyte inhibitory receptor 1 (LIR-1) and low Siglec-7, NKG2A and Interleukin-18Rα expression, killed tumor targets and responded to cells expressing HLA-E (a NKG2C ligand). In addition, they were poor Interferon-γ producers in response to Interleukin-12 and Interleukin-18. The impaired response to these cytokines, together with their highly differentiated profile, may reflect their skewing toward an adaptive condition specialized in controlling human cytomegalovirus. In conclusion, in pediatric patients receiving a type of allograft different from umbilical cord blood transplantation, human cytomegalovirus also induced memory-like natural killer cells, possibly contributing to controlling infections and reinforcing anti-leukemia effects.
Introduction
Natural killer (NK) cells are innate lymphocytes that play an important role in anti-viral and anti-tumor responses.1 Their function is finely regulated by an array of both activating and inhibitory surface receptors2–4 and can be strongly influenced by several other factors, such as exposure to cytokines and/or PAMPs,5 developmental stage,6 and licensing.7,8 A fundamental role is played by HLA-class I specific inhibitory receptors including: killer Ig-like receptors (KIRs) distinguishing among allotypic determinants of the HLA-A, -B and -C;9 the HLA-E-specific CD94/NKG2A heterodimer10 and the leukocyte inhibitory receptor 1 (LIR-1 or ILT2) broadly recognizing HLA class I alleles.11 Activating KIRs, as well as CD94/NKG2C, represent the activating counterpart of HLA-I specific inhibitory receptors, although the ligand specificity is known only for selected receptors (i.e. KIR2DS1, KIR2DS4 and CD94/NKG2C).10,12–14
Since NK cells are the first lymphocyte population to emerge after hematopoietic stem cell transplantation (HSCT), their role in early recovery of immunity after the allograft is considered crucial, contributing to protection from both tumor recurrence and viral infections before the full restoration of T cell immunity. In KIR/KIR-L mismatched haplo-HSCT recipients, alloreactive NK cells, generated 6–8 weeks after HSCT,15 are capable of killing residual tumor cells, thus critically improving patients outcome.16,17 The first wave of NK cells after HSCT is represented by immature CD56bright CD94/NKG2Abright NK cells, while more differentiated CD56dim KIR+ NKG2A− NK cells, containing alloreactive NK cells, only emerge later.15,18,19 To reduce the time window required for fully competent NK cell generation, a new method of graft manipulation has been developed and applied; this approach is based on the elimination of αβ+ T cells (to prevent graft-versus-host disease, GvHD) and of B cells (to avoid EBV-related post-transplant lymphoproliferative disorders). Notably, together with high numbers of CD34+ HSC, this graft contains donor-derived, mature NK cells and γδ T cells20 which may confer prompt protection against both leukemia recurrence and infections.21
As recently shown, NK cell reconstitution after HSCT can be highly accelerated by human cytomegalovirus (HCMV) infection/reactivation.22,23 Indeed, in patients receiving umbilical cord blood transplantation (UCBT), HCMV infection induced a rapid development of mature NK cells characterized by the KIR+NKG2A− phenotype. Importantly, these cells expressed NKG2C. Although the exact mechanism(s) involved in HCMV-induced NKG2C+ NK cell expansion has not been clarified, it is likely that NKG2C may play a role in HCMV recognition and in promoting the expansion and/or maturation of NKG2C+ cells,24 as well as in the control of HCMV infection, as suggested in the case of a T cell deficient patient.25 In addition, a correlation between early HCMV reactivation and a reduced incidence of leukemia relapse has been reported in adult patients with acute myeloid leukemia (AML) receiving allo-HSCT.26
In the present study, we analyzed the impact of HCMV reactivation on the development of NK cells in a cohort of pediatric patients affected by hematological malignancies who received α/β+ T cell- and B cell-depleted HSCT. We observed a great expansion of memory-like NK cells in HCMV-reactivating/infected patients that express NKG2C, a putative receptor for HCMV and CD57, a marker of terminal differentiation.27,28
Methods
Patients and samples
A cohort of 27 pediatric patients affected by hematological malignancies (mainly acute lymphoblastic leukemia, ALL) was enrolled in a phase I/II trial (ClinicalTrials.gov Identifier NCT01810120) and received HLA-haploidentical HSCT after removal of both αβ+T cells and CD19+ B cells.20 Patients were transplanted between November 2010 and May 2012. Among them, 13 experienced HCMV infection after transplantation. Of these 13 patients, 12 had a positive HCMV serology, and 1 had a negative HCMV serology. However, for the sake of brevity, we consider all 13 patients together as the HCMV-reactivating group throughout this article. The clinical characteristics of patients and details on graft composition are summarized in the Online Supplementary Table S1. The assessment of HCMV serology, episodes of HCMV infection/reactivation and therapy are detailed in the Online Supplementary Methods and summarized in the Online Supplementary Table S2.
Peripheral blood samples were collected at 1, 3, 6 and 12 months after transplantation. Peripheral blood mononuclear cells (PBMC) were separated from blood samples by ficoll-hypaque gradients (Sigma-Aldrich, St. Louis, MO, USA) and used directly for flow cytometry analyses and functional assays, or frozen and subsequently thawed for further investigations, whenever indicated. NK cell reconstitution was analyzed at the above time points, from month 1 to month 12 for 21 patients, and from month 1 to month 6 for 6 patients.
PBMC collected from adult healthy donors (HD) were used as controls. Frozen samples from HSC donors peripheral blood (PB) or leukapheresis were also analyzed.
To compare NK cell subsets differentiation, PBMC from 10 pediatric UCBT recipients and 5 pediatric recipients of positively selected CD34+HSC from a HLA-haploidentical parent were also analyzed.
Patients were transplanted at the Bambino Gesù Children’s Hospital, Rome, Italy. Patients’ parents gave their informed consent to participation in this study, which was approved by the Azienda Ospedaliera Universitaria San Martino (Genoa, Italy), by the University of Genoa (Genoa, Italy) and by the Bambino Gesù Children’s Hospital (Rome, Italy) ethics committees and was conducted in accordance with the tenants of the Declaration of Helsinki.
Monoclonal antibodies and flow cytometry, functional assays, KIR-ligands and KIR genes profile analyses and statistical analysis
See Online Supplementary Methods for details.
Results
HCMV reactivation/infection accelerates NK cell maturation in αβ+T/B cell-depleted HSCT pediatric patients
We analyzed NK cell reconstitution in 27 pediatric patients undergoing αβ+T/B cell-depleted HSCT and compared, at different time intervals post-HSCT, data in children who experienced HCMV reactivation (or primary infection in 1 case) (n=13) with those of children who did not (n=14). In all cases, reactivation/infection occurred within month 2 after HSCT and the virus was cleared by month 6.
The cells infused with this type of transplantation contain not only CD34+ HSC, but also donor-derived NK and γδ T cells (see Online Supplementary Table S1 for details). Thus, at early time points after transplantation, peripheral blood NK cells contain mature NK cells together with HSC-derived NK cells. Although, due to technical limitation, the mature NK cells could not be distinguished from de novo generated NK cells, a remarkable difference could be detected between patients who either did or did not reactivate HCMV.
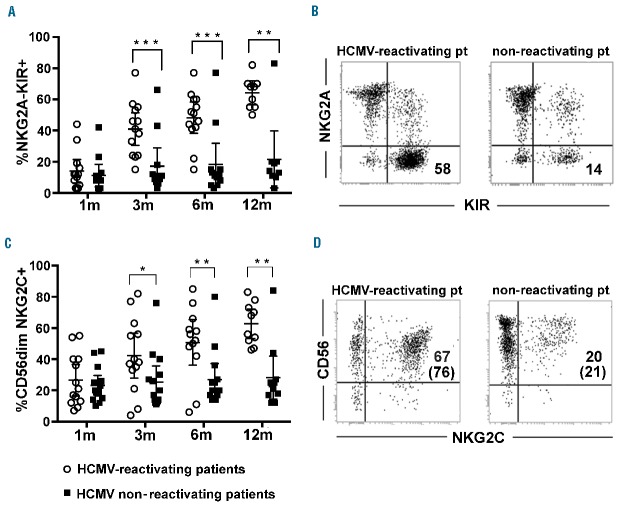
HCMV reactivation/infection accelerated the differentiation of mature NK cells, as shown by the higher frequency of KIR+NKG2A− NK cells by month 3 after HSCT in HCMV-reactivating patients (Figure 1A). Major differences emerged at 6 months after HSCT between HCMV-reactivating and non-reactivating patients (two representative patients are shown in Figure 1B). In line with previous studies,22,23,29 HCMV reactivation induced a strong imprinting in NK cell development not only by accelerating KIR+NKG2A− NK cell differentiation, but also by inducing a remarkable increase of CD56dim NKG2C+ NK cells (Figure 1C,D).
Figure 1.
HCMV induces rapid differentiation of NKG2A−KIR+ NKG2C+ NK cells in patients receiving αβ+T/B cell-depleted haplo-HSCT. Freshly collected PBNK cells from the various patients were analyzed by multicolor immunofluorescence and FACS analysis at different time intervals after HSCT. NK cells were gated from PBMC samples as CD3−CD19− lymphocytes. In (A) the expression of NKG2A in combination with KIRs was analyzed and the percentages of NKG2A−KIR+ NK cells in patients who did (empty circles, n=13) or did not (filled black squares, n=14) experience HCMV after transplantation are reported at 1, 3, 6 and 12 months after HSCT. 95% CI for the mean and statistical significance are indicated (*P<0.05; ** P<0.01; *** P<0.001). In (B) reciprocal expression of NKG2A and KIR are shown for two representative patients, one reactivating (left panel) and the other non-reactivating HCMV (right panel), at 6 months after transplantation. The percentage of NKG2A-KIR+ NK cells is indicated in the lower right quadrant. In (C), after gating on CD56dim NK cells, the percentages of CD56dim NK cells expressing NKG2C are reported, at the different time points, in patients experiencing (empty circles, n=13) or not (filled black squares, n=14) HCMV reactivation after transplantation. 95% CI for the mean and statistical significance are indicated. In (D) NK cells from two representative patients are shown at 6 months after HSCT. The percentages of NKG2C+ NK cells are depicted in the upper right quadrants. Numbers in brackets represent the percentages of NKG2C+ NK cells by gating on the CD56dim NK cell subset.
Notably, two patients in the HCMV-reactivating group did not expand NKG2C+ NK cells, while two others in the HCMV non-reactivating group developed high proportions of NKG2C+ NK cells and were also characterized by high percentages of NKG2A−KIR+ NK cells (see dots in individual plots in Figure 1A,C).
In line with our previous study showing the emergence of high proportions of hypofunctional and phenotypically aberrant CD56−CD16+ NK cells in HCMV-reactivating adult recipients of UCBT,22 in our pediatric cohort we also found the presence of this peculiar NK cell subset in significantly higher frequencies in HCMV-reactivating patients than in non-reactivating ones (Figure 2A). However, the proportion of these cells at month 6 was significantly lower in the αβ+T/B cell-depleted cohort of HCMV-reactivating patients (Figure 2B and Online Supplementary Figure S1) than in the other two cohorts analyzed in parallel (namely, one undergoing UCBT and the other receiving only megadoses of positively selected CD34+ cells from haploidentical donors).
Figure 2.
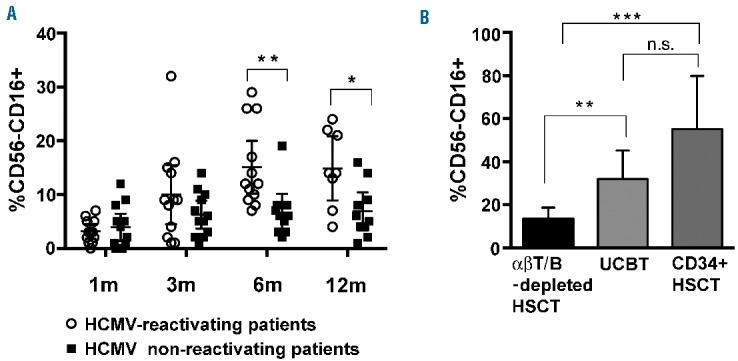
Modest accumulation of aberrant CD56−CD16+ NK cells in patients undergoing HCMV reactivation after αβ+ T/B cell-depleted transplant as compared to patients receiving UCBT or purified CD34+ cells. PBNK cells from the various patients were analyzed for the expression of CD56 and CD16 at 1, 3, 6 and 12 months after HSCT. In (A), the percentages of CD56−CD16+ NK cells in patients experiencing HCMV (empty circles, n=13) or not (filled black squares, n=14) after transplantation are reported at the different time points. 95% CI for the mean and statistical significance are indicated (*P<0.05; ** P<0.01; *** P<0.001). In (B), the percentages of CD56−CD16+ NK cells measured in αβ+ T/B cell-depleted haplo-HSCT (n=13) patients are compared to those measured in two different groups of pediatric patients reactivating HCMV after transplantation who received either cord blood transplantation (UCBT) (n=5) or positively selected CD34+ HSC (CD34+ haplo-HSCT) (n=5). The values reported correspond to 6 months after HSCT for all patients. 95% CI for the mean and statistical significance are indicated.
High proportions of “memory-like” NKG2C+ CD57+ CD56dim NK cells develop in patients experiencing HCMV reactivation
In both solid organ transplanted patients and HSCT recipients, HCMV infection can induce the expansion of NKG2C+ CD57+ NK cells.23,30 This subset is present at variable proportions, also in HCMV-seropositive (HCMV+) healthy individuals,31 and may possibly be endowed with “memory” properties. Thus, we analyzed the development of such NKG2C± CD57± NK cell subsets in our cohort of patients.
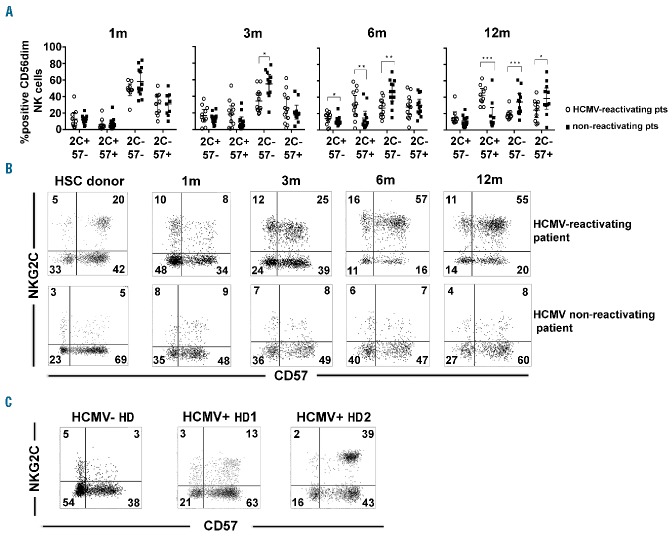
In Figure 3A, the distribution of different NKG2C/CD57 NK cell subsets (gated on CD56dim NK cells) is reported at different time points after HSCT for patients who did or did not reactivate HCMV (the gating strategy is shown in Online Supplementary Figure S2A). By month 3, a substantial increase of NKG2C+CD57+ NK cells was detectable in HCMV-reactivating patients. More marked differences were found at month 6, when much higher proportions of NKG2C+CD57+ cells, paralleled by a sharp decrease of NKG2C− CD57− NK cells, were detectable in HCMV-reactivating patients.
Figure 3.
Different NK cell subsets identified by NKG2C/CD57 expression: progressive expansion of memory-like NKG2C+CD57+ NK cells in patients reactivating HCMV after HSCT. PBNK cells from the various patients were analyzed for the expression of NKG2C and CD57 at 1, 3, 6 and 12 months after HSCT. After gating on CD56+CD3−CD19− lymphocytes, CD56dim NK cells were evaluated. In (A) the size of the different subsets identified by NKG2C and CD57 on CD56dim NK cells (i.e. NKG2C+CD57−, NKG2C+CD57+, NKG2C−CD57− and NKG2C−CD57+, indicated for brevity as 2C and 57) in patients experiencing (empty circles, n=13) or not (filled black squares, n=14) HCMV reactivation after transplantation are shown from month 1 to month 12 after HSCT. 95% CI for the mean and statistical significance are indicated (*P<0.05; ** P<0.01; *** P<0.001). In (B), reciprocal expression of NKG2C and CD57 on CD56dim NK cells from two representative patients, one reactivating (upper panels), and the other non-reactivating HCMV (lower panels), is shown at different time intervals after transplantation in comparison to NK cells isolated from their respective HSC donors (left panels). The percentages of positive cells are indicated in each quadrant. In (C) reciprocal expression of NKG2C and CD57 on CD56dim NK cells from three HDs (one HCMV−, left panel; two HCMV+, middle and right panels) are shown. The percentages of positive cells are indicated in each quadrant.
Figure 3B shows data from two representative patients, one reactivating and the other not reactivating HCMV. A progressive expansion of the NKG2C+CD57+ NK cell subset (gating on the CD56dim subset) can be observed in the patient reactivating HCMV. Further analysis of PBNK cells from HCMV+ and HCMV− healthy donors (HDs) (Figure 3C and Online Supplementary Figure S2B) showed that the NKG2C+CD57+ NK cell subset was present in significantly lower frequencies in HCMV+ HDs than in HCMV-reactivating HSCT patients, both at month 6 and 12 (Online Supplementary Figure S2C).
When PBNK cells from the various HSC donors of HCMV-reactivating patients were analyzed, we found that NKG2C+CD57+ NK cells were present at a low median frequency (11%, Online Supplementary Figure S2D) although all the HSC donors analyzed were HCMV+ (Online Supplementary Table S1). Thus, 3–6 months after HSCT, in most HCMV-reactivating patients, the frequency of NKG2C+CD57+ NK cells exceeded that measured in their respective donors (Figure 3B, Online Supplementary Figure S2D and data not shown). In view of this observation, we can suggest that NKG2C+CD57+ NK cells emerging in HCMV-reactivating patients are mostly de novo generated.
Memory-like NK cells from recipients of αβ+T/B cell-depleted HSCT are characterized by the KIR+ NKG2A-Siglec7− LIR1+/− IL-18Ralow NCRlow surface phenotype
We analyzed in more detail the phenotype of the HCMV-induced NKG2C+CD57+ CD56dim NK cell subset, focusing on HCMV-reactivating patients at month 6 after HSCT, i.e. when these cells were maximally expanded. HCMV+ HDs were analyzed for comparison. Representative dot plots indicating the gating strategy are shown in Figure 4A.
Figure 4.
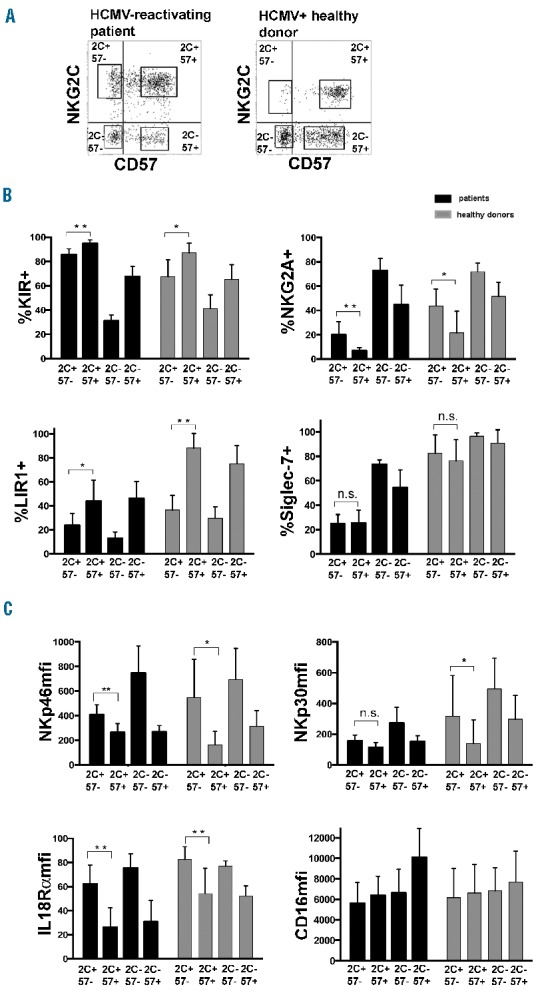
NKG2C+ CD57+ NK cells expanded in patients receiving αβ+ T/B cell-depleted HSCT and experiencing HCMV are characterized by a KIR+ NKG2A− Siglec7− LIR1+/−IL18Ralow NCRlow surface phenotype. PBNK cells collected at 6 months after HSCT from HCMV-reactivating patients and from HCMV+ HDs were analyzed for the expression of the indicated surface markers, after gating on the different CD56dim NK cell subsets identified by NKG2C and CD57 (i.e. NKG2C+CD57−, NKG2C+CD57+, NKG2C−CD57− and NKG2C-CD57+, indicated for brevity as 2C and 57). In (A) the gating strategy is shown for a representative patient and a donor, in (B) 95% CI for the mean percentage of positive CD56dim NK cells is shown for HCMV-reactivating patients (black bars, n=8) and for HCMV+ HDs (grey bars, n=7). In (C) 95% CI for the median fluorescence intensity (mfi) is similarly shown. Statistical significance was calculated for NKG2C+CD57− vs. NKG2C+CD57+ NK cell subsets for each surface marker (*P<0.05; ** P<0.01; *** P<0.001).
Both NKG2C+CD57− and NKG2C+CD57+ NK cells were mostly KIR+NKG2A− in both transplanted patients and HCMV+ HDs (Figure 4B). Notably, expanded NKG2C+ KIR+ NK cells preferentially expressed KIRs specific for the respective donor KIR ligands (Online Supplementary Figure S3 and data not shown). As previously reported in UCBT patients, Siglec-7 was significantly down-regulated in NKG2C+ NK cells isolated from patients, but only marginally in NK cells from HCMV+ HDs (Figure 4B).22 Importantly, Siglec-7 down-regulation was correlated with HCMV recurrence. Indeed, non-reactivating patients did not significantly down-regulate this marker at any time points after HSCT (Online Supplementary Figure S4), with the exception of the two outliers mentioned before.
The analysis of LIR-1 in our cohort of patients revealed a more frequent expression in NKG2C+CD57+ than in NKG2C+CD57− NK cells. This different expression was more evident in HCMV+ HDs, where most NKG2C+CD57+ NK cells were LIR-1+ (Figure 4B), in line with previous studies.31
The NKG2C+CD57− and NKG2C+CD57+ NK cells subsets of both HSCT recipients and HDs displayed remarkable differences in the expression levels of IL-18Rα (Figure 4C). This is in line with previous reports that terminally differentiated CD57+ NK cells express low levels of different cytokine receptors.27,28 Interestingly, both the proportion of IL-18Rα (data not shown) and the median surface intensity (Figure 4C) were lower in patients than in HDs in all CD56dim NK cell subsets. Figure 4C also shows that NKG2C+CD57+ (both in patients and HDs) display lower expression of NKp46 and NKp30 as compared to NKG2C+ CD57− NK cells. No significant differences were detected for other triggering receptors including CD16, DNAM1, NKG2D and 2B4 (Figure 4C and Online Supplementary Figure S5).
Memory-like NKG2C+CD57+ NK cells respond efficiently to tumor cells and HLA-E+ targets, but show impaired IFN-γ production in response to rhIL-12 plus rhIL-18 stimulation
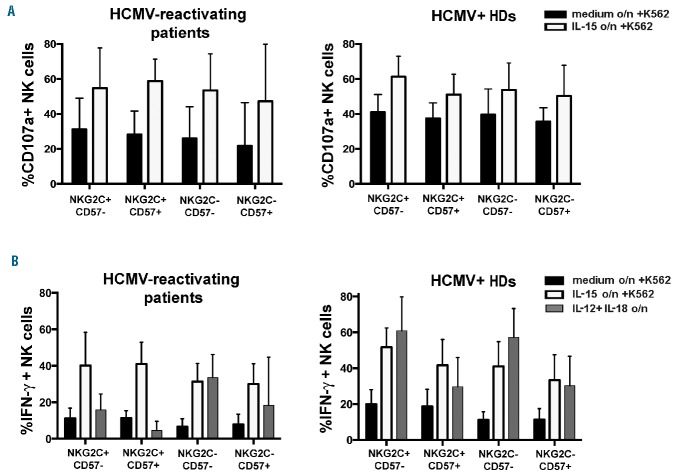
We next evaluated the functional capabilities of the expanded NKG2C+CD57+ memory-like NK cell subset (in comparison with the other subsets identified by the expression of NKG2C/CD57) by assessing degranulation and IFN-γ production upon K562 stimulation and/or exposure to cytokines. Figure 5A (left panel) shows that the different NK subsets (analyzed by gating on CD56dim NK cells) displayed comparable levels of CD107a degranulation, further increased by overnight exposure to rhIL-15. Figure 5A (right panel) shows that, in the absence of exogenous rhIL-15, PBNK cells from HCMV+ HDs displayed slightly higher degranulation as compared to patients.
Figure 5.
Memory-like NKG2C+CD57+ NK cells from patients are capable of both degranulating and producing IFN-γ in response to tumor targets, but show impaired IFN-γ production in response to rhIL-12 plus rhIL-18 stimulation. Freshly drawn PBMC from HCMV-reactivating patients (n=8) at 6 months after HSCT, and from HCMV+ HDs (n=6) were cultured overnight in the presence or in the absence of rhIL-15 or rhIL-12 plus rhIL-18. Then PB cells were incubated with either medium alone (data not shown) or K562 for 3 hours. In (A), after incubation with K562, CD107a expression was evaluated in the different NKG2C/CD57 CD56dim NK cell subsets shown in Fig.4. Black bars represent cells cultured overnight in medium alone, while white bars represent cells cultured overnight in rhIL-15. In B), parallel cultures were assessed for intracellular IFN-γ production upon overnight culture with medium alone (black bars) or rhIL-15 (white bars) followed by stimulation for 3 hours in the presence of K562 or upon overnight exposure to rhIL-12 plus rhIL-18 (grey bars). 95% CI for the mean of CD107a/IFN-γ positive NK cells is shown for each subset. Left panels show data relative to patients and right panels show data relative to HDs.
Regarding the production of IFN-γ, upon stimulation with K562, both the NKG2C+CD57− and NKG2C+CD57+ NK cell subsets (both in patients and HDs) were slightly better producers than NKG2C− NK cell subsets. Also, IFN-γ production was increased upon overnight exposure to rhIL-15 (Figure 5B).
In parallel experiments, the ability of the various NKG2C/CD57 subsets to produce IFN-γ was assessed after overnight incubation in the presence of rhIL-12 plus rhIL-18. Remarkably, under these conditions, NKG2C+CD57+ memory-like NK cells from patients resulted as poor IFN-γ producers (Figure 5B), while the less differentiated NKG2C−CD57− NK cells were the best producers. Intermediate levels of IFN-γ were produced by the NKG2C+CD57− and NKG2C−CD57+ NK subsets. These differences in response to rhIL-12 plus rhIL-18 might reflect, at least in part, the levels of expression of IL-18Rα (Figure 4C) in the different NKG2C/CD57 subsets. It is of note, in this context, that all the NK subsets analyzed expressed IL-12R at similar levels (data not shown). A lower production of IFN-γ, in response to rhIL-12 plus rhIL-18, was also detected in NKG2C+CD57+ NK cells from HDs. However, down-regulation of IL-18Rα expression was less marked and IFN-γ production was only partially compromised. At variance with IFN-γ production, exposure to rhIL-12 plus rhIL-18 induced comparable levels of degranulation in the different NKG2C/CD57 NK cell subsets after stimulation with K562 (Online Supplementary Figure S6).
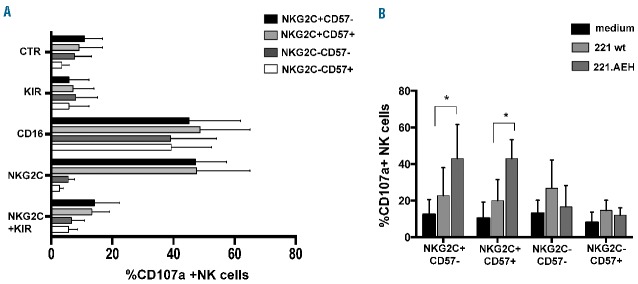
In another set of experiments, NKG2C+ NK cells from HCMV-reactivating patients were assessed for degranulation in reverse ADCC assays, either in the presence or in the absence of specific anti-NKG2C mAb. Antibody-mediated triggering of NKG2C efficiently induced degranulation in both NKG2C+CD57− and NKG2C+CD57+ NK cell subsets at levels comparable to those induced by anti- CD16 mAbs (Figure 6A). The simultaneous mAb-mediated triggering of NKG2C and LIR-1 did not affect degranulation induced by anti-NKG2C mAb alone (data not shown). On the contrary, the simultaneous mAb-mediated triggering of NKG2C and KIR almost completely abolished NKG2C-triggered degranulation (Figure 6A). Similar results were obtained in the analysis of HCMV+ HD NK cells (Online Supplementary Figure S7).
Figure 6.
Both anti-NKG2C mAbs and HLA-E+ target cells efficiently trigger degranulation of the memory-like NKG2C+CD57+ NK cell subset of HCMV-reactivating transplanted patients. Thawed PBMC from HCMV-reactivating patients, collected at 6 months after HSCT, were cultured in the presence of rhIL-15. In (A), after overnight culture, cells were incubated for 1h with the FcγR+ murine cell line p815 either in the presence or in the absence of anti-CD16, anti-NKG2C and anti-KIR specific mAbs alone or in combination (for each patient an anti-KIR mAb recognizing the KIR that was most expanded on NKG2C+CD57+ cells was used, i.e the KIR specific for the corresponding donor KIR ligand). CD107a expression is shown for each NK cell subset as 95% CI for the mean summarizing data for n=7 HCMV-reactivating HSCT patients. (dark bars: NKG2C+CD57−, light grey: NKG2C+CD57+, dark grey: NKG2C-CD57-, white bars: NKG2C-CD57+). CTR indicates NK cells cultured in the presence of p815 and in the absence of mAbs. In B), after 3 days of culture with rhIL-15, cells were incubated in medium alone (black bars) or with 221wt (light grey bars) or with 221 expressing HLA-E (221.AEH, dark grey bars). CD107a expression is shown for each NK cell subset as 95% CI for the mean summarizing data for n=5 HCMV-reactivating HSCT patients. Statistical significance is indicated (*P<0.05).
Next, we examined the ability of NKG2C+ NK cell subsets (either CD57+ or CD57−) to recognize HLA-E, a specific ligand of NKG2C,10 by assessing their degranulation, in CD107a assays, in the presence of the HLA-E expressing (transfected) 721.221 cell line (221.AEH).32 As shown in Figure 6B, both NKG2C+CD57− and NKG2C+CD57+ NK cell subsets displayed enhanced degranulation as compared to 221wt (i.e. lacking HLA-E surface expression, Online Supplementary Figure S8A), indicating that NKG2C recognizes HLA-E on target cells and induces activation/degranulation of NKG2C+ cells. This finding was further substantiated by masking experiments performed using a specific anti-NKG2C mAb. In the presence of this mAb, degranulation of NKG2C+ NK cells in response to 221.AEH was inhibited (data not shown). On the contrary, NKG2C− CD57− and NKG2C−CD57+ NK cells that express high levels of NKG2A (Figure 4B), displayed decreased degranulation against 221.AEH than 221wt, in line with the inhibitory effect mediated by NKG2A upon interaction with HLA-E.
Similar results were obtained in parallel experiments with HCMV+ HDs. However, in this case, NKG2C+ NK cells displayed a lesser increase in degranulation when comparing 221wt to 221.AEH (Online Supplementary Figure S8B). This is likely due to the higher levels of NKG2A expressed on the surface of NKG2C+ NK cells from HDs (Figure 4B). In line with this possibility, when degranulation was evaluated after gating on NK cells lacking NKG2A (Online Supplementary Figure S8C), the increment became significant also in HDs (Online Supplementary Figure S8D).
Discussion
NK cells play a crucial role in early immunity following HSCT in leukemic patients;33 therefore the possibility to improve and to control NK cell maturation and function in HSCT recipients may result in important clinical benefits.
We have recently documented that in UCBT recipients HCMV infection/reactivation early after HSCT results in a remarkable acceleration of NK cell reconstitution and maturation.22 In agreement with this study, we also found that in pediatric patients receiving a different cell composition in the HLA-haploidentical HSCT setting, depleted of TCR-αβ+T and CD19+ B cells, HCMV reactivation could accelerate the development of mature NK cells characterized by the KIR+NKG2A−NKG2C+Siglec7− phenotypic signature. This is noteworthy, as compared to pediatric patients receiving either UCBT or positively-selected CD34+ HSC from a HLA-haploidentical relative, this cohort of patients had reduced proportions of hypofunctional CD56−CD16+ NK cells. Since these cells usually develop in subjects infected by HCMV when T cell immunity is impaired,22,34 their reduced presence suggests that NK and γδ T cells contained in the graft may exert a protective role against severe infections.
It is well documented that HCMV infection influences NK cell maturation and induces a long-term reconfiguration of the NK cell receptor repertoire.35–37 This imprinting, induced by HCMV infection, also suggested that NK cells might keep the memory of past infections, thus sharing features with the cells of the adaptive immune system. Indeed, in mice, the expansion and persistence of memory Ly49H+ NK cells, endowed with specific anti-MCMV properties, has been clearly documented.38 It is possible that NKG2C+ NK cells that expand in humans after HCMV infection and preferentially acquire CD57 may represent the human counterpart of murine memory Ly49H+ NK cells.
In the present study, we show that most pediatric patients reactivating HCMV display a progressive expansion of this putative memory NK cell population expressing both NKG2C and CD57. The frequency of NKG2C+ NK cells (both CD57+ and CD57−) at 6 and 12 months after HSCT was higher than in both adult HCMV-seropositive HDs and in the respective HSC donors (Online Supplementary Figure S2, Figure 3B). This may reflect a recent or ongoing HCMV infection occurring in patients, but could also depend on the status of immunosuppression allowing a better imprinting of NK cells.5,37 Notably, we found a correlation between the duration of the antiviral treatment (Online Supplementary Table S2) and the percentage of Siglec-7 negative NK cells, which was only a tendency at month 3 (Spearman r=0,47 P=0,14) and became significant at month 6 (Spearman r=0,6 P=0,04), possibly indicating that the loss of Siglec-7 might be a marker of an efficient anti-HCMV response.
The phenotypic characterization of NK cells developing in our patients reactivating HCMV revealed that NKG2C+CD57+ NK cells represent a highly differentiated subset, displaying lower levels of expression of Siglec-7, IL-18Rα, NKG2A, NKp46 and NKp30 and higher levels of KIRs and LIR-1 than NKG2C+CD57− NK cells. It is likely that NKG2C+CD57− NK cells first emerge in response to HCMV infection and rapidly shift to a more differentiated CD57+LIR-1+ phenotype. It cannot be ruled out that LIR-1 expression may be progressively acquired by NKG2C+ NK cells following HCMV infection, and represent a viral evasion strategy. Indeed, the HCMV-derived viral glycoprotein UL-18 is a high affinity ligand for LIR-1.39 Although in reverse ADCC experiments LIR-1 did not substantially inhibit NKG2C activation, it is possible that, in vivo, UL-18 expressed by infected cells may efficiently engage LIR-1 and weaken NKG2C-mediated signaling.
Whether the NKG2C+CD57+ NK cell subset can persist for a long time after resolution of infection, or is continuously replenished by differentiating NKG2C+CD57− NK cells36,40 is still unknown. In support of the first hypothesis is the finding that HCMV infection can induce resistance to cell death in NK cells developing after UCBT.41 In some patients, we could observe an increase in NK cell numbers after HCMV infection (Online Supplementary Table S3) that was followed by the acquisition of a memory-like phenotype, suggesting that both proliferation and differentiation are likely contributing to the generation of this subset.
In our cohort of patients, the expanded memory-like NKG2C+CD57+ NK cells were functionally competent in terms of cytokine production and cytotoxicity/degranulation in response to tumor targets. On the other hand, they displayed poor capabilities of producing IFN-γ in response to rhIL-12 plus rhIL-18. This may be consequent, at least in part, to the reduced expression of IL-18Rα. However, we cannot exclude the involvement of other mechanisms affecting the signaling pathway downstream of the receptors. The diminished responsiveness to cytokines of NKG2C+CD57+ NK cells may reflect their specialization in controlling HCMV infection and their memory-like signature, in agreement with recent findings in mice.42 Indeed, in mice, MCMV-induced memory Ly49H+ NK cells show an impaired response to cytokines alone (IL-12 and IL-18). Whether this poor response was determined by the reduced expression of cytokine receptors, or by an altered signaling downstream of the receptors, has not been established. On the other hand, these MCMV-induced memory Ly49H+ NK cells were characterized by a higher responsiveness to m157 antigen (the specific viral ligand for Ly49H) in the presence of cytokines, as compared to naive Ly49H+ NK cells.42 In this context, we also show that cytokine-treated NKG2C+ CD57+ NK cells can efficiently degranulate in response to HLA-E+ targets (HLA-E is a ligand of NKG2C) (Figure 6).10 It is of note that differently to mice, the putative viral ligands recognized by NKG2C, expressed by HCMV-infected cells, have not been identified. They may be HLA-E molecules bound to viral peptides (e.g. UL40-derived peptides),37,43 as well as other undefined molecules.
Interestingly, two patients receiving grafts from HCMV-seropositive donors containing donor-derived NKG2C+ NK cells did not experience HCMV reactivation after transplantation, but displayed a significant expansion of highly differentiated NKG2C+ NK cells. These data suggest that donor-derived transplanted NK cells may persist in the recipient and favor anti-viral responses. Since these patients received grafts from seropositive donors, it is likely that transplanted NKG2C+ NK cells, primed by a previous encounter with HCMV in the donor, had undergone expansion in response to viral antigens present in low levels in infected peripheral tissues of the recipient (subclinical HCMV reactivation). This would be in line with previous data in recipients receiving T cell-replete HSCT.44,45 Interestingly, one of these two patients (pt #26) expanding NKG2C+ NK cells, experienced infection with viruses other than HCMV, namely adenovirus and BK, early after HSCT (Online Supplementary Table S1). Thus, it cannot be ruled out that these viral infections could have favored the expansion of HCMV-primed, donor-derived NKG2C+ NK cells, in agreement with a previous study showing that hantavirus infection could induce the expansion of NKG2C+ NK cells in HCMV+ individuals.46
The response to HCMV may also be influenced by the number of donor-derived mature NK cells contained in the graft, which is highly variable among patients receiving this type of HSCT (Online Supplementary Table S1). It is possible that protection from HCMV reactivation is achieved only with suitable numbers of NKG2C+KIR+ NK cells in the graft. Notably, the two patients displaying high frequencies of NKG2C+ NK cells and the absence of HCMV reactivation received grafts containing high numbers of donor-derived NK cells (pts #26 and #27, Online Supplementary Table S1).
Thus, in HCMV-reactivating patients, as well as in the few non-reactivating ones who were transplanted with seropositive donors, the proportions of mature NKG2C+CD57+ NK cells were significantly higher than those of non-reactivating patients. The capability of killing patient leukemia blasts and of protecting patients from acute HCMV infection, and also from other viral infections, should be investigated in assays against autologous leukemia blasts and autologous infected targets. However, it is conceivable that these cells, which respond efficiently against tumors and HLA-E+ targets, may play a beneficial role. Indeed AML blasts (and to a lesser extent ALL blasts) express HLA-E at significant levels47,48 and could be directly targeted by NKG2C+NK cells (especially when a KIR/KIR-L mismatch occurs in the graft-versus-host direction). Along this line, a protective role for NKG2C+CD57+ CD56dim NK cells emerging after HSCT in HCMV-reactivating recipients has been suggested recently, although in a different transplantation setting.49
Interestingly, it has recently been shown that NKG2C+CD57+ NK cells, isolated from HCMV+ individuals, are characterized by an epigenetic remodeling at the IFN-γ locus, which is similar to the one found in memory CD8+ T cells or Th1 cells. This epigenetic imprinting could be responsible for the enhanced IFN-γ production observed in NKG2C+ NK cells, and may be involved in the regulation of NK cell adaptive immune system mechanisms.40 Moreover, very recently, an altered pattern of expression of signalling proteins has been described in HCMV-induced memory-like NK cells.50 In particular, HCMV infection could promote the generation of adaptive NK cells that lack the expression of FcεRγ, EAT-2 and SyK. These molecular characteristics depend on given DNA methylation patterns that memory NK cells share in part with CTLs. Such epigenetic alterations could be responsible for the functional skewing shown by HCMV-induced NK cells that appear to be specialized in target cell recognition, especially via ADCC mechanisms, and impaired in cytokine-induced responses (with IL-12 and IL-18 at least). Indeed, previous studies showed that memory-like NKG2C+CD57+NK cells, isolated from HCMV+ HDs, can efficiently kill HCMV-infected targets in the presence of anti-HCMV antibodies, i.e. through CD16 cross-linking.31,51 Notably, in HSCT recipients, memory-like NKG2C+CD57+ NK cells displayed efficient mAb-mediated CD16 triggering (Figure 6A) and could kill infected cells via ADCC.
The precise definition of the signals capable of inducing this selective imprinting confined to NKG2C+ NK cells would be important for providing a molecular basis for the regulation and, possibly, the manipulation of adaptive features in innate cells.
A larger cohort of patients should be investigated in future studies to definitively establish whether HCMV reactivation actually confers beneficial effects against infections and leukemia relapses, as suggested by other studies.26,49
In conclusion, we show that HCMV reactivation in pediatric patients receiving a novel type of haplo-HSCT (αβ+T/B cell-depleted), deeply influence NK cell maturation and induce the emergence of memory-like NK cells. Learning to harness the recently unraveled adaptive features of NK cells may be revealed as being useful in order to achieve better immune recovery in HSCT recipients.
Acknowledgements
Investigator Grants n. 15704 (A.M.), 15283 (L.M.), 15925 (A.B.) and Special Project 5×1000 n. 9962 (A.M., L.M. and F.L.) from Associazione Italiana Ricerca sul Cancro; PRIN 2010 (Progetto di Rilevante Interesse Nazionale to F.L., A.M.) and RF-2010-2316606 (L.M., F.L., D.P.) from The Ministry of Health; progetto Cordon de Vie (F.L.) and Progetto Ricerca Ateneo 2013 (M.D.C.).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/3/371
References
- 1.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moretta A, Bottino C, Vitale M, et al. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–648. [DOI] [PubMed] [Google Scholar]
- 3.Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19: 197–223. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. [DOI] [PubMed] [Google Scholar]
- 5.Della Chiesa M, Marcenaro E, Sivori S, Carlomagno S, Pesce S, Moretta A. Human NK cell response to pathogens. Seminars in immunology. 2014;26(2):152–160. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol. 2013;34(12):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30(4): 143–149. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. [DOI] [PubMed] [Google Scholar]
- 9.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5(3):201–214. [DOI] [PubMed] [Google Scholar]
- 10.Braud VM, Allan DSJ, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–799. [DOI] [PubMed] [Google Scholar]
- 11.Colonna M, Navarro F, Bellon T, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186(11):1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moretta A, Sivori S, Vitale M, et al. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med. 1995;182(3):875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. Journal of immunology. 2007;179(2):854–868. [DOI] [PubMed] [Google Scholar]
- 14.Graef T, Moesta AK, Norman PJ, et al. KIR2DS4 is a product of gene conversion with KIR3DL2 that introduced specificity for HLA-A*11 while diminishing avidity for HLA-C. J Exp Med. 2009;206(11):2557–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locatelli F, Pende D, Mingari MC, et al. Cellular and molecular basis of haploidentical hematopoietic stem cell transplantation in the successful treatment of high-risk leukemias: role of alloreactive NK cells. Front Immunol. 2013;4(15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pende D, Marcenaro S, Falco M, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113(13):3119–3129. [DOI] [PubMed] [Google Scholar]
- 17.Ruggeri L, Aversa F, Martelli MF, Velardi A. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol Rev. 2006;214:202–218. [DOI] [PubMed] [Google Scholar]
- 18.Foley B, Felices M, Cichocki F, Cooley S, Verneris MR, Miller JS. The biology of NK cells and their receptors affects clinical outcomes after hematopoietic cell transplantation (HCT). Immunol Rev. 2014;258(1):45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beziat V, Descours B, Parizot C, Debre P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One. 2010;5(8): e11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood. 2014;124(5):822–826. [DOI] [PubMed] [Google Scholar]
- 21.Airoldi I, Bertaina A, Prigione I, et al. gammadelta T cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood. 2015; 125(15):2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della Chiesa M, Falco M, Podesta M, et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2012;119(2):399–410. [DOI] [PubMed] [Google Scholar]
- 23.Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2011; 119(11):2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guma M, Budt M, Saez A, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107(9):3624–3631. [DOI] [PubMed] [Google Scholar]
- 25.Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112(3): 914–915. [DOI] [PubMed] [Google Scholar]
- 26.Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplant is associated with a decreased relapse-risk: evidence for a putative virus-versus-leukemia effect AML patients. Blood. 2011;118(5):1402–1412. [DOI] [PubMed] [Google Scholar]
- 27.Bjorkstrom NK, Riese P, Heuts F, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116(19):3853–3864. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Verges S, Milush JM, Pandey S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116(19):3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Della Chiesa M, Falco M, Bertaina A, et al. Human cytomegalovirus infection promotes rapid maturation of NK cells expressing activating killer Ig-like receptor in patients transplanted with NKG2C−/− umbilical cord blood. Journal of immunology. 2014;192(4): 1471–1479. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Verges S, Milush JM, Schwartz BS, et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108(36):14725–14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z, Sinzger C, Frascaroli G, et al. Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity. J Virol. 2013;87(13):7717–7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. Journal of immunology. 1998;160(10):4951–4960. [PubMed] [Google Scholar]
- 33.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. [DOI] [PubMed] [Google Scholar]
- 34.Mavilio D, Lombardo G, Benjamin J, et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102(8):2886–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–3671. [DOI] [PubMed] [Google Scholar]
- 36.Beziat V, Liu LL, Malmberg JA, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121(14):2678–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Botet M, Muntasell A, Vilches C. The CD94/NKG2C+ NK-cell subset on the edge of innate and adaptive immunity to human cytomegalovirus infection. Seminars in immunology. 2014;26(2):145–151. [DOI] [PubMed] [Google Scholar]
- 38.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman TL, Heikema AP, Bjorkman PJ. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity. 1999;11(5):603–613. [DOI] [PubMed] [Google Scholar]
- 40.Luetke-Eversloh M, Hammer Q, Durek P, et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS pathogens. 2014;10(10):e1004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felices M, Lenvik TR, Ankarlo DE, et al. Functional NK cell repertoires are maintained through IL-2Ralpha and Fas ligand. Journal of immunology. 2014;192(8):3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Min-Oo G, Lanier LL. Cytomegalovirus generates long-lived antigen-specific NK cells with diminished bystander activation to heterologous infection. J Exp Med. 2014;211(13):2669–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomasec P, Braud VM, Rickards C, et al. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287(5455):1031. [DOI] [PubMed] [Google Scholar]
- 44.Foley B, Cooley S, Verneris MR, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. Journal of immunology. 2012;189(10):5082–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kheav VD, Busson M, Scieux C, et al. Favorable impact of natural killer cell reconstitution on chronic graft-versus-host disease and cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014;99(12): 1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjorkstrom NK, Lindgren T, Stoltz M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen S, Dhedin N, Vernant JP, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105(10):4135–4142. [DOI] [PubMed] [Google Scholar]
- 48.Majumder D, Bandyopadhyay D, Chandra S, Mukherjee N, Banerjee S. Lack of HLA-E surface expression is due to deficiency of HLA-E transcripts in the malignant hematopoietic cells of leukemic patients. Leukemia research. 2006;30(2):242–245. [DOI] [PubMed] [Google Scholar]
- 49.Cichocki F, Cooley S, Davis Z, et al. CD56CD57NKG2C NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 2015; 10.1038/leu.2015.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costa-Garcia M, Vera A, Moraru M, Vilches C, Lopez-Botet M, Muntasell A. Antibody-Mediated Response of NKG2Cbright NK Cells against Human Cytomegalovirus. Journal of immunology. 2015;194(6):2715–2724. [DOI] [PubMed] [Google Scholar]