Wild-type MLL infant acute lymphoblastic leukemia (ALL) patients account for approximately 20% of all infant ALL cases. Although this group of patients generally fares better than MLL-rearranged infant ALL patients, their prognosis is still worse than that of non-infant pediatric B-ALL patients. Extensive characterization of this specific patient group largely remains unacknowledged. In this study, we aim to obtain a clinical and molecular profile of this patient group in order to find new opportunities to optimize treatment. We report on a large cohort of 78 wild-type MLL infant ALL samples using clinical parameters, array-comparative genomic hybridization analysis, and gene expression profiling. The frequency of DNA copy number variations and molecular genetic lesions in genes involved in B-cell development are lower in wild-type MLL infant ALL than older children with ALL. Wild-type MLL infant ALL presented with higher white blood cell (WBC) counts and a more immature immunophenotype than pediatric (non-infant) B-ALL patients. The strongest predictor of outcome in wild-type MLL infant ALL was the level of MEIS1 expression, which may indicate new opportunities for novel strategies in treating wild-type MLL infant ALL.
Acute lymphoblastic leukemia (ALL) in infants (<1 year of age) is a rare but highly aggressive type of leukemia, typically characterized by the presence of MLL-rearrangements occurring in approximately 80% of these patients.1 The prognosis for MLL-rearranged infant ALL patients is highly unfavorable.2 In contrast, infant ALL patients carrying wild-type (or germline) MLL genes fare significantly better, with reported event-free survival (EFS) of 60%–74%.1,2 However, these rates are much lower than the 5-year survival rates in older children with ALL (approx. 90%).3 We recently showed that wild-type MLL infant ALL specifies a gene expression pattern that is different from both MLL-rearranged infant ALL and from pediatric non-infant precursor B-ALL.4 In an unsupervised clustering analysis, wild-type MLL infant ALL samples even appeared more closely related to MLL-rearranged infant ALL than to pediatric precursor B-ALL cases. Also, infant ALL patients who do not carry MLL translocations share the same cytogenetic abnormalities as older children with ALL, albeit with a different distribution: a lower incidence of the favorable abnormalities ETV6-RUNX1 and high hyperdiploidy, and a higher incidence of unfavorable abnormalities, including BCR-ABL1.5
In the present study, we aimed to obtain a clinical and genetic profile of a relatively large cohort of wild-type MLL infant ALL patients all treated according to INTERFANT treatment protocols (i.e. Interfant-99 or Interfant-06), in order to find a common denominator in this group that can ultimately be used to optimize treatment for these patients. The results are compared to data obtained in MLL-rearranged infant ALL patients (enrolled in INTERFANT studies) and pediatric (non-infant) precursor ALL patients uniformly treated according to the Dutch Childhood Oncology Group (DCOG) ALL-10 protocol. Patients enrolled in this study from the INTERFANT-99 study (n=61) have been presented elsewhere.5
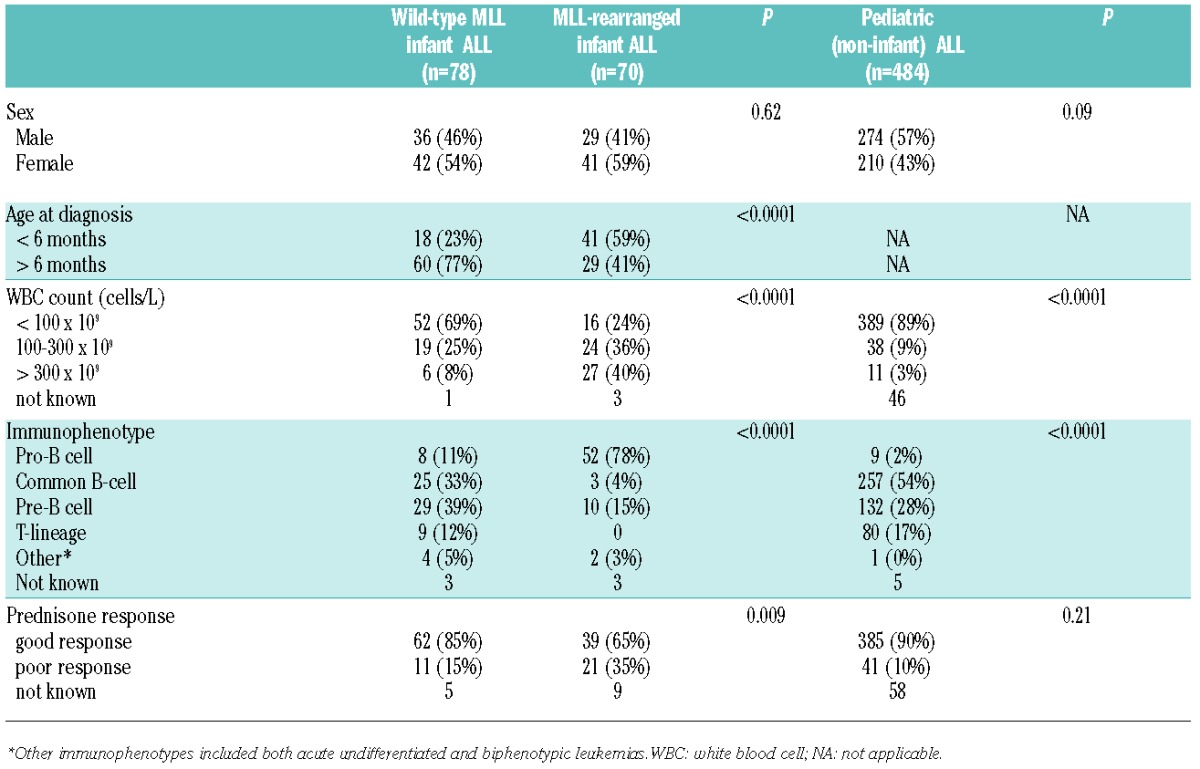
Clinical parameters known to predict outcome in MLL-rearranged infant ALL were compared between infant ALL patients carrying wild-type MLL genes (n=78) and MLL-rearranged infant ALL cases (n=70), as well as between wild-type MLL infant ALL patients and pediatric (non-infant) ALL patients (n=484). The adverse prognostic factors analyzed included: age under six months, WBC counts more than 300×109 leukemic cells/L, a pro-B (CD10-) immunophenotype, and a poor in vivo prednisone window response (Table 1). Compared with MLL-rearranged infant ALL cases, infant ALL patients carrying wild-type MLL genes were significantly more often diagnosed at over six months of age, presented with more favorable WBC counts, more mature (pre-B or common) immunophenotypes, and generally responded well to a 7-day window of prednisone monotherapy (Table 1).
Table 1.
Clinical characteristics and prognostic factors of wild-type MLL infant ALL patients.
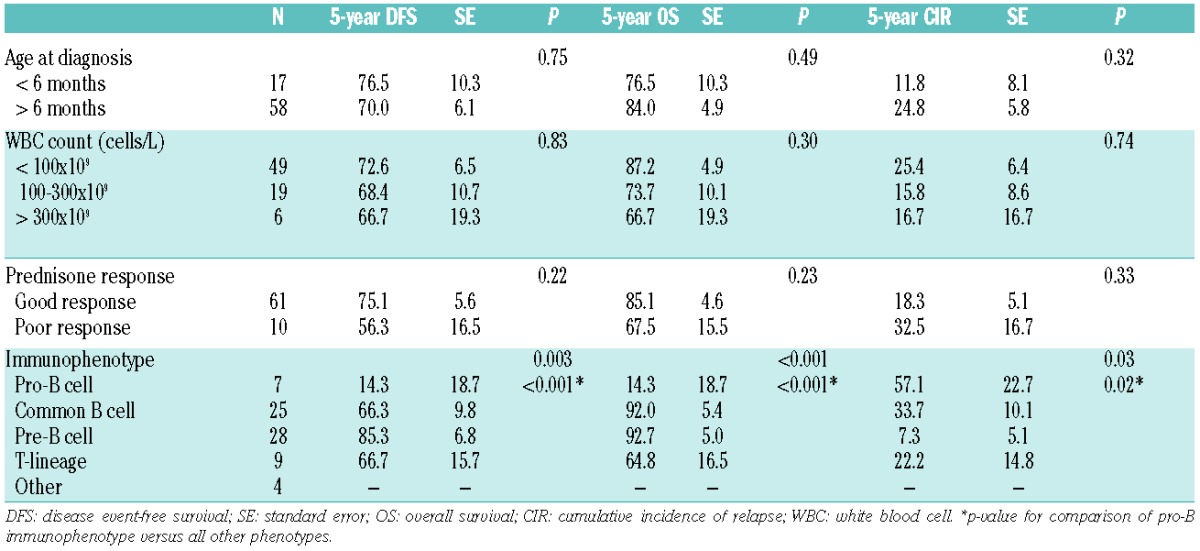
Next we assessed the prognostic relevance of these predictive parameters in terms of disease-free survival (DFS), overall survival (OS), and cumulative incidence of relapse (CIR) five years after diagnosis in wild-type MLL infant ALL patients (n=76) for whom clinical follow-up data were available (Table 2). Overall, 5-year DFS (standard error, SE) was 71.3 (5.3), 5-year OS 82.2 (4.5), and 5-year CIR 21.9 (4.9). Neither age under six months at diagnosis, nor WBC counts more than 300×109 leukemic cells/L were predictive of clinical outcome within this group. In contrast, a poor prednisone response was marginally associated with an inferior outcome (although not significantly so), whereas an immature pro-B immunophenotype was highly predictive of a poor clinical outcome (P<0.001). The 5-year OS in the wild-type MLL infant ALL patients diagnosed with pro-B ALL was 14.3 (18.7), whereas this was 92.0 (5.4) and 92.7 (5.0) in wild-type MLL infants diagnosed with common BALL and pre-B ALL, respectively (P<0.001).
Table 2.
Univariate analysis of prognostic factors in wild-type MLL infant ALL patients.
In order to identify additional prognostic factors for wild-type MLL infant ALL, we applied significance analysis of microarrays (SAM) to screen our gene expression profiles (Affymetrix HU133plus2.0 GeneChips) for genes predictive for clinical outcome. Gene expression profiles were available for 36 wild-type MLL infant ALL patients and clinical follow-up data were available for 30. Of these 30 patients, 8 experienced an event. Interestingly, two probe sets appeared highly predictive of clinical outcome: i) the level of MEIS1 expression (Affymetrix probe set 242172_at); and ii) PENK (Affymetrix probe set 213791_at). Patients expressing low levels of MEIS1 (i.e. below the median MEIS1 level of the entire patient group, n=16) had a superior outcome over patients expressing high levels (i.e. above the median, n=14): 5-year DFS (SE) was 87.5 (8.3) versus 50.0 (13.4; P=0.01), while 5-year OS was 100.0 versus 71.4 (12.1; P=0.02) for low and high MEIS1-expression, respectively. Remarkably, differential gene expression analysis between patients with high MEIS1-expression (n=18) and patients with low MEIS1-expression (n=18) could not identify differentially expressed genes other than MEIS1 itself. Analysis of prognostic factors showed a significant difference in terms of immunophenotype, with more immature phenotype more frequent in the wild-type infant ALL patients with high MEIS1 expression (P=0.009) (Online Supplementary Table S1). Strikingly, we could not detect a prognostic value of high-level MEIS1 expression in the pediatric (non-infant) ALL patients.
Interestingly, high-level expression of MEIS1 is also closely associated with prognostically unfavorable MLL-rearranged leukemias.6,7 Hence, the prognostic relevance of MEIS1 expression in wild-type MLL infant ALL patients may imply transformation events that, to some extent, resemble that of MLL-rearranged infant ALL cases. Furthermore, the strong influence on clinical outcome of MEIS1 expression suggests that infant ALL expressing high levels of MEIS1 represent a highly aggressive leukemia that require very few co-operative genetic lesions during leukemogenesis and/or leukemia maintenance.
Kang et al. have shown that FLT3, IRX2 and TACC2 expression is highly predictive of EFS in infant ALL.8 That we did not find a significant result from FLT3, IRX and TACC2 as predictors of outcome could have been due to the fact that we only used wild-type MLL patients or that patient numbers were too low to provide a significant result.
In order to detect submicroscopic deletions and amplifications in the DNA, we performed array-comparative genomics hybridization (array-CGH) and multiplex ligation-dependent probe amplification (MLPA) on a cohort of wild-type MLL infant ALL patients (n=31 and n=32, respectively) for whom genomic DNA was available (Online Supplementary Table S2).
The results from array-CGH were compared with data from a group of pediatric (non-infant) B-ALL patients (n=115) (Table 3) selected with a relatively high frequency of B-others and low number of high hyperdiploid patients, and this should be taken into account when interpreting the results. The frequency of structural aberrations found by array-CGH (i.e. partial deletions/amplifications, and translocations) was much lower in wild-type MLL infants than in non-infant ALL patients (45% vs. 98%, respectively; P<0.001) (Table 3). The frequency of patients with numerical aberrations (i.e. gain or loss of complete chromosomes) among wild-type MLL infant ALL patients (23%) was comparable to the frequency of numerical aberrations in non-infant pediatric ALL patients (29%).
Table 3.
Distribution of DNA copy-number variations in wild-type MLL infant acute lymphoblastic leukemia (ALL) and pediatric non-infant precursor B-ALL patients.
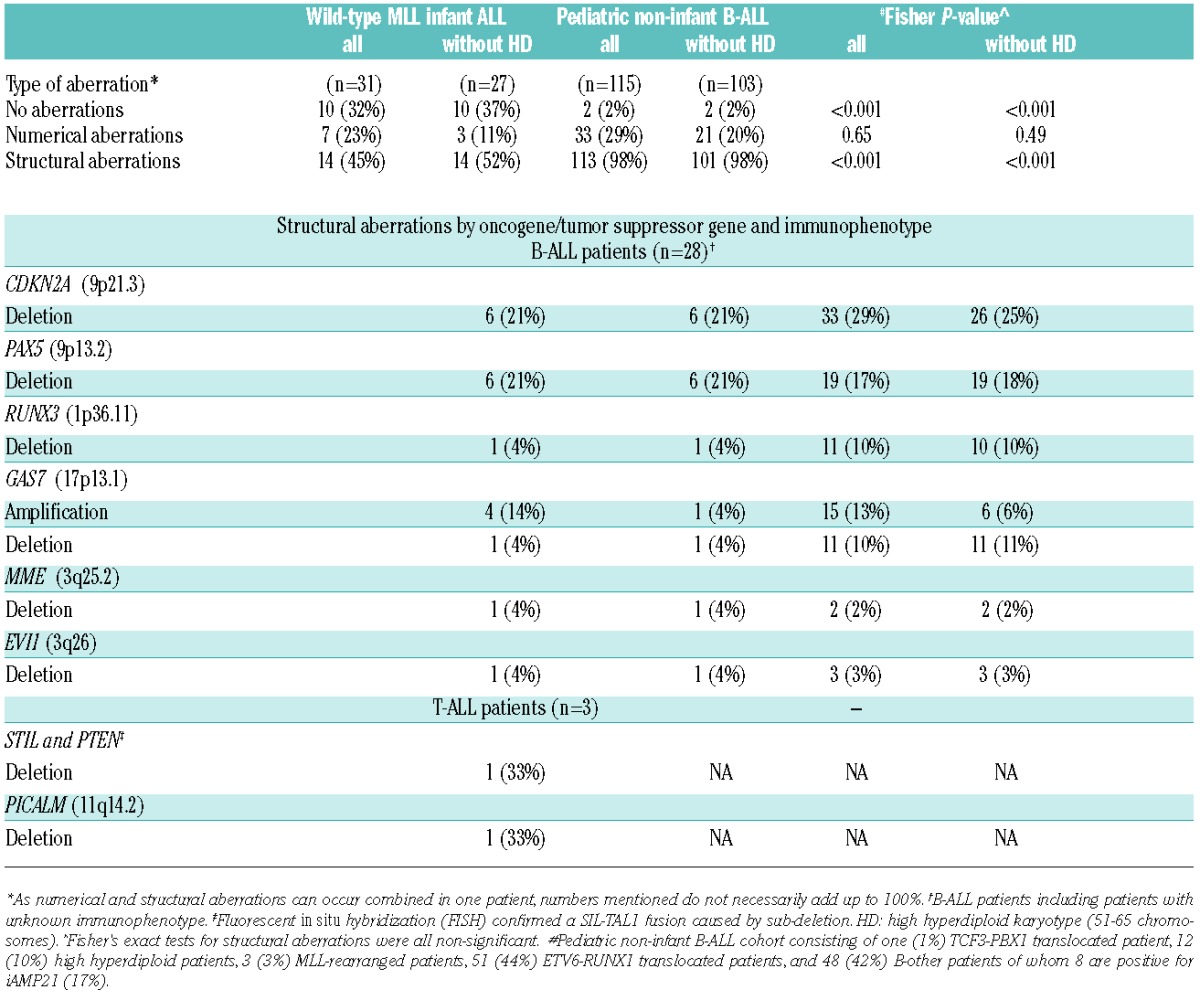
MLPA analysis was carried out using specific probes for single gene alterations including CDKN2A, CDKN2B, IKZF1, PAX5 and ETV6 (Online Supplementary Table S3). No alterations of IKZF1 were detected in any of the wild-type MLL infant ALL samples (n=32) tested, whereas 17% of the pediatric non-infant B-ALL patients are known to carry an IKZF1 deletion.9 A deletion of CDKN2A and CDKN2B on 9p21.3 was found in 6 (19%) of the infants. In contrast, deletions of CDKN2A and CDKN2B were found in 35% and 34% of the pediatric non-infant ALL samples, respectively. Deletions of PAX5 were present together with CDKN2A and CDKN2B in 4 wild-type MLL infant ALL patients, whereas the PAX5 deletion was the only observed abnormality in 2 infants as determined by MLPA. The incidence of ETV6 deletions was markedly lower in infants: 3% versus 26% of the pediatric precursor B-ALL patients (P=0.002); however, when ETV6-RUNX1 cases were excluded, only 11% of precursor B-ALL patients had ETV6 deletions (11%; P=0.2).
In conclusion, wild-type MLL infant ALL has a different clinical and molecular profile to pediatric (non-infant) precursor B-ALL and is characterized by a higher incidence of poor prognostic factors and fewer genetic alterations. High MEIS1 expression is highly predictive of poor outcome in wild-type MLL infant ALL.
Acknowledgments
We thank the members and participating hospitals of the Interfant-99 and Interfant-06 treatment protocols for supporting this study by providing leukemic samples. Members are: Campbell, M (PINDA), Felice, M (Argentina), Ferster, A (CLCG), Hann, I and Vora, A (UKCCSG), Hovi, L (NOPHO), Janka-Schaub, G (COALL), Li, CK (Hong Kong), Mann, G (BFM-A), Mechinaud, F (Fralle), Pieters, R (DCOG), Locatelli, F and Biondi, A (AIEOP), Rubnitz J (SJCRH), Schrappe, M (BFM-G), Silverman, L (DFCI), Stary, J (CPH), Suppiah, R (ANZCHOG), Szczepanski, T (PPLLSG), Valsecchi, M and de Lorenzo, P (Interfant Trial Data Center).
Footnotes
Funding: this project was financially supported by Stichting Kinderen Kankervrij (KiKa) and the Dutch Cancer Society; Pediatric Oncology Foundation Rotterdam (KOCR) and the European Union’s Seventh Framework Programme (FP7/2007-2013) under the ENCCA project (HEALTH-F2-2011-261474); by Associazione Italiana per la Ricerca sul Cancro, Fondazione Cariplo and MIUR.
The online version of the article contains a data supplement.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Pieters R, Schrappe M, De Lorenzo P, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370(9583):240–250. [DOI] [PubMed] [Google Scholar]
- 2.Hilden JM, Dinndorf PA, Meerbaum SO, et al. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: report on CCG 1953 from the Children’s Oncology Group. Blood. 2006;108(2):441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14):1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stam RW, Schneider P, Hagelstein JA, et al. Gene expression profiling-based dissection of MLL translocated and MLL germline acute lymphoblastic leukemia in infants. Blood. 2010;115(14):2835–2844. [DOI] [PubMed] [Google Scholar]
- 5.De Lorenzo P, Moorman AV, Pieters R, et al. Cytogenetics and outcome of infants with acute lymphoblastic leukemia and absence of MLL rearrangements. Leukemia. 2014;28(2):428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeoh EJ, Ross ME, Shurtleff SA, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1(2): 133–143. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong SA, Hsieh JJ, Korsmeyer SJ. Genomic approaches to the pathogenesis and treatment of acute lymphoblastic leukemias. Curr Opin Hematol. 2002;9(4):339–44. [DOI] [PubMed] [Google Scholar]
- 8.Kang H, Wilson CS, Harvey RC, et al. Gene expression profiles predictive of outcome and age in infant acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2012;119(8):1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Veer A, Waanders E, Pieters R, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122(15):2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]


