Abstract
Background:
Interleukin-10 (IL-10) is a inhibiting inflammatory cytokine that plays an important role in immune suppressive microenvironment in multiple myeloma (MM). Whether the level of serum IL-10 could predict treatment response and survival outcomes or not needs to be investigated in MM patients.
Methods:
The level of IL-10 in serum was measured using enzyme-linked immunosorbent assay in 188 patients with newly diagnosed MM.
Results:
The best cutoff value for IL-10 in predicting survival is 169.69 pg ml−1 with an area under the curve (AUC) value of 0.747 (P<0.001). In all, 92 patients (48.9%) were classified as high-IL-10 group (>169.96 pg ml−1) and 96 patients (51.1%) as low-IL-10 group (⩽169.96 pg ml−1). The overall response rate (ORR) was 79.2% in low-IL-10 group, significantly higher than that in high-IL-10 group (53.3%, P<0.001). Patients in low-IL-10 group had significantly better survival compared with those in high-IL-10 group (3-year PFS rate: 69.3% vs 13.3%, P<0.001; 3-year OS rate: 93.6% vs 51.9%, P<0.001). Multivariate analysis revealed that serum IL-10 level >169.96 pg ml−1 at diagnosis and certain cytogenetic abnormalities were two adverse factors for PFS and OS.
Conclusions:
Our data suggest that serum IL-10 at diagnosis is a novel, powerful predictor of prognosis for MM.
Keywords: multiple myeloma, interleukin-10, immunosuppression, prognosis, biomarker
Multiple myeloma (MM) is the second most common adult haematological malignancy (Smith and Yong, 2013). It is characterised by the expansion of a plasma cell clone that localises to the bone marrow (BM), causing bone lesions, hypercalcaemia, anaemia, and proteinaemia (Smith and Yong, 2013). Multiple studies have demonstrated that elevated cytokines levels promote development and proliferation of MM and result in worse prognosis, for instance, interleukin (IL)-16 (Alexandrakis et al, 2004; Atanackovic et al, 2012) and IL-17 (Noonan et al, 2010; Song et al, 2013).
Interleukin-10 is a pleiotrophic cytokine referred to stimulate and suppress immune response (Moore et al, 2001). However, the prognostic role of IL-10 and its relationship to tumour propagation or aggressive characteristics in MM have not yet been defined. Interleukin-10 has been proven to inhibit various immune functions such as macrophage activation, cytokine production, and antigen presentation (Howard et al, 1992). Moreover, IL-10 has been shown to play a key role in initiating and promoting certain types of malignancies (Holland and Zlotnik, 1993). With respect to the pathological role of IL-10 in MM, previous studies have reported that IL-10 induces both plasma cell proliferation and angiogenesis in MM (Alexandrakis et al, 2015). Abnormal level of IL-10 secreted by T regulatory cells or produced from myeloma cells can modulate antitumour host immune response, including the abrogation of DC function, by constitutive activation of STAT3 in MM (Muthu Raja et al, 2012; Nguyen-Pham et al, 2012). In addition, IL-10 abolishes all-trans retinoic acid (ATRA)-induced growth inhibition of myeloma cells (Otsuki et al, 2002). Furthermore, experimental studies suggested that IL-10 plays an important role in induction of chemoresistance in non-Hodgkin's lymphoma and thyroid cancer (Stassi et al, 2003; Gupta et al, 2012). Accordingly, we hypothesised that pretreatment serum levels of IL-10 predict the treatment resistance and progression of MM.
The prognosis of MM has significantly improved because of the development of treatment protocols (Kumar et al, 2008). To get a deeper is beneficial to longer duration of disease control (Chanan-Khan et al, 2008; Harousseau et al, 2009a). With the advent of novel proteasome inhibitors and immunomodulatory drugs, most patients can achieve a better treatment response and improved progression-free survival (PFS) (Kumar et al, 2008). However, MM still remains to be an incurable disease and nearly all patients eventually relapse and succumb to MM. One of the biggest challenge in treating relapsed MM is drug resistance. Thus, alternative treatment modalities using distinct mechanisms to overcome drug resistance is an area of active research. Furthermore, biomarkers that can predict treatment response and prognosis will be of great help in choosing optimal drugs to treat MM. Therefore, the identification of specific risk factors remains an important issue. In this study, we characterised the serum levels of IL-10 and evaluated its impact on the treatment response and outcome in MM.
Materials and methods
Patient selection
A total of 188 patients with symptomatic MM were enrolled in our study, and all patients were treated in Sun Yat-sen University Cancer Center between January 2005 and December 2014. These patients were identified by the hospital discharge registry system and electronic medical record. The inclusion criteria of this retrospective clinical study were as follows: (1) newly diagnosed with symptomatic MM based on the diagnostic criteria from World Health Organisation (WHO); (2) had either measurable monoclonal protein (M protein) in blood or urine; (3) previously untreated patients; (4) no previous or concomitant tumour; (5) available serum samples obtained before the primary treatment and stored at −80 °C; and (6) complete clinical information and long-term follow-up data were available. All patients were staged according to the International Staging System (ISS) staging. This study was approved by the institutional review board and ethics committees of Sun Yat-Sen University Cancer Center. Written informed consent for blood samples and medical information were obtained from all patients and healthy volunteers. The study was performed in agreement with the guidelines of the Declaration of Helsinki.
Treatments and response evaluation
In total, 104 patients received DVD (doxil, vincristine, and dexamethasone) (Dimopoulos et al, 2003); 84 patients received bortezomib-based regimens such as PAD (bortezomib, doxorubicin, and dexamethasone) (Sonneveld et al, 2012) and VTD (bortezomib, thalidomide, and dexamethasone) (Cavo et al, 2010). Patients were given at least four cycles of treatment, followed by autologous stem cell transplantation (if eligible) or maintenance with thalidomide. Treatment responses were evaluated after each cycle according to the International Myeloma Working Group (IMWG) criteria (Durie et al, 2006).
Serum IL-10 measurement
The level of serum IL-10 was tested with Sandwich enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Emeryville, CA, USA). All the samples of venous blood were collected from patients at diagnosis and 14 healthy subjects. Serum was collected by centrifuging the blood samples and stored at −80 °C until further assay. A routine ELISA method was used to carry out the test according to the manufacturer's protocol. Briefly, standards and samples were added to a microplate that had been precoated with a mouse monoclonal antibody specific to IL-10. Any unbound antibody or enzyme reagent was removed by washing with wash buffer. Then, a substrate solution was added into each well; colour development was terminated by stop solution; and the resulting absorbance was read at 450 nm using a spectrophotometer (Tecan, Mannedorf, Switzerland). IL-10 concentrations were determined based on a standard curve that was established using the recombinant IL-10 protein. Each sample was analysed in triplicate, and the results were averaged.
Statistical analysis
Serum IL-10 concentration was presented as median (min, max). The correlation of serum IL-10 concentration with various clinicopathologic parameters was assessed using the Mann–Whitney U-test and χ2 test or Fisher's exact test was used for categorical values. The cutoff concentration for serum IL-10 in predicting survival was determined using the receiver operating characteristics (ROC) curve analysis. Overall survival (OS) was measured from the date of diagnosis to the date of death or last follow-up visit. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of disease progression, relapse, death, or last follow-up visit. Kaplan–Meier method was used to calculate the probability of survival, and survival curves were tested using log-rank test. Univariate and multivariate analyses were performed using Cox proportional hazards model. Two-sided P-values of <0.05 were considered statistically significant. Statistical analysis was performed with SPSS software (SPSS Standard version 19.0, SPSS Inc., Chicago, IL, USA).
Results
Patients' characteristics and correlation with serum IL-10 measurement level
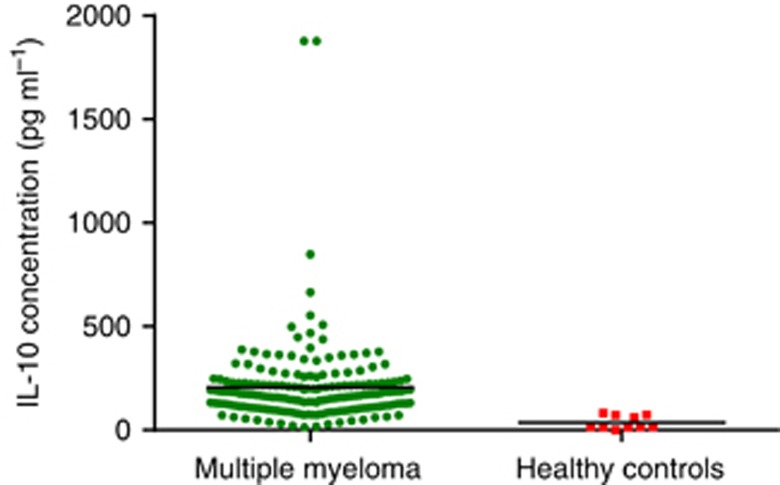
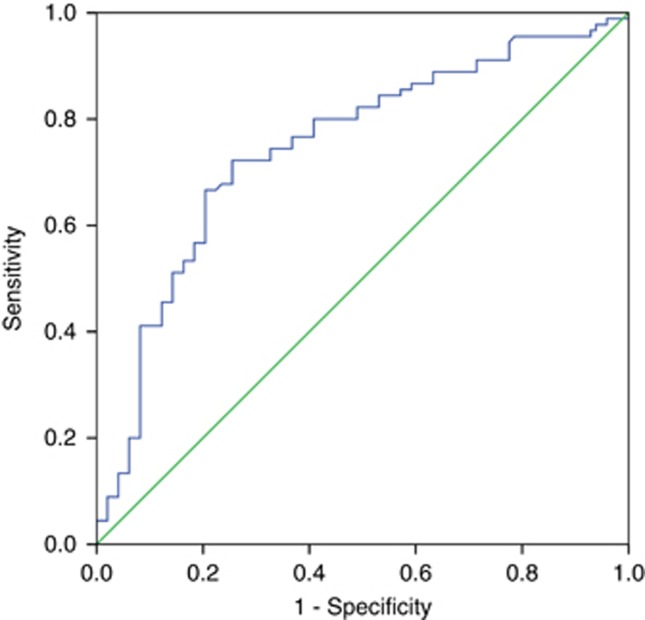
In total, 188 patients (132 male, 56 female; median age 59 years) met the inclusion criteria. The clinical characteristics of 118 patients are listed in Table 1. More than half (57.4%) were <65 years old. Fifty-four patients (55.3%) had a favourable performance status (ECOG PS 0–1). The stages were balanced among stages I (23.9%), II (44.1%), and III (32.0%%). The types of monoclonal protein were: IgG for 90 patients, IgA for 58 patients, and light chain disease for 40 patients. Elevated LDH levels were observed for 45 cases (23.9%). Seventy-two patients (38.3%) presented with genetic abnormalities. The mean concentration of serum IL-10 for all patients was 201.96 pg ml−1 with a median of 167.19 pg ml−1 (range 13.65–1877.44 pg ml−1). Serum IL-10 was detected in 10 healthy volunteers, and the median concentration was 17.54 pg ml−1 (range 0–82.35 pg ml−1), significantly lower than that of MM patients (P<0.001, Figure 1). The ROC curve analysis for the optimal cutoff point of serum IL-10 in prediction of survival was performed. The most discriminative cutoff concentration of serum IL-10 was 169.96 pg ml−1 with an area under the curve (AUC) value of 0.747 (P<0.001, Figure 2).The sensitivity and specificity were 72.2% and 72.4%, respectively. According to the results of ROC analysis, we used the IL-10 level >169.96 pg ml−1 as the cutoff value in the present study. In terms of this cutoff value, 96 patients (51.1%) were categorised into the low-IL-10 group (⩽169.96 pg ml−1) and 92 patients (48.9%) were classed into the high-IL-10 group (>169.96 pg ml−1). As is shown in Table 1, the serum IL-10 level was significantly higher in patients with poor PS, high ISS stage, elevated LDH levels, or no complete response (CR) after treatment. Moreover, we have analysed the correlation between IL-10 levels and other markers of tumour burden (M-protein levels, BM plasma cell numbers, and so on) and found that IL-10 levels correlated with them (data shown in Supplementary Table 1). However, there was no significant correlation between serum IL-10 level and age, gender, type of myeloma, and genetic abnormalities. No significant difference in IL-10 levels was found between DVD and bortezomib-based chemotherapy.
Table 1. Correlation of serum IL-10 level with clinical characteristics in MM patients.
| Characteristics | N=188 | Median concentration, pg ml−1 (range) | P-value |
|---|---|---|---|
| Age | 0.873 | ||
| <65 | 108 | 164.69 (23.08–553.54) | |
| ⩾65 | 80 | 179.91 (13.65–1877.44) | |
| Gender (male) | 132 | 165.66 (13.65–1877.44) | 0.608 |
| ECOG PS | 0.004 | ||
| 0–1 | 104 | 161.19 (18.54–465.78) | |
| ⩾2 | 84 | 187.76 (13.65–1877.44) | |
| ISS stage | 0.028 | ||
| I | 45 | 118.65 (13.65–465.78) | |
| II | 83 | 173.75 (18.54–449.54) | |
| III | 60 | 194.61 (65.45–1877.44) | |
| Type of myeloma | 0.301 | ||
| IgG | 90 | 164.16 (18.54–449.54) | |
| IgA | 58 | 174.29 (13.65–1877.44) | |
| Light chain | 40 | 173.75 (23.08–553.54) | |
| Serum LDH | 0.006 | ||
| ⩽ULN | 143 | 164.84 (13.65–1877.44) | |
| >ULN | 45 | 187.54 (65.45–1877.44) | |
| Genetic abnormalities | 0.072 | ||
| Yesa | 72 | 196.78 (18.54–498.43) | |
| No | 116 | 164.50 (13.65–1877.44) | |
| Treatment regimens | 0.376 | ||
| Bortezomib based | 84 | 162.73 (23.08–498.43) | |
| DVD | 104 | 174.28 (13.65–1877.44) | |
| Treatment response | <0.001 | ||
| CR | 36 | 112.46 (23.08–389.48) | |
| Less than CR | 152 | 174.29 (13.65–1877.44) |
Abbreviations: CR=complete response; DVD=doxil, vincristine, and dexamethasone; ECOG PS=Eastern Cooperative Oncology Group Performance Status; Ig=immunoglobulin; IL-10=interleukin-10; ISS=International Staging System; LDH=lactate dehydrogenase; MM=multiple myeloma; ULN=upper limit of normal.
Patients with abnormalities of 13q14, 1q21, 14q32, and 17p13.
Figure 1.
Serum IL-10 measurement in healthy subjects and in patients of our cohort collected at diagnosis. Serum IL-10 levels were significantly higher in patients with MM than in healthy volunteers (P<0.001).
Figure 2.
The ROC curve analysis for the optimal cutoff point of serum IL-10. The most discriminative cutoff concentration of serum IL-10 was 169.96 pg ml−1 with an AUC value of 0.747. The sensitivity and specificity were 72.2% and 72.4%, respectively.
Treatment response and correlation with IL-10 level
The primary treatment modalities were as follows: (1) 66 cases (35.1%) received chemotherapy followed by autologous stem cell transplantation (ASCT) and (2) 122 cases (64.9%) received chemotherapy alone; the treatment details and responses are listed in Table 2. No significant difference was found in the treatment modalities between the patients displaying IL-10 levels ⩽169.96 pg ml−1 and patients with IL-10 levels >169.96 pg ml−1 (P=0.542). After the initial treatment involving ASCT, 36 of the 188 treated patients (19.1%) achieved CR. The CR rate in the low-IL-10 group was significantly higher than that in the high-IL-10 group (26.0% vs 12.0%, respectively, P=0.016). The overall response rate (ORR) in the low-IL-10 group was significantly higher than that in the high-IL-10 group (79.2% vs 53.3%, respectively, P<0.001). The ORR was significantly higher in patients treated with bortezomib-based regimens than those with DVD regimen (75.0% vs 47.1%, P<0.001).
Table 2. Primary treatment and response in patients with multiple myeloma.
| Low-IL-10 group, n (%) | High-IL-10 group, n (%) | P-value | |
|---|---|---|---|
|
Treatment | |||
| Treatment modalities | 0.542 | ||
| CT followed by ASCT | 36 (37.5) | 30 (32.6) | |
| CT alone | 60 (62.5) | 62 (67.4) | |
| Chemotherapy regimens | 0.080 | ||
| Bortezomib based | 49 (51.4) | 35 (38.0) | |
| DVD | 47 (48.6) | 57 (62.0) | |
|
Treatment response | |||
| CR | 25 (26.0) | 11 (12.0) | 0.016 |
| VGPR | 20 (20.8) | 16 (17.4) | 0.582 |
| ORR (⩾PR) | 76 (79.2) | 49 (53.3) | <0.001 |
Abbreviations: ASCT=autologous stem cell transplantation; CR= complete response; CT=chemotherapy; DVD=doxil, vincristine, and dexamethasone; IL-10=interleukin-10; ORR=overall response rate; PR=partial response; VGPR=very good partial response.
Survival and prognostic factors
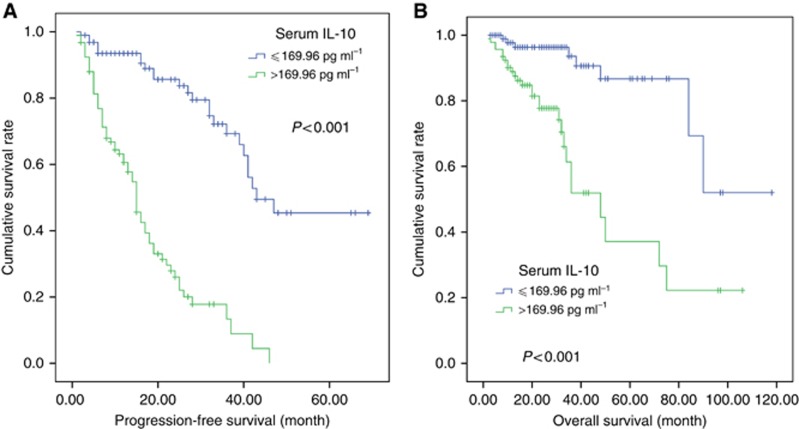
At a median follow-up of 23 months (2–118), the 3-year PFS and OS rates for all 188 patients were 41.8 (95% CI 32.4–51.2%) and 75.9% (95% CI 66.9–84.9%), respectively. Patients in the low-IL-10 group had significantly better survival compared with those in the high-IL-10 group (3-year PFS rate: 69.3% vs 13.3%, P<0.001, Figure 3A; 3-year OS rate: 93.6% vs 51.9%, P<0.001, Figure 3B). In the patients who received chemotherapy alone (n=122, 64.9%), high level of IL-10 was associated with shorter PFS and OS (P<0.001 and P<0.001, respectively) and was also related to inferior PFS and OS (P<0.001 and P=0.022, respectively) in the patients who received chemotherapy followed by ASCT (n=66, 35.1%). Similarly, among patients receiving bortezomib-containing therapy and those receiving DVD, the impact of IL-10 level was significant, similar to the overall population. Additionally, bortezomib-based regimens resulted in longer PFS but showed no significant effect on OS compared with DVD in overall patients. Significant survival differences were found between the patients who gained good treatment response (CR+VGPR) after treatment and those less than VGPR.
Figure 3.
Survival outcome of patients based on the serum IL-10 level. (A) Progression-free survival (PFS) of patients according to serum IL-10 level (⩽169.96 vs >169.96 pg l−1). (B) Overall survival (OS) of patients according to baseline IL-10 level (⩽169.96 vs >169.96 pg l−1).
Table 3 displays the results of the univariate and multivariate analyses of the potential predictors of OS and PFS. Factors that were statistically significant predictors of OS and PFS were included in the multivariate analysis. Multivariate analysis using the forward conditional Cox regression model revealed that serum IL-10 level >169.96 pg ml−1 at diagnosis and certain cytogenetic abnormalities were two adverse factors for PFS and OS. Treatment regimen (DVD compared with bortezomib-based regimen) was an independent prognostic factor for PFS but not for OS based on our analysis results.
Table 3. Univariate and multivariate analyses of factors associated with PFS and OS of all patients.
|
PFS |
OS |
|||||
|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||
| Parameters | P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value |
| Age ⩾65 years | 0.710 | 0.118 | ||||
| Gender, male | 0.211 | 0.072 | ||||
| ECOG PS score (>2) | 0.029 | 0.053 | ||||
| ISS stage | 0.025 | 0.022 | ||||
| LDH >ULN | 0.013 | 0.002 | ||||
| FISHa | <0.001 | 2.610 (1.670–4.082) | <0.001 | <0.001 | 4.145 (1.836–9.358) | 0.001 |
| Treatment regimensb | 0.003 | 1.784 (1.147–2.775) | 0.010 | 0.060 | ||
| IL-10 level (>164.50 pg ml−1) | <0.001 | 5.885 (3.552–9.750) | <0.001 | <0.001 | 4.708 (2.084–10.637) | <0.001 |
Abbreviations: CI=confidence interval; ECOG PS=Eastern Cooperative Oncology Group Performance Status; FISH=interphase fluorescence in situ hybridization; HR=hazard ratio; IL-10=interleukin-10; ISS=International Staging System; LDH=lactate dehydrogenase; OS= overall survival; PFS= progression-free survival; ULN=upper limit of normal.
Patients with abnormalities of 13q14, 1q21, 14q32, and 17p13 compared with no FISH abnormalities.
Doxil, vincristine, and dexamethasone (DVD) regimen compared with bortezomib-based regimen.
Disscusion
Previous studies have confirmed the role of IL-10 in various haematological malignancies, including Hodgkin's lymphoma, non-Hodgkin's lymphoma, and MM (Visco et al, 2004; Gupta et al, 2012; Alexandrakis et al, 2015). A clinical study in diffuse large B-cell lymphoma indicated that increasing serum IL-10 level was significantly associated with shorter survival (Gupta et al, 2012). However, the prognostic role of IL-10 in MM remains unclear. In this study, we found that the serum concentration of IL-10 in MM patients was much higher than in normal healthy people. In MM patients, the levels of serum IL-10 significantly correlated with clinicopathological features, such as poor PS, high ISS stage, elevated LDH levels, and no CR after chemotherapy. Furthermore, elevated serum concentration of IL-10 was related to poor responses to treatment. According to the Cox regression model that included the serum levels of IL-10, treatment regimens, genetic abnormalities, LDH level, ISS stage, and ECOG PS score, it was concluded that serum IL-10 and certain cytogenetic abnormalities were independent prognostic factors for both PFS and OS.
In the present study, we measured serum IL-10 levels in healthy volunteers and patients with MM. We found that IL-10 was fairly low in the healthy controls (median concentration 17.54 pg ml−1), but it showed a relative higher level in patients with MM, with a median value of 167.19 pg ml−1. The results of the current study are consistent with the previous study of Pappa et al (2007) in which serum IL-10 levels were reported as a specific tumour biomarker for MM patients. Interleukin-10 is a cytokine that can significantly enhance the proliferation of B cells, being involved in their terminal differentiation into plasma cells. Thus, it is easy to explain the correlation between IL-10 and elevated serum LDH and response to treatment.
According to the ROC curve analyses, 169.96 pg ml−1 was an optimal cutoff value for distinguishing between poor outcomes and good outcomes. Patients with low IL-10 levels (⩽169.96 pg ml−1) had higher ORR rates (79.2%) than those with high IL-10 levels (>169.96 pg ml−1; 53.3%); P<0.001). Specifically, 3-year PFS and OS rates in the group with low IL-10 level were clearly longer than those in the group with high IL-10 level (3-year PFS rate: 69.3% vs 13.3%, P<0.001; 3-year OS rate: 93.6% vs 51.9%, P<0.001). Multivariate analysis also indicated that IL-10 was an independent prognostic factor for PFS and OS. All these data confirmed that serum IL-10 was closely correlated with treatment response and prognosis in MM, implying a significant role for IL-10 in the pathogenesis and development of this disease. A possible role for IL-10 in MM was first suggested by the fact that IL-10 stimulated the proliferation of cytokine-dependent cell lines and freshly explanted myeloma cell from patients with active MM through the Janus kinase/signal transducer and activator of transcription pathway as well as the mitogen-activated protein (MAP) kinase and phosphatidylinositol 3-kinase/AKT pathways in the absence of IL-6 (Lu et al, 1995; Palumbo and Anderson, 2011; Manier et al, 2012). The mechanisms underlying the relationship between elevated serum IL-10 levels and poor prognosis are not clear; however, several potential explanations have been proposed. Functionally, IL-10 supports cell expansion and maintenance by the following. (1) Induces strong proliferative signals through a gp130 cytokine oncostain (OSM) that is frequently produced by myeloma cells. Myeloma cells failed to express OSM receptors but IL-10, by inducing it, confers on them the sensitivity to OSM (Gu et al, 1996). (2) Prevents cell death by downregulating membrane Fas and increasing Bcl-2 and Bcl-xL levels (Otsuki et al, 2002). (3) Intensifies the immunosuppressive state in local tumour microenvironment. Human myeloma-associated monocytes/macrophages (MAM) with activation of TLR2/6-dependent TPL2 pathway constitute the predominant source of IL-10 (Hope et al, 2014) that may directly affect the infiltrating T cells by rendering them functionally inactive and maintain the immaturity of dendritic cells that leads to impair local host immune responses and then weakens the antitumour immunity (Hattori et al, 2003). In addition, IL-10 upregulates programmed death ligand 1 (PD-L1) expression on macrophages that is critical for immune tolerance induction (Getts et al, 2011). Further studies, however, are needed to elucidate the cellular mechanisms of IL-10-mediated signalling in MM.
Patients achieving CR are reported to have a significantly better prognosis, regardless of whether it is in the newly diagnosed patients or relapse/refractory patients (Chanan-Khan et al, 2008; Niesvizky et al, 2008; Harousseau et al, 2009b). In this study, we found that good treatment response (CR+PR) is a significantly favourable prognostic factor independent of the treatment regimens in MM. Moreover, our result showed that the ORR was significantly higher in patients treated with bortezomib-based regimens than those with DVD regimen (75.0% vs 47.1%, P<0.001). Higher ORR only brought about longer PFS but failed to improve OS. For the past few years, a number of prospective randomised clinical trials demonstrated that bortezomib-based regimens improved the ORR but could not prolong the OS compared with conventional regimens such as VAD (vincristine, adriamycin, and dexamethasone) (Harousseau et al, 2010; Sonneveld et al, 2012). Similarly, our data from this retrospective study with large sample showed that bortezomib-based regimens was only superior to DVD in PFS. Therefore, although the efficacy was markedly improved with the extensive use of the novel drug such as bortezomib, it is still needed to develop more novel antimyeloma drugs with different mechanism. Sun et al (2015) found that IL-10 and PD-1 cooperate to limit the activity of tumour-specific CD8+ T cells and IL-10 blockade adds to PD-1 blockade to further enhance the expansion and functions of tumour-specific CD8+ T cells. Such findings may have significant implications in terms of targeting IL-10 signalling using blocking antibodies to further improve the clinical efficacy of PD-1 blockade in the treatment of MM.
In conclusion, this is the first study that confirms the close relationship of serum IL-10 with several clinical features of MM, including elevated LDH levels, high ISS stage, and poor PS that can be easily measured in clinical practice, and it may be a significant independent prognostic factor for this disease. Future prospective studies are warranted to confirm our findings.
Acknowledgments
We thank the patients and their families and all the investigators, including the physicians, nurses, and laboratory technicians in this study. Our work was supported by the following funds: National Natural Science Foundation of China (Contract/Grant Number 81272620); Science and Technology Projects of Guangdong Province (Contract/Grant Number 2014A020212577), and Medical Research Foundation of Guangdong Province (Contract/Grant Number A2015008).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Alexandrakis MG, Goulidaki N, Pappa CA, Boula A, Psarakis F, Neonakis I, Tsirakis G (2015) Interleukin-10 induces both plasma cell proliferation and angiogenesis in multiple myeloma. Pathol Oncol Res 21(4): 929–934. [DOI] [PubMed] [Google Scholar]
- Alexandrakis MG, Passam FH, Kyriakou DS, Christophoridou AV, Perisinakis K, Hatzivasili A, Foudoulakis A, Castanas E (2004) Serum level of interleukin-16 in multiple myeloma patients and its relationship to disease activity. Am J Hematol 75(2): 101–106. [DOI] [PubMed] [Google Scholar]
- Atanackovic D, Hildebrandt Y, Templin J, Cao Y, Keller C, Panse J, Meyer S, Reinhard H, Bartels K, Lajmi N, Sezer O, Zander AR, Marx AH, Uhlig R, Zustin J, Bokemeyer C, Kroger N (2012) Role of interleukin 16 in multiple myeloma. J Natl Cancer Inst 104(13): 1005–1020. [DOI] [PubMed] [Google Scholar]
- Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, Di Raimondo F, Crippa C, Zamagni E, Palumbo A, Offidani M, Corradini P, Narni F, Spadano A, Pescosta N, Deliliers GL, Ledda A, Cellini C, Caravita T, Tosi P, Baccarani M (2010) Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet 376(9758): 2075–2085. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, Neuwirth R, Anderson KC, Richardson PG (2008) Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol 26(29): 4784–4790. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Pouli A, Zervas K, Grigoraki V, Symeonidis A, Repoussis P, Mitsouli C, Papanastasiou C, Margaritis D, Tokmaktsis A, Katodritou I, Kokkini G, Terpos E, Vyniou N, Tzilianos M, Chatzivassili A, Kyrtsonis MC, Panayiotidis P, Maniatis A (2003) Prospective randomized comparison of vincristine, doxorubicin and dexamethasone (VAD) administered as intravenous bolus injection and VAD with liposomal doxorubicin as first-line treatment in multiple myeloma. Ann Oncol 14(7): 1039–1044. [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV (2006) International uniform response criteria for multiple myeloma. Leukemia 20(9): 1467–1473. [DOI] [PubMed] [Google Scholar]
- Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, King NJ, Miller SD (2011) Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol 187(5): 2405–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu ZJ, Costes V, Lu ZY, Zhang XG, Pitard V, Moreau JF, Bataille R, Wijdenes J, Rossi JF, Klein B (1996) Interleukin-10 is a growth factor for human myeloma cells by induction of an oncostatin M autocrine loop. Blood 88(10): 3972–3986. [PubMed] [Google Scholar]
- Gupta M, Han JJ, Stenson M, Maurer M, Wellik L, Hu G, Ziesmer S, Dogan A, Witzig TE (2012) Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood 119(12): 2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harousseau JL, Attal M, Avet-Loiseau H (2009. a) The role of complete response in multiple myeloma. Blood 114(15): 3139–3146. [DOI] [PubMed] [Google Scholar]
- Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, Lenain P, Hulin C, Facon T, Casassus P, Michallet M, Maisonneuve H, Benboubker L, Maloisel F, Petillon MO, Webb I, Mathiot C, Moreau P (2010) Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol 28(30): 4621–4629. [DOI] [PubMed] [Google Scholar]
- Harousseau JL, Avet-Loiseau H, Attal M, Charbonnel C, Garban F, Hulin C, Michallet M, Facon T, Garderet L, Marit G, Ketterer N, Lamy T, Voillat L, Guilhot F, Doyen C, Mathiot C, Moreau P (2009. b) Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 Trials. J Clin Oncol 27(34): 5720–5726. [DOI] [PubMed] [Google Scholar]
- Hattori E, Okumoto K, Adachi T, Takeda T, Ito J, Sugahara K, Watanabe H, Saito K, Saito T, Togashi H, Kawata S (2003) Possible contribution of circulating interleukin-10 (IL-10) to anti-tumor immunity and prognosis in patients with unresectable hepatocellular carcinoma. Hepatol Res 27(4): 309–314. [DOI] [PubMed] [Google Scholar]
- Holland G, Zlotnik A (1993) Interleukin-10 and cancer. Cancer Invest 11(6): 751–758. [DOI] [PubMed] [Google Scholar]
- Hope C, Ollar SJ, Heninger E, Hebron E, Jensen JL, Kim J, Maroulakou I, Miyamoto S, Leith C, Yang DT, Callander N, Hematti P, Chesi M, Bergsagel PL, Asimakopoulos F (2014) TPL2 kinase regulates the inflammatory milieu of the myeloma niche. Blood 123(21): 3305–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, O'Garra A, Ishida H, de Waal Malefyt R, de Vries J (1992) Biological properties of interleukin 10. J Clin Immunol 12(4): 239–247. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA (2008) Improved survival in multiple myeloma and the impact of novel therapies. Blood 111(5): 2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZY, Zhang XG, Rodriguez C, Wijdenes J, Gu ZJ, Morel-Fournier B, Harousseau JL, Bataille R, Rossi JF, Klein B (1995) Interleukin-10 is a proliferation factor but not a differentiation factor for human myeloma cells. Blood 85(9): 2521–2527. [PubMed] [Google Scholar]
- Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM (2012) Bone marrow microenvironment in multiple myeloma progression. J Biomed Biotechnol 2012: 157496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765. [DOI] [PubMed] [Google Scholar]
- Muthu Raja KR, Kubiczkova L, Rihova L, Piskacek M, Vsianska P, Hezova R, Pour L, Hajek R (2012) Functionally suppressive CD8 T regulatory cells are increased in patients with multiple myeloma: a cause for immune impairment. PLoS One 7(11): e49446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Pham TN, Lee YK, Kim HJ, Lee JJ (2012) Immunotherapy using dendritic cells against multiple myeloma: how to improve? Clin Dev Immunol 2012: 397648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesvizky R, Richardson PG, Rajkumar SV, Coleman M, Rosinol L, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Boral AL, Esseltine DL, Anderson KC, Blade J (2008) The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 APEX trial in relapsed multiple myeloma. Br J Haematol 143(1): 46–53. [DOI] [PubMed] [Google Scholar]
- Noonan K, Marchionni L, Anderson J, Pardoll D, Roodman GD, Borrello I (2010) A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood 116(18): 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki T, Yata K, Sakaguchi H, Kurebayashi J, Matsuo Y, Uno M, Fujii T, Eda S, Isozaki Y, Yawata Y, Yamada O, Wada H, Sugihara T, Ueki A (2002) Interleukin 10 abolishes the growth inhibitory effects of all-trans retinoic acid on human myeloma cells. Br J Haematol 116(4): 787–795. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Anderson K (2011) Multiple myeloma. N Engl J Med 364(11): 1046–1060. [DOI] [PubMed] [Google Scholar]
- Pappa C, Miyakis S, Tsirakis G, Sfiridaki A, Alegakis A, Kafousi M, Stathopoulos EN, Alexandrakis MG (2007) Serum levels of interleukin-15 and interleukin-10 and their correlation with proliferating cell nuclear antigen in multiple myeloma. Cytokine 37(2): 171–175. [DOI] [PubMed] [Google Scholar]
- Smith D, Yong K (2013) Multiple myeloma. BMJ 346: f3863. [DOI] [PubMed] [Google Scholar]
- Song XN, Yang JZ, Sun LX, Meng JB, Zhang JQ, Lv HY, Kong LJ (2013) Expression levels of IL-27 and IL-17 in multiple myeloma patients: a higher ratio of IL-27:IL-17 in bone marrow was associated with a superior progression-free survival. Leuk Res 37(9): 1094–1099. [DOI] [PubMed] [Google Scholar]
- Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, Zweegman S, Vellenga E, Broyl A, Blau IW, Weisel KC, Wittebol S, Bos GM, Stevens-Kroef M, Scheid C, Pfreundschuh M, Hose D, Jauch A, van der Velde H, Raymakers R, Schaafsma MR, Kersten MJ, van Marwijk-Kooy M, Duehrsen U, Lindemann W, Wijermans PW, Lokhorst HM, Goldschmidt HM (2012) Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol 30(24): 2946–2955. [DOI] [PubMed] [Google Scholar]
- Stassi G, Todaro M, Zerilli M, Ricci-Vitiani L, Di Liberto D, Patti M, Florena A, Di Gaudio F, Di Gesu G, De Maria R (2003) Thyroid cancer resistance to chemotherapeutic drugs via autocrine production of interleukin-4 and interleukin-10. Cancer Res 63(20): 6784–6790. [PubMed] [Google Scholar]
- Sun Z, Fourcade J, Pagliano O, Chauvin JM, Sander C, Kirkwood JM, Zarour HM (2015) IL10 and PD-1 cooperate to limit the activity of tumor-specific CD8+ T cells. Cancer Res 75(8): 1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visco C, Vassilakopoulos TP, Kliche KO, Nadali G, Viviani S, Bonfante V, Medeiros LJ, Notti P, Rassidakis GZ, Peethambaram P, Wilder R, Witzig T, Gianni M, Bonadonna G, Pizzolo G, Pangalis GA, Cabanillas F, Sarris AH (2004) Elevated serum levels of IL-10 are associated with inferior progression-free survival in patients with Hodgkin's disease treated with radiotherapy. Leuk Lymphoma 45(10): 2085–2092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.