Abstract
Background:
Medullary thyroid carcinoma (MTC) accounts for ∼5% of all thyroid malignancies. To date, surgery is the first-line therapy with curative intention. However, for advanced MTC, conventional chemotherapeutic agents do not provide convincing results. Therefore, the identification of biomarkers that can be antagonised by small-molecule therapeutics may lead to novel encouraging treatment options.
Methods:
Seventy-nine patients with surgically resected and histologically confirmed MTC were included in this study. Tissue microarrays were constructed to assess the relationship between inhibitor of apoptosis proteins (IAPs) survivin or XIAP expression levels and clinicopathological variables as well as overall survival.
Results:
High survivin or XIAP expression was associated with an advanced T-stage and metastatic disease. Whereas tissue expression levels of survivin correlated with serum calcitonin levels, XIAP was overexpressed in the subgroup of patients with sporadic MTC. Both IAPs were negatively associated with patient survival in the multivariate Cox regressions analysis (survivin: hazard ratio (HR) 1.62; 95% confidence interval (CI): 1.21–2.16; P=0.001; XIAP: HR 1.78; 95% CI: 1.16–2.72; P=0.008).
Conclusions:
Survivin and XIAP demonstrate distinct expression patterns in MTCs, which are associated with advanced disease and poor prognosis. We thus provide first evidence that both IAPs might serve as viable targets in patients with MTC.
Keywords: survivin, XIAP, medullary thyroid carcinoma, inhibitor of apoptosis protein, biomarker, apoptosis
Medullary thyroid carcinoma (MTC) originates from the calcitonin-secreting, non-iodine-retaining parafollicular C cells. It is the third most common thyroid malignancy and accounts for ∼5% of all thyroid carcinomas (Ball, 2009). Although 75% of all MTCs occur sporadically, ∼25% of all patients harbour a germline mutation of the REarranged during Transfection (RET) proto-oncogene (Donis-Keller et al, 1993; Takahashi, 1995). The RET proto-oncogene is located on chromosome 10q11.2 and is essential for the regulation of cell cycle progression, migration and differentiation (Blume-Jensen and Hunter, 2001; Qi et al, 2011). Importantly, mutations of the RET gene are associated with three distinct clinical syndromes: multiple endocrine neoplasia type 2 A (70%) and B (10%) (MEN2A/B) and the non-MEN (20%), also called familial MTC (FMTC). Although these germline mutations conclude in different syndrome-specific diseases such as pheochromocytoma and primary hyperparathyroidism for MEN2A and B, FMTC shows no other sign or symptom apart from MTC (Eng et al, 1996; Leboulleux et al, 2004; Wells et al, 2013).
However, for both groups of patients the adequate therapy upon establishing the diagnosis remains the radical thyroidectomy with or without lymphadenectomy depending on the clinical findings and the serum level of calcitonin (American Thyroid Association Guidelines Task Force et al, 2009; Dralle et al, 2013). Owing to the large group of patients that present with advanced metastatic disease at the time of initial diagnosis, adjuvant therapeutic strategies have been in the focus of researchers worldwide (Tuttle et al, 2010). Hence, the better understanding of MTC tumour biology has led to the identification of new promising molecular targets like the tyrosine kinase receptors (Hu et al, 2014). These therapeutic targets can be affected by small molecular compounds, creating new options for the patients as well as the clinical team (Wu et al, 2011). However, despite first encouraging results of targeted therapy approaches, the prognosis of patients with distant metastases remains poor, with 10-year survival rates of 21% (American Thyroid Association Guidelines Task Force et al, 2009).
Consequently, there is still an urgent need to identify novel druggable molecular targets that are of relevance for MTC tumour biology. In this context, a group of antiapoptotic proteins referred to as the inhibitor of apoptosis protein (IAP) family has attracted considerable attention. Inhibitor of apoptosis proteins are a family of proteins defined by up to three baculoviral IAP repeat (BIR) domains (Miller, 1999) that are necessary for protein–protein interaction with a number of proapoptotic factors (Gyrd-Hansen and Meier, 2010). They have an important role in cell death, mitosis, migration, metastasis and inflammation (Bertrand et al, 2009; Krieg et al, 2009; Krieg and Reed, 2010; Mehrotra et al, 2010; de Almagro and Vucic, 2012). So far, eight members of the human IAP family have been identified: NAIP (BIRC1), c-IAP1 (BIRC2), c-IAP2 (BIRC3), XIAP (BIRC4), survivin (BIRC5), Apollon/Bruce (BIRC6), ML-IAP (BIRC7) and ILP-2 (BIRC8) (de Almagro and Vucic, 2012).
Two of the most renowned IAP-family members survivin and XIAP synergistically inhibit apoptosis by forming a complex that prevents XIAP from proteasomal degradation, resulting in an enhanced inhibition of caspases (Vaux and Silke, 2003). Moreover, survivin-XIAP interaction promotes tumour cell invasion and metastasis by inducing nuclear translocation of transcription factor nuclear factor-κB (NF-κB), which leads to the activation of cell motility kinases FAK (focal adhesion kinase) and Src (sarcoma) (Mehrotra et al, 2010). Whereas XIAP additionally inhibits the Ripoptosome, a ∼2 MDa large cell death-inducing platform that mediates apoptosis and necroptosis in response to genotoxic stress (Tenev et al, 2011), survivin is involved in the formation of the chromosomal passenger complex, making it a key regulator of cell cycle progression that is essential for completion of mitosis (Rosa et al, 2006).
Importantly, overexpression of XIAP and survivin has been demonstrated to be associated with a poor prognosis in several types of tumours such as carcinomas of the colon, stomach, breast and kidney, making them potential biomarkers for these tumour entities (Mizutani et al, 2007; Goossens-Beumer et al, 2014; Gu et al, 2014; Xu et al, 2014). In addition, overexpression of survivin and survivinΔEx3 splice variant has been demonstrated in thyroid malignancies, including a small number of MTC tissue specimens (Waligórska-Stachura et al, 2014).
Thus, the aim of our study was to determine the expression of survivin and XIAP in surgically resected specimens of MTC and corresponding normal thyroid tissue. Considering the synergistic function of XIAP and survivin, we aimed to assess the prognostic value of both IAPs in a rigorous manner according to the REporting recommendations for tumour MARKer prognostic studies (REMARK), as well as their association with clinicopathological characteristics of MTC patients (McShane et al, 2005).
Materials and methods
Patient selection
Patients who underwent curative surgery for histologically confirmed MTC at the Department of Surgery (A), University Hospital Duesseldorf between 1986 and 2003 were retrospectively reviewed. All types of primary thyroid surgery, with or without lymph node dissection, were included (i.e., thyroidectomy, hemithyroidectomy, subtotal resection). Exclusion criteria were incomplete pathological report or clinical data as well as macroscopic incomplete resection, or insufficient tumour material for further analysis. In addition, patients lost to follow-up or who died because of postoperative complications within the first 30 days after surgery were excluded for survival analysis. The study was conducted according to Good Clinical Practice, the Declaration of Helsinki and local rules as well as regulations of the country. In addition, strict anonymity of all study data were established and maintained. Moreover, the study was carried out with an ethical approval from the institutional ethics committee of the Medical Faculty, Heinrich Heine University Duesseldorf (reference number: 3821).
Clinicopathological data
Overall survival data were obtained from our prospectively maintained clinical database. Clinical parameters including age at first diagnosis, gender, date and type of surgery, serum calcitonin levels before surgery, family history and results from genotyping with regard to sporadic and inherited MTC, initial tumour stage including TNM status, affected lobe and clinical follow-up were retrospectively reviewed. Pathological findings were directly obtained from the original histopathology reports. All tumours were staged according to the 7th edition of the UICC classification (Ito et al, 2012). Samples from earlier surgeries were adjusted to this staging system to ensure conformity and reproducibility.
Tissue microarray and immunohistochemistry
All formalin-fixed paraffin-embedded tissue blocks were retrieved from the Institute of Pathology, University Hospital Duesseldorf. A total of nine TMA paraffin blocks were constructed for this study. They contained each two representative tissue cores of the primary tumour, one tissue core of normal thyroid gland, two tissue samples of a lymph node metastasis if present and one tissue sample of distant metastasis if available for each respective patient. Accordingly, for each patient up to six cylinders of 1.0 mm diameter were taken from their respective donor blocks and placed in paraffin recipient blocks, with 0.5 mm distance between the cylinders. Finally, one TMA block consisted of a maximum number of 10 different patients and their respective tissue samples.
For immunohistochemical staining of survivin, XIAP and calcitonin, TMAs were cut into slides of 4 μm thickness. The staining was performed using the ZytoChem Plus HRP-DAB Kit (Zytomed Systems, Berlin, Germany) as described previously (Cupisti et al, 2014). Briefly, after deparaffinisation and rehydration, antigen unmasking was performed at 95 °C for 30 min using a 3% trisodium citrate dihydrate buffer equilibrated at pH 6, followed by incubation for 20 min at room temperature. Endogenous peroxidase was inactivated by incubating the slides in 3% H2O2 in phosphate-buffered saline (PBS, pH 7.4) for 10 min at room temperature. Sections were then rinsed three times for 2 min in PBS with 0.1% Tween-20 (Sigma-Aldrich, St Louis, MO, USA), followed by blocking of unspecific protein binding sites using reagent 1. After washing in PBS with 0.1% Tween-20, immunostaining was performed for 60 min at room temperature with rabbit polyclonal anti-survivin (NB500-201; 1 : 750 dilution; Novus, Littleton, CO, USA), mouse monoclonal anti-XIAP (Clone 48; 1 : 50 dilution; BD Biosciences, San Jose, CA, USA) and rabbit polyclonal anti-calcitonin antibody (Clone Poly29118, 1 : 100 dilution; Biolegend, San Diego, CA, USA), respectively. Isotype control was conducted using mouse IgG1κ (MOPC-21; 1 : 50 dilution; Abcam, Cambridge, UK) and rabbit immunoglobulin fraction (Code X0903; 1 : 1000 dilution; Dako, Glostrup, Denmark). After three washing steps in PBS with 0.1% Tween-20, slides were incubated with biotinylated secondary antibody and streptavidin–HRP conjugate. Finally, colour development was achieved by incubation with 3,3′-diaminobenzidine high contrast and counterstaining with haematoxylin. To verify that all of the extracted MTC tissue cores contained tumour, we performed immunohistochemical staining against calcitonin. For each immunohistochemical staining procedure, a tissue slide of pretested human colon and renal cell carcinoma, known to express survivin or XIAP intensively, served as a positive control.
For TMA analyses, survivin and XIAP staining intensity and percentage of stained cells were scored by two independent investigators (TW and YT) according to the immunoreactivity score (IRS) reported by Remmele et al (1986) with slight modifications: intensity was graded as absent (0), weak/low (1), moderate (2) and high (3); percentage of stained cells was graded <5% (0), 5–25% (1), 25–50% (2), 50–75% (3) and >75% (4). The product of the two attributes equalled the IRS ranging from 0 to 12.
Statistical analysis
Differences in expression levels of survivin or XIAP according to clinicopathological variables were examined using the nonparametric Mann–Whitney test. Categorical data were analysed using the Fisher's exact test.
For survival analysis, survivin and XIAP were categorised into the groups of high expression (⩾median IRS) and low expression (<median IRS). Clinicopathological variables were compared as follows: T1/2 vs T3/4, UICC I/II vs UICC III/IV and age as well as calcitonin (pmol l−1) based on their median (⩾median vs <median). Overall survival was defined as the period from the date of surgery until death of any causes or until the date of the last follow-up at which survivors were censored. For univariate survival analysis, survival curves were evaluated using Kaplan–Meier curves and assessed using the log-rank (Mantel Cox) test (GraphPad Software, La Jolla, CA, USA). Cox regression analysis was used for multivariate survival analyses, estimating hazard ratios (HRs) with 95% confidence intervals (CIs). The regression analysis was first performed with all available variables and then focussed using a stepwise variable selection procedure based on the Akaike information criterion (AIC). Internal validation was conducted using Bootstrap analysis with 500 replicates.
In addition, as an exploratory analysis, we performed a survival regression tree analysis to identify subgroups of patients with a high risk of death. This technique combines an algorithm for recursive partitioning together with a well-defined theory of permutation tests. Multiple test procedures are applied to determine whether a significant association between any of the covariables and the response variable can be stated. The resulting partitioning regression analysis is graphically displayed as a classification tree. The partitioning nodes are displayed by an optimal cut-off point for continuous covariables and with a classification split for categorical covariables. Each node split is assessed with a P-value calculated by a permutation test.
Data analysis was performed using the Statistical Software R version 3.1.0 (R Development Core Team, 2014). A P-value <0.05 was considered to indicate statistical significance.
Results
Patients and outcome
Based on our inclusion and exclusion criteria, a total number of 79 patients could be included in this study (Supplementary Figure 1). Patients' clinical and pathological characteristics are summarised in Table 1. Because five patients were lost to follow-up, a total number of 74 patients were included for survival analysis. Median overall survival of these patients was 166 months (range 5–287 months).
Table 1. Patient characteristics (n=79).
| Variables | No. of patients (%) |
|---|---|
| Total | 79 |
| Age | |
| Median (range); years | 49 (6–83) |
|
Gender | |
| Male | 38 (48) |
| Female | 41 (52) |
| Genetic | |
| Sporadic | 37 (47) |
| MEN2A | 34 (43) |
| Unknown | 8 (10) |
| Basal calcitonin levels prior to surgery | |
| <12 pg ml−1 | 47 (60) |
| >12 pg ml−1 | 12 (15) |
| Unknown | 20 (25) |
| Type of surgery | |
| Hemithyroidectomy | 11 (14) |
| With unilateral ND | 3 (4) |
| Subtotal thyroidectomy | 3 (4) |
| With bilateral ND | 2 (2) |
| Total thyroidectomy without ND | 14 (18) |
| With unilateral ND | 16 (20) |
| With bilateral ND | 30 (38) |
| Side affected | |
| Unilateral | 65 (82) |
| Bilateral | 14 (18) |
| Tumour stage | |
| T1/2 | 60 (76) |
| T3/4 | 19 (24) |
| Lymph node metastasis | |
| N0 | 37 (47) |
| N1a/b | 42 (53) |
| Distant metastasis | |
| M0 | 55 (70) |
| M1 | 24 (30) |
| UICC stage | |
| UICC I/II | 36 (46) |
| UICC III/IV | 43 (54) |
Abbreviations: ND=neck dissection; UICC=Union internationale contre le cancer.
Calcitonin basal blood levels were available for 59 patients before surgery. The median level being 459 pg ml−1. The genetic background of MTCs, whether sporadic or inherited, was known for 71 patients, whereas there was no clear indication for eight patients. The type of surgery differed depending on the given recommendation of the respective period of time. The vast majority, however, was treated with a total thyroidectomy with or without neck dissection.
Survivin and XIAP expression correlate with advanced tumour stage
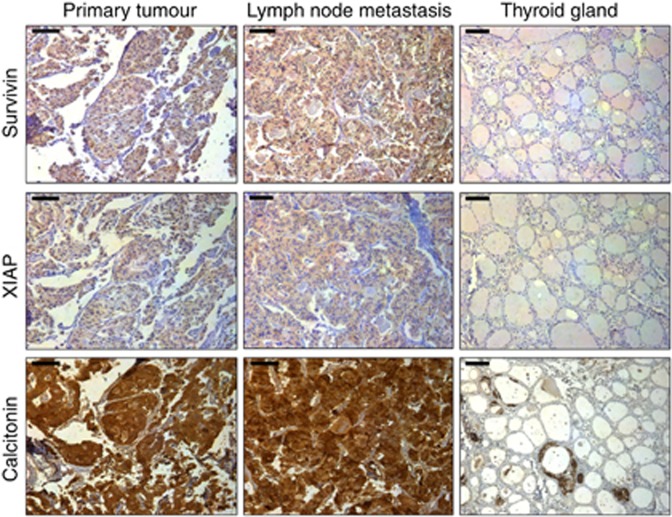
All included cores of primary tumour tissue as well as metastases stained positive for calcitonin, confirming the diagnosis of MTC. In addition, parafollicular C cells stained positively in normal thyroid tissue specimen (Figure 1). Next, we analysed the distribution of survivin and XIAP within the normal thyroid gland, MTCs and the corresponding lymph node or distant metastases. Whereas survivin and XIAP were strongly expressed in MTC with a predominately cytoplasmic localisation, normal thyroid tissue stained negative for both IAPs (Figure 1). However, when comparing primary MTC with their corresponding lymph node or distant metastasis, no difference in expression levels became evident (data not shown).
Figure 1.
Representative tissue samples with immunohistochemical staining for survivin, XIAP and calcitonin in MTC (left), respective lymph node metastases (middle) and normal thyroid gland (right). All shown samples were classified as strong expression for the respective targets in accordance with the IRS. The bar at the top left corner indicates 50 μm.
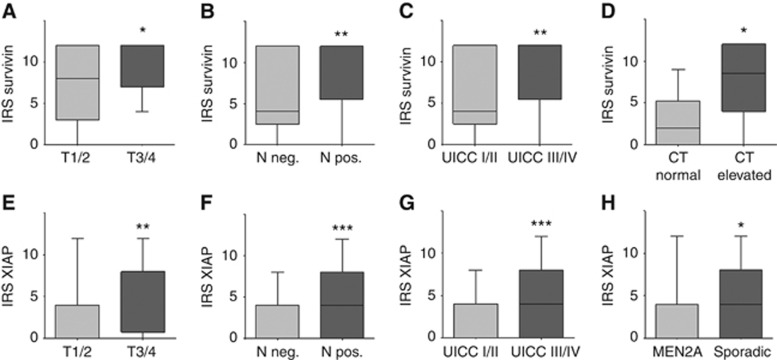
To explore a potential association between the two IAPs and clinicopathological parameters, two statistical approaches were applied. First, we compared the IRS across groups for each clinicopathological variable (i.e., UICC I+II vs UICC III+IV; Figure 2).
Figure 2.
(A–H) Expression levels of survivin and XIAP and their association with clinicopathological variables in MTC. Boxplots display the median IRS with the upper and lower quartile, as well as maximum and minimum stratified according to the respective clinicopathological variable. CT=calcitonin. *P<0.05; **P<0.01; ***P<0.001.
Using this approach, we found a strong correlation between high survivin (P=0.007) and XIAP (P=0.001) expression levels and advanced UICC III/IV tumour stages (Figure 2C and G). Accordingly, IRSs for survivin were significantly higher in patients with T3/4 tumours (median: 12; range: 4–12) when compared with T1/2 MTCs (median: 8; range: 0–12; P=0.032; Figure 2A). Additionally, tumour probes from patients with lymph node or distant metastasis showed a distinct higher expression of survivin compared with tumour probes from patients with non-metastasised disease stages (P=0.007 and 0.007; Figure 2B and C). Interestingly, we also found a strong association between basal calcitonin blood levels and survivin expression. Patients with a high calcitonin blood level at the time of admission showed a significantly higher survivin expression in their respective primary tumour compared with patients with normal calcitonin blood levels (P=0.047; Figure 2D).
Similar results were obtained for the XIAP expression analysis. T3/4 samples demonstrated a significantly higher IRS for XIAP compared with T1/2 tumour samples (P=0.002; Figure 2E). Again, a lymph node-positive status was also accompanied by significantly higher expression of XIAP in the primary tumour (P=0.001; Figure 2F). Notably, patients with sporadic MTC also exhibited a distinct higher expression of XIAP as compared with the MEN2A-associated MTC (P=0.032; Figure 2H).
Second, by categorising the respective expression levels according to the median into high (⩾median) and low (<median), we could confirm the association of high survivin and XIAP expression with more advanced tumour stages as well as an association of XIAP with sporadic MTC (Table 2).
Table 2. Correlation between survivin or XIAP expression and clinicopathological factors in MTC.
|
Survivin expression |
XIAP expression |
|||||
|---|---|---|---|---|---|---|
| Variables | Low, n=32 (%) | High, n=47 (%) | P-value | Low, n=42 (%) | High, n=37 (%) | P-value |
| Age, mean±s.d. | 48.5±18.7 | 50.5±18.6 | 0.3021 | 49±20.7 | 49±19.3 | 0.9849 |
| Gender | ||||||
| Male | 15 (47) | 23 (49) | 19 (45) | 19 (51) | ||
| Female | 17 (53) | 24 (51) | 1.0 | 23 (55) | 18 (49) | 0.6550 |
| Tumour stage | ||||||
| T1/2 | 29 (91) | 31 (66) | 37 (88) | 23 (62) | ||
| T3/4 | 3 (9) | 16 (34) | 0.0153 | 5 (12) | 14 (38) | 0.0089 |
| Lymph node metastasis | ||||||
| N0 | 21 (66) | 16 (34) | 26 (62) | 11 (30) | ||
| N1a/b | 11 (34) | 31 (66) | 0.0068 | 16 (38) | 26 (70) | 0.0065 |
| Distant metastasis | ||||||
| M0 | 29 (91) | 30 (64) | 27 (64) | 28 (76) | ||
| M1 | 3 (9) | 17 (36) | 0.0084 | 15 (36) | 9 (24) | 0.3311 |
| UICC stage | ||||||
| UICC I/II | 20 (62.5) | 16 (34) | 25 (60) | 11 (30) | ||
| UICC III/IV | 12 (37.5) | 31 (66) | 0.0208 | 17 (40) | 26 (70) | 0.0124 |
| Low, n=24 (%) | High, n=35 (%) | Low, n=27 (%) | High, n=32 (%) | |||
| Calcitonin basal blood level | ||||||
| Normal | 9 (37.5) | 3 (9) | 5 (19) | 7 (22) | ||
| Elevated | 15 (62.5) | 32 (91) | 0.0095 | 22 (81) | 25 (78) | 1.0 |
| Low, n=32 (%) | High, n=39 (%) | Low, n=38 (%) | High, n=33 (%) | |||
| Genetic | ||||||
| Sporadic | 13 (41) | 24 (62) | 15 (39) | 22 (67) | ||
| MEN2A | 19 (59) | 15 (38) | 0.0980 | 23 (61) | 11 (33) | 0.0321 |
Abbreviations: MTC=medullary thyroid carcinoma; XIAP=X-Linked inhibitor of apoptosis protein; UICC=Union internationale contre le cancer.
Survivin and XIAP are independent markers of poor prognosis in MTC
For survival analysis, the following variables were included: age, gender, sporadic and inherited form, calcitonin basal blood level, tumour stage, lymph node status, distant metastasis and UICC stage as well as survivin and XIAP expression levels according to the IRS.
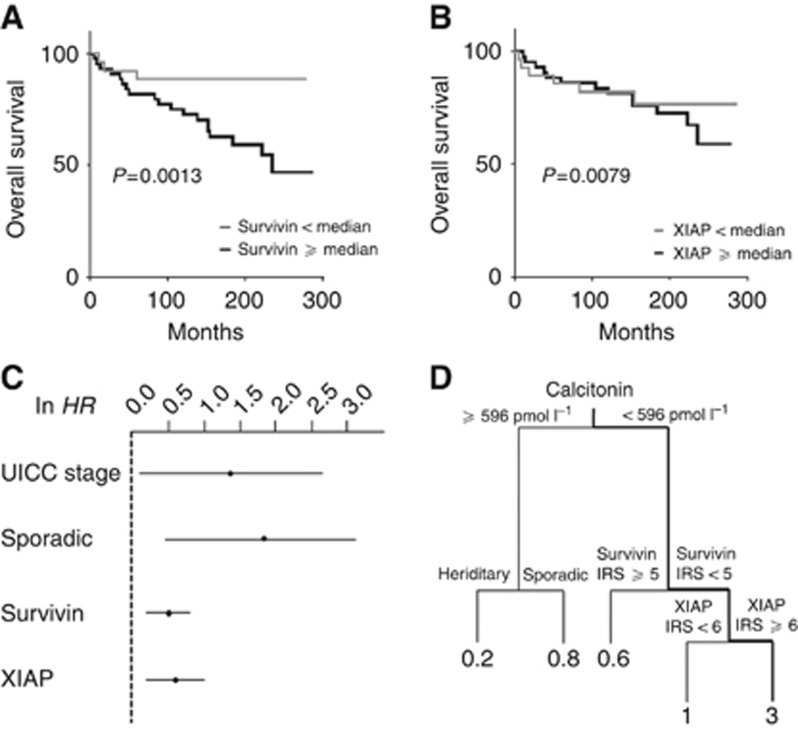
In the univariate analysis, high survivin (HR=3.765; 95% CI: 1.166–6.568; P=0.021) but not XIAP (HR=1.239; 95% CI: 0.484–3.140; P=0.662) expression was significantly associated with poor prognosis (Figure 3A and B, Table 3A). Clinicopathological variables such as old age at diagnosis, sporadic MTC, lymph node metastasis, M1 stage and the UICC stage predicted a worse outcome.
Figure 3.
Kaplan–Meier curves for overall survival according to the IRS for survivin and XIAP. Based on the IRS of (A) survivin or (B) XIAP, specimens were classified into samples with high (⩾median) and low (<median) expression levels. (C) Summarises HR and CI of the multivariate analysis after ln transformation. (D) Survival regression tree analysis was performed to identify subgroups of patients with a high risk of death. Numbers under the vertical lines represent HRs of the given constellation.
Table 3A. Overall survival: univariate analysis.
| Variables | HR | CI (lower–upper 95%) | P-value |
|---|---|---|---|
| Age | 2.939 | 1.256–6.823 | 0.0132 |
| Sex | 1.488 | 0.6425–3433 | 0.3535 |
| T1/2 vs T3/4 | 2.176 | 0.9341–7.322 | 0.0696 |
| N0 vs N1a/b | 5.007 | 1.785–9.519 | 0.0010 |
| M0 vs M1 | 4.873 | 3.511–32.44 | 0.0001 |
| UICC I/II vs UICC III/IV | 4.187 | 1.461–7.780 | 0.0046 |
| Sporadic vs MEN2A | 5.094 | 1.558–9.886 | 0.0039 |
| Calcitonin basal blood level | 3.306 | 0.7676–14.24 | 0.1085 |
| Survivin expression | 3.765 | 1.166–6.568 | 0.0214 |
| XIAP expression | 1.239 | 0.4843–3.140 | 0.6618 |
Abbreviations: CI=confidence interval; HR=hazard ratio; XIAP=X-linked inhibitor of apoptosis protein; UICC=Union internationale contre le cancer.
Next, we performed multivariate analysis using a stepwise variable selection procedure based on the AIC. Accordingly, advanced UICC stages III and IV (HR=3.95; 95% CI: 1.143–13.645; P=0.030) as well as sporadic disease (HR=5.815; 95% CI: 1.548–21.840; P=0.009) and high survivin expression (HR=1.616; 95% CI: 1.207–2.162; P=0.001) were found to be independent prognostic factors for MTC (Table 3B). Interestingly, high XIAP expression levels became significant on multivariate analysis (HR=1.776; 95% CI: 1.162–2.715; P=0.008), reflecting a prognostic relevance of XIAP in MTC disease. For a better visualisation, the regression estimates of the multivariate analysis are illustrated in Figure 3C after ln transformation.
Table 3B. Overall survival: multivariate analysis after stepwise variable selection.
| Variables | HR | CI (lower–upper 95%) | P-value |
|---|---|---|---|
| UICC I/II vs UICC III/IV | 3.950 | 1.143–13.645 | 0.030 |
| Sporadic vs MEN2A | 5.815 | 1.548–21.840 | 0.009 |
| Survivin expression | 1.616 | 1.207–2.162 | 0.001 |
| XIAP expression | 1.776 | 1.162–2.715 | 0.008 |
Abbreviations: CI=confidence interval; HR=hazard ratio; XIAP=X-linked inhibitor of apoptosis protein; UICC=Union internationale contre le cancer.
Correlation between biomarkers
To further elucidate a possible interaction between survivin, XIAP and other clinicopathological markers, we performed a regression tree analysis. This test procedure allows the identification of specific biomarker constellations under which patients have a high risk of death. Interestingly, we found a subgroup of patients in our cohort that was associated with a distinct deterioration of their prognosis. Patients with a calcitonin basal blood level at the time of diagnosis below 596 pg ml−1, low survivin expression (IRS<5), but high XIAP expression (IRS⩾6) had three times the risk of dying over the observed period of time, as compared with the other patients (Figure 3D).
Discussion
Over the past years, great achievements have been made, bringing scientific benchwork to the patient's bed site and improving therapeutic results as well as quality of life. The progress of identifying strong prognostic biomarkers and later targeting them with their respective antagonists has led to paramount changes in patient treatment strategies (Miura et al, 2011; Rödel et al, 2012).
Approximately 50% of all patients with MTC harbour distant metastasis at the time of diagnosis, and systemic therapeutic agents are in high demand to treat the spreading disease (Hu et al, 2014). At the beginning of the twenty-first century, only conventional chemotherapeutics, mostly based on dacarbazin and doxorubicin, have been used in the treatment of advanced MTC (Vitale et al, 2001). However, in the past decade a new insight into the biology of MTC has been gained, which enabled oncologists for novel approaches in targeted therapy. Owing to the known biology of RET oncogene mutations in MTC, the main focus has been placed on tyrosine kinase inhibitors, for both hereditary and sporadic MTC (Lalami and Awada, 2011). However, only 40–50% of all patients with sporadic MTC have a somatic RET mutation and a consequently activated tyrosine kinase (Elisei et al, 2008). Therefore, especially for the remaining patients, research dealing with the commonly altered signalling pathways in oncogenesis is of great importance.
In this regard, inhibition of apoptosis is a hallmark feature of carcinoma cells. The regulation as well as the maintenance of a reasonable homeostasis in tissues and organs is a key physiological operation, largely depending on the complex interdependency of proliferation and apoptosis. However, during oncogenesis an imbalance between these two poles leads to an uncontrolled proliferation and metastatic spread (Obexer and Ausserlechner, 2014; Wan et al, 2014). In this context, several antiapoptotic proteins such as the IAP-family members have attracted considerable attention during the past decades. IAPs comprise a group of BIR-domain containing proteins that inhibit apoptosis, induce resistance to conventional chemotherapy or radiotherapy and are overexpressed in a wide variety of tumour entities (de Almagro and Vucic, 2012).
Thus far, little is known about the role of IAPs in the oncogenesis of MTC. To our knowledge, our study is the first that focused on the stage-dependent expression levels of survivin and XIAP in MTC and analysed their prognostic relevance. For our cohort, we could show that survivin and XIAP are two independent novel prognostic biomarkers in MTC. They both demonstrated a strong negative correlation with patients' overall survival in the multivariate analysis. Besides their association with a worse outcome in MTC patients, both survivin and XIAP correlated with more advanced tumour stages and a metastatic phenotype. Importantly, these results are in line with recent meta-analyses that underscored not only the prognostic relevance but also the association of survivin with a metastasised disease for colorectal and gastric carcinoma patients (Krieg et al, 2013a, 2013b). A recent study by Selemetjev et al (2014) also demonstrated the close correlation between survivin and the presence of lymph node metastasis in papillary thyroid carcinoma. Similar results have been published for XIAP in several solid tumour entities, underscoring the relation between XIAP and a metastatic phenotype as well as poor prognosis (Ramp et al, 2004; Shi et al, 2008; Yim et al, 2014). In addition, Mehrotra et al (2010) demonstrated that survivin in a complex with XIAP was essential for the formation of distant metastasis. Functionally, this aspect is independent of their antiapoptotic properties and is caused by an activation of cell motility kinases FAK and Src via NF-κB.
Remarkably, high survivin expression in MTC was associated with elevated calcitonin basal blood levels, whereas this was not evident for XIAP. The observation that high serum calcitonin correlates with increased expression levels of survivin in MTC specimens supports the recently published data by Thomas and Shah (2005) who demonstrated in prostate carcinoma cell lines an induction of survivin by calcitonin, causing chemoresistance in vitro (Thomas and Shah, 2005; Thomas et al, 2007). This interdependency between survivin and calcitonin may be of unique importance in MTC as parafollicular C cells, the sole producers of the peptide hormone calcitonin, are the hosts from which MTC originates. Thus, the relationship between autocrine and paracrine signalling in which tumour cells secrete calcitonin that, upon binding to its receptor, induces antiapoptotic or promitotic signalling pathways are of great interest for the oncogenesis in MTC. This self-preserving cycle of autocrine secretion and self-stimulation may prove to be a key regulatory step and warrants further research in this field.
Interestingly, patients with sporadic MTC exhibited significantly higher expression levels of XIAP compared with the hereditary form. This emphasises again that certain subgroups of a carcinoma may establish different molecular pathways by which they ensure their survival and progression. However, as shown in the decision tree, it sometimes might be the combination of certain biomarkers and the actual given condition of the individual patient that increases the risk of death considerably. Although the decision tree is solely an exploratory statistical method, it still underscores the importance of a broad panel of prognostic biomarkers to describe and foresee the tumour's biology, which is as individual as the patient. Especially, grouping of patients with different characteristics will lead us to a more profound understanding of the disease and ultimately to a more individualised medicine and the chance to tailor our therapeutic approaches to the individual needs of our patients (Schwaederle et al, 2015).
Encouraging results have been published concerning the antagonising effect of chemical compounds against survivin and XIAP in subgroups of thyroid carcinoma. Mehta et al (2015) demonstrated that the survivin inhibitor YM155 could significantly reduce cellular proliferation in anaplastic thyroid carcinoma cell lines, inhibit proliferation and metastasis in vivo and prolong survivial. Similarly for XIAP, Hussain et al (2015) showed that antagonising of XIAP by small-molecule Embelin caused growth inhibition and apoptosis in papillary thyroid carcinoma cell lines and induced tumour regression in a nude mouse model. Thus, both studies underscore the functional aspects of survivin and XIAP as novel biomarkers and their potential therapeutic implications.
Despite the limitations of the retrospective design of our study, our results fit perfectly into the emerging landscape of IAP interaction. Both biomarkers with their distinct expression profiles and negative prognostic implications can therefore be considered as new viable therapeutic options in MTC. However, more research, especially on the functional aspects of survivin and XIAP in MTC, needs to be carried out to further improve our understanding of their role in MTC biology and to enable us for a more personalised therapeutic approach in the future.
Acknowledgments
The study was supported, in part, by a grant from the Deutsche Forschungsgemeinschaft (KR 3496/2-1 to AK).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- American Thyroid Association Guidelines Task Force, Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA (2009) Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19: 565–612. [DOI] [PubMed] [Google Scholar]
- Ball DW (2009) American Thyroid Association guidelines for management of medullary thyroid cancer: an adult endocrinology perspective. Thyroid 19: 547–550. [DOI] [PubMed] [Google Scholar]
- Bertrand MJM, Doiron K, Labbé K, Korneluk RG, Barker PA, Saleh M (2009) Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity 30: 789–801. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411: 355–365. [DOI] [PubMed] [Google Scholar]
- Cupisti K, Lehwald N, Anlauf M, Riemer J, Werner TA, Krieg A, Witte J, Chanab A, Baldus SE, Krausch M, Raffel A, Herdter C, Schott M, Knoefel WT (2014) Encapsulation status of papillary thyroid microcarcinomas is associated with the risk of lymph node metastases and tumor multifocality. Horm Metab Res 46: 138–144. [DOI] [PubMed] [Google Scholar]
- de Almagro MC, Vucic D (2012) The inhibitor of apoptosis (IAP) proteins are critical regulators of signaling pathways and targets for anti-cancer therapy. Exp Oncol 34: 200–211. [PubMed] [Google Scholar]
- Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, Howe JR, Moley JF, Goodfellow P, Wells SA (1993) Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet 2: 851–856. [DOI] [PubMed] [Google Scholar]
- Dralle H, Musholt TJ, Schabram J, Steinmüller T, Frilling A, Simon D, Goretzki PE, Niederle B, Scheuba C, Clerici T, Hermann M, Kußmann J, Lorenz K, Nies C, Schabram P, Trupka A, Zielke A, Karges W, Luster M, Schmid KW, Vordermark D, Schmoll H-J, Mühlenberg R, Schober O, Rimmele H, Machens A German Societies of General and Visceral Surgery, Endocrinology, Nuclear, Medicine, Pathology, Radiooncology, Oncological Hematology and the German Thyroid Cancer Patient Support Organization Ohne Schilddrüse leben e.V. (2013) German Association of Endocrine Surgeons practice guideline for the surgical management of malignant thyroid tumors. Langenbecks Arch Surg 398: 347–375. [DOI] [PubMed] [Google Scholar]
- Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, Agate L, Vivaldi A, Faviana P, Basolo F, Miccoli P, Berti P, Pacini F, Pinchera A (2008) Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 93: 682–687. [DOI] [PubMed] [Google Scholar]
- Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, Gagel RF, van Amstel HK, Lips CJ, Nishisho I, Takai SI, Marsh DJ, Robinson BG, Frank-Raue K, Raue F, Xue F, Noll WW, Romei C, Pacini F, Fink M, Niederle B, Zedenius J, Nordenskjöld M, Komminoth P, Hendy GN, Mulligan LM (1996) The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 276: 1575–1579. [PubMed] [Google Scholar]
- Goossens-Beumer IJ, Zeestraten ECM, Benard A, Christen T, Reimers MS, Keijzer R, Sier CFM, Liefers GJ, Morreau H, Putter H, Vahrmeijer AL, van de Velde CJH, Kuppen PJK (2014) Clinical prognostic value of combined analysis of Aldh1, Survivin, and EpCAM expression in colorectal cancer. Br J Cancer 110: 2935–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Jin S, Wang F, Hua Y, Yang L, Shu Y, Zhang Z, Guo R (2014) Clinicopathological significance of PI3K, Akt and survivin expression in gastric cancer. Biomed Pharmacother 68: 471–475. [DOI] [PubMed] [Google Scholar]
- Gyrd-Hansen M, Meier P (2010) IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer 10: 561–574. [DOI] [PubMed] [Google Scholar]
- Hu MI, Ying AK, Jimenez C (2014) Update on medullary thyroid cancer. Endocrinol Metab Clin N Am 43: 423–442. [DOI] [PubMed] [Google Scholar]
- Hussain AR, Bu R, Ahmed M, Jehan Z, Beg S, Al-Sobhi S, Al-Dayel F, Siraj AK, Uddin S, Al-Kuraya KS (2015) Role of X-linked inhibitor of apoptosis as a prognostic marker and therapeutic target in papillary thyroid carcinoma. J Clin Endocrinol Metab 100: E974–E985. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kihara M, Hirokawa M, Takamura Y, Kobayashi K, Miya A, Miyauchi A (2012) Validity of 6th edition of UICC TNM classification system for medullary thyroid carcinoma: a proposal for intraoperative evaluation of T category. Endocr J 59: 407–416. [DOI] [PubMed] [Google Scholar]
- Krieg A, Baseras B, Tomczak M, Verde PE, Stoecklein NH, Knoefel WT (2013. a) Role of survivin as prognostic and clinicopathological marker in gastric cancer: a meta-analysis. Mol Biol Rep 40: 5501–5511. [DOI] [PubMed] [Google Scholar]
- Krieg A, Correa RG, Garrison JB, Le Negrate G, Welsh K, Huang Z, Knoefel WT, Reed JC (2009) XIAP mediates NOD signaling via interaction with RIP2. Proc Natl Acad Sci USA 106: 14524–14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A, Reed JC (2010) IAPs and their emergent role in NLR signaling. Cell Cycle 9: 426–427. [DOI] [PubMed] [Google Scholar]
- Krieg A, Werner TA, Verde PE, Stoecklein NH, Knoefel WT (2013. b) Prognostic and clinicopathological significance of survivin in colorectal cancer: a meta-analysis. PLoS One 8: e65338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalami Y, Awada A (2011) Recurrent thyroid cancer: a molecular-based therapeutic breakthrough. Curr Opin Oncol 23: 235–240. [DOI] [PubMed] [Google Scholar]
- Leboulleux SS, Baudin EE, Travagli J-PJ, Schlumberger MM (2004) Medullary thyroid carcinoma. Clin Endocrinol (Oxf) 61: 299–310. [DOI] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics (2005) Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 97: 1180–1184. [DOI] [PubMed] [Google Scholar]
- Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC (2010) IAP regulation of metastasis. Cancer Cell 17: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Zhang L, Boufraqech M, Liu-Chittenden Y, Zhang Y, Patel D, Davis S, Rosenberg A, Ylaya K, Aufforth R, Li Z, Shen M, Kebebew E (2015) Inhibition of survivin with YM155 induces durable tumor response in anaplastic thyroid cancer. Clin Cancer Res 21: 4123–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LK (1999) An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell Biol 9: 323–328. [DOI] [PubMed] [Google Scholar]
- Miura K, Fujibuchi W, Ishida K, Naitoh T, Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, Shibata C, Sasaki I (2011) Inhibitor of apoptosis protein family as diagnostic markers and therapeutic targets of colorectal cancer. Surg Today 41: 175–182. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Nakanishi H, Li YN, Matsubara H, Yamamoto K, Sato N, Shiraishi T, Nakamura T, Mikami K, Okihara K, Takaha N, Ukimura O, Kawauchi A, Nonomura N, Bonavida B, Miki T (2007) Overexpression of XIAP expression in renal cell carcinoma predicts a worse prognosis. Int J Oncol 30: 919–925. [PubMed] [Google Scholar]
- Obexer P, Ausserlechner MJ (2014) X-linked inhibitor of apoptosis protein – a critical death resistance regulator and therapeutic target for personalized cancer therapy. Front Oncol 4: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X-P, Ma J-M, Du Z-F, Ying R-B, Fei J, Jin H-Y, Han J-S, Wang J-Q, Chen X-L, Chen C-Y, Liu W-T, Lu J-J, Zhang J-G, Zhang X-N (2011) RET germline mutations identified by exome sequencing in a Chinese multiple endocrine neoplasia type 2A/familial medullary thyroid carcinoma family. PLoS One 6: e20353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2014) R: A Language and Environment for Statistical Computing. The R foundation for statistical computing: Vienna, Austria, Available from http://www.R-project.org/. [Google Scholar]
- Ramp U, Krieg T, Caliskan E, Mahotka C, Ebert T, Willers R, Gabbert HE, Gerharz CD (2004) XIAP expression is an independent prognostic marker in clear-cell renal carcinomas. Hum Pathol 35: 1022–1028. [DOI] [PubMed] [Google Scholar]
- Remmele W, Hildebrand U, Hienz HA, Klein PJ, Vierbuchen M, Behnken LJ, Heicke B, Scheidt E (1986) Comparative histological, histochemical, immunohistochemical and biochemical studies on oestrogen receptors, lectin receptors, and Barr bodies in human breast cancer. Virchows Arch A 409: 127–147. [DOI] [PubMed] [Google Scholar]
- Rosa J, Canovas P, Islam A, Altieri DC, Doxsey SJ (2006) Survivin modulates microtubule dynamics and nucleation throughout the cell cycle. Mol Biol Cell 17: 1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel F, Sprenger T, Kaina B, Liersch T, Rödel C, Fulda S, Hehlgans S (2012) Survivin as a prognostic/predictive marker and molecular target in cancer therapy. Curr Med Chem 19: 3679–3688. [DOI] [PubMed] [Google Scholar]
- Schwaederle M, Zhao M, Lee JJ, Eggermont AM, Schilsky RL, Mendelsohn J, Lazar V, Kurzrock R (2015) Impact of precision medicine in diverse cancers: a meta-analysis of phase II clinical trials. J Clin Oncol 33: 3817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemetjev S, Dencic TI, Marecko I, Jankovic J, Paunovic I, Savin S, Cvejic D (2014) Evaluation of survivin expression and its prognostic value in papillary thyroid carcinoma. Pathol Res Pract 210: 30–34. [DOI] [PubMed] [Google Scholar]
- Shi Y-H, Ding W-X, Zhou J, He J-Y, Xu Y, Gambotto AA, Rabinowich H, Fan J, Yin X-M (2008) Expression of X-linked inhibitor-of-apoptosis protein in hepatocellular carcinoma promotes metastasis and tumor recurrence. Hepatology 48: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M (1995) Oncogenic activation of the ret protooncogene in thyroid cancer. Crit Rev Oncog 6: 35–46. [DOI] [PubMed] [Google Scholar]
- Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P (2011) The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell 43: 432–448. [DOI] [PubMed] [Google Scholar]
- Thomas S, Muralidharan A, Shah GV (2007) Knock-down of calcitonin receptor expression induces apoptosis and growth arrest of prostate cancer cells. Int J Oncol 31: 1425–1437. [PubMed] [Google Scholar]
- Thomas S, Shah G (2005) Calcitonin induces apoptosis resistance in prostate cancer cell lines against cytotoxic drugs via the Akt/survivin pathway. Cancer Biol Ther 4: 1226–1233. [DOI] [PubMed] [Google Scholar]
- Tuttle RM, Ball DW, Byrd D, Daniels GH, Dilawari RA, Doherty GM, Duh Q-Y, Ehya H, Farrar WB, Haddad RI, Kandeel F, Kloos RT, Kopp P, Lamonica DM, Loree TR, Lydiatt WM, McCaffrey J, Olson JA, Parks L, Ridge JA, Shah JP, Sherman SI, Sturgeon C, Waguespack SG, Wang TN, Wirth LJ (2010) Medullary carcinoma. Compr Cancer 8: 512–30. [DOI] [PubMed] [Google Scholar]
- Vaux DL, Silke J (2003) Mammalian mitochondrial IAP binding proteins. Biochem Biophys Res Commun 304: 499–504. [DOI] [PubMed] [Google Scholar]
- Vitale G, Caraglia M, Ciccarelli A, Lupoli G, Abbruzzese A, Tagliaferri P, Lupoli G (2001) Current approaches and perspectives in the therapy of medullary thyroid carcinoma. Cancer 91: 1797–1808. [DOI] [PubMed] [Google Scholar]
- Waligórska-Stachura J, Andrusiewicz M, Sawicka-Gutaj N, Biczysko M, Jankowska A, Kubiczak M, Czarnywojtek A, Wrotkowska E, Ruchała M (2014) Survivin delta Ex3 overexpression in thyroid malignancies. PLoS One 9: e100534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Liu T, Hou X, Dun Y, Guan P, Fang H (2014) Antagonists of IAP proteins: novel anti-tumor agents. Curr Med Chem 21: 3877–3892. [DOI] [PubMed] [Google Scholar]
- Wells SA, Pacini F, Robinson BG, Santoro M (2013) Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab 98: 3149–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LS, Roman SA, Sosa JA (2011) Medullary thyroid cancer: an update of new guidelines and recent developments. Curr Opin Oncol 23: 22–27. [DOI] [PubMed] [Google Scholar]
- Xu Y-C, Liu Q, Dai J-Q, Yin Z-Q, Tang L, Ma Y, Lin X-L, Wang H-X (2014) Tissue microarray analysis of X-linked inhibitor of apoptosis (XIAP) expression in breast cancer patients. Med Oncol 31: 764. [DOI] [PubMed] [Google Scholar]
- Yim JH, Kim WG, Jeon MJ, Han JM, Kim TY, Yoon JH, Hong SJ, Song DE, Gong G, Shong YK, Kim WB (2014) Association between expression of X-linked inhibitor of apoptosis protein and the clinical outcome in a BRAF V600E-prevalent papillary thyroid cancer population. Thyroid 24: 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.