Abstract
Background:
Where people die can influence a number of indicators of the quality of dying. We aimed to describe the place of death of people with cancer and its associations with clinical, socio-demographic and healthcare supply characteristics in 14 countries.
Methods:
Cross-sectional study using death certificate data for all deaths from cancer (ICD-10 codes C00-C97) in 2008 in Belgium, Canada, Czech Republic, England, France, Hungary, Italy, Mexico, the Netherlands, New Zealand, South Korea, Spain (2010), USA (2007) and Wales (N=1 355 910). Multivariable logistic regression analyses evaluated factors associated with home death within countries and differences across countries.
Results:
Between 12% (South Korea) and 57% (Mexico) of cancer deaths occurred at home; between 26% (Netherlands, New Zealand) and 87% (South Korea) occurred in hospital. The large between-country differences in home or hospital deaths were partly explained by differences in availability of hospital- and long-term care beds and general practitioners. Haematologic rather than solid cancer (odds ratios (ORs) 1.29–3.17) and being married rather than divorced (ORs 1.17–2.54) were most consistently associated with home death across countries.
Conclusions:
A large country variation in the place of death can partly be explained by countries' healthcare resources. Country-specific choices regarding the organisation of end-of-life cancer care likely explain an additional part. These findings indicate the further challenge to evaluate how different specific policies can influence place of death patterns.
Keywords: cancer, place of death, death certificates, end-of-life care, cross-national comparison
Although life extension and outright cures are possible now for many types of cancers, it remains one of the leading causes of death worldwide. Advanced cancer patients form a large group among people who could benefit from a care approach aimed at comfort and quality of life (Smith et al, 2012). One important aspect of this approach is respect for patient choices in terms of the places where they receive ongoing care, spend the last days of their lives and ultimately die (Davies and Higginson, 2004). Evidence across many studies suggests that, for the majority of people with cancer and their caregivers, home is the preferred place of death (Gomes et al, 2013). Previous research indicates that dying in one's home environment is better aligned with patient's well-being as it benefits control, autonomy, dignity and continuity of care and involves lower healthcare costs and less risk of iatrogenic events and overly aggressive treatments (Marie Curie Cancer Care, 2012; Higginson et al, 2013; Boockvar et al, 2014).
At the population level, place of death is a valid indicator of where care is provided in the final hours or days of life (Earle, 2003). Cancer is a leading cause of death worldwide, and so knowing where people with cancer die and understanding the determinants of dying in a particular place are important public health issues, relevant not only to a commitment to patient preferences but also to efforts to avoid unnecessary hospitalisations (House of Commons Health Committee, 2004; Federale Evaluatiecel Palliatieve Zorg, 2008; Pennec et al, 2013). Several countries have developed policies and programmes to strengthen palliative and end-of-life care at home and to facilitate dying in the place of choice (Department of Health, 2008; Wright et al, 2008; Van Beek et al, 2013).
Population-level monitoring of place of death trends provides descriptive information that, if complemented with more in-depth understanding, can help inform public health policy regarding the allocation of end-of-life care resources and to support improvement strategies. Adding a cross-national comparison provides an opportunity to examine differences in place of death between countries with different levels of palliative care integration and different policies related to, amongst others, cancer care, long-term care and end-of-life care. This can help generate hypotheses regarding the influence of different factors related to policy and healthcare organisation and strategies for influencing where people die (Hantrais, 2008). Although previous studies have looked at cross-national differences in place of death in Europe (Cohen et al, 2010; Houttekier et al, 2010), no studies using individual data have made comparisons across continents.
The aim of this study was to describe the place of death of cancer patients and associated characteristics in 14 countries across 4 continents. We investigated where people with cancer die in 14 countries across 4 continents and how this differs from where people with a non-cancer condition die, to what extent differences in patient characteristics and healthcare supply explain variations between countries in place of death and to what extent the place of death of cancer patients is associated with patient characteristics and healthcare supply in each of the countries studied.
Materials and Methods
Study design
This study is part of the International Place of Death project, which collected complete death certificate data for a period of 1 year in 14 countries (Table 1). We obtained full country data except for Spain, where the data are from the Andalusia province, and Canada where the data do not include the Quebec province. An exploration of all candidate partners showed that, at the time of the data collection (2011–2013), 2008 was the most recent year for which data were available in most targeted countries. In the USA, 2007 was the most recent available year and in Andalusia (Spain), place of death was not recorded before 2010. We also obtained a number of clinical, socio-demographic and residential characteristics of the deceased, factors already identified as being associated with the place of death in people with cancer.(Gomes and Higginson, 2006) The project lead coordinated all data requests to ensure similar variables and data and pooled all data into one common database. Ethics approval was not required as we studied anonymised death certificate data.
Table 1. Deaths from cancer and socio-demographic characteristics in 2008a in 14 countries.
|
Type of cancer (% of all deaths) |
Age (% of all cancer deaths |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Abbreviations | Total number of deaths | Deaths from cancer N (% of all deaths) | Respiratory | Gastro-intestinal | Genitourinary | Haemato-logical | other | Sex (% of all cancer deaths % women | 0–59 | 60–79 | 80 and above |
|
Continental Western Europe | ||||||||||||
| France | FR | 541 135 | 153 576 (28.4) | 5.8 | 8.5 | 4.6 | 2.4 | 7.1 | 41.2 | 19.3 | 47.4 | 33.4 |
| Italy | IT | 578 192 | 164 297 (28.4) | 6.1 | 9.8 | 4.1 | 2.4 | 6.0 | 43.3 | 13.1 | 51.7 | 35.3 |
| Spain (Andalusia)a | ES | 57 380 | 15 194 (26.5) | 6.1 | 8.6 | 4.8 | 1.9 | 5.1 | 36.9 | 18.4 | 52.6 | 29.0 |
| Belgium | BE | 102 924 | 26 749 (26.0) | 6.6 | 7.1 | 4.1 | 2.1 | 6.1 | 43.4 | 17.0 | 50.7 | 32.4 |
| Netherlands | NL | 135 136 | 40 750 (30.2) | 7.5 | 9.0 | 5.0 | 2.2 | 6.4 | 46.3 | 18.0 | 52.6 | 29.4 |
|
Central/Eastern Europe | ||||||||||||
| Czech Republic | CZ | 101 804 | 26 996 (26.5) | 5.6 | 8.8 | 4.7 | 1.7 | 5.8 | 44.4 | 19.9 | 57.2 | 22.9 |
| Hungary | HU | 130 027 | 32 111 (24.7) | 6.9 | 8.0 | 3.4 | 1.4 | 5.0 | 44.3 | 27.2 | 53.5 | 19.3 |
|
UK | ||||||||||||
| England | ENG | 475 763 | 128 802 (27.1) | 6.1 | 7.6 | 4.7 | 2.1 | 6.6 | 47.7 | 13.7 | 50.9 | 35.4 |
| Wales | WAL | 32 066 | 8681 (27.1) | 6.5 | 7.8 | 4.5 | 2.0 | 6.3 | 47.0 | 13.1 | 52.2 | 34.6 |
|
Oceania | ||||||||||||
| New Zealand | NZ | 29 312 | 8454 (28.8) | 5.8 | 8.8 | 4.9 | 2.6 | 6.8 | 46.8 | 17.9 | 51.1 | 31.0 |
|
North America | ||||||||||||
| Canadab | CA | 182 134 | 51 622 (28.3) | 7.3 | 7.7 | 4.4 | 2.6 | 6.4 | 47.3 | 17.9 | 49.9 | 32.2 |
| United Statesa | US | 2 428 342 | 563 569 (23.2) | 6.7 | 5.6 | 3.5 | 2.3 | 5.1 | 48.0 | 20.6 | 49.6 | 29.8 |
|
Latin America | ||||||||||||
| Mexico | MX | 528 093 | 65 812 (12.5) | 1.4 | 4.0 | 2.8 | 1.4 | 2.9 | 51.0 | 35.5 | 45.8 | 18.8 |
|
East Asia | ||||||||||||
| South Korea | KR | 247 757 | 69 297 (28.0) | 6.3 | 15.3 | 2.2 | 1.4 | 2.8 | 36.4 | 26.2 | 56.7 | 17.0 |
| Total | 5 570 065 | 1 355 910 (24.3) | ||||||||||
Percentages may not add up to total due to rounding.
Reference year is 2007 in USA and 2010 in Spain (Andalusia).
The data for Canada exclude the province of Quebec.
Data
All included countries have similar death certification: generally the attending physician certifies the sex of the deceased, the cause or causes of death and the day and place of death. The authorities in each country check these certificates for inconsistencies and code death causes according to the WHO's International Classification of Diseases 10th Revision (ICD-10). The death certificates of some countries contain socio-demographic information, while in other countries it can be gained through links with other databases (e.g., census data). A minimum number of socio-demographic variables were thus obtained. Available healthcare resource statistics per capita were also obtained for the healthcare regions within the countries studied and linked to the region of residence of each deceased cancer patient (Supplementary Table S1, Supplementary Material).
Measures
The place of death was derived from the death certificate and comprised at minimum the categories home, hospital and nursing home/care home in all countries except Hungary where only hospital and other location were distinguished and Mexico where nursing home/care home is not a recorded category because there are no such facilities. We henceforth refer to nursing home/care as ‘long-term care facility'.
As independent variables we included individual socio-demographic (age, sex, marital status) and clinical characteristics (dying from cancer, including respiratory, gastrointestinal, genitourinary, haematologic and other cancers, or from a non-cancer condition excluding deaths from external causes such as accident and homicide), degree of urbanisation of the region of residence and healthcare supply measures that were identified as relevant in the literature on place of death of people with cancer (Gomes and Higginson, 2006). The codes of the municipality/local authority of residence were matched with available data on urbanisation levels and healthcare supply. The healthcare supply data included the number of hospital beds per 10 000 inhabitants, the number of family physicians or general practitioners per 10 000 inhabitants and the number of long-term care beds per 1000 inhabitants aged 65 years or over, each per health region of residence of the deceased. In some countries, the individual's region of residence was not identified. For those countries we used nationally aggregated measures of healthcare supply.
Statistical analysis
In the 14 countries all deaths with cancer as an underlying or primary cause of death (ICD-10 codes C00-C97) were selected. The cause of death was recorded as solid vs haematological cancer. Descriptive statistics were used to describe the place of death. Relative risks (unadjusted risk ratios) were calculated to compare the chances of dying in a certain setting for those with cancer and all those without cancer (excluding deaths from external causes).
To evaluate to what extent the country differences in place of death could be accounted for by clinical and socio-demographic characteristics or healthcare supply, a hierarchical binary logistic regression using all cancer deaths with place of death (home vs elsewhere) as the dependent variable and country as an independent variable (Model 1) was expanded by entering cancer type (18 types of cancer), age, sex and marital status (Model 2), and then density of hospital beds, long-term beds and general practitioners (Model 3) into the model. This method was chosen as it allows evaluating how certain groups of variables explain part of the variation between countries.
To evaluate to what extent the place of death of cancer patients was associated with patient characteristics and healthcare supply in each of the countries studied a multivariable binary logistic regression analysis (home vs elsewhere) for all cancer patients was conducted for each country with various independent candidate variables (see Supplementary Material for more information).
All models were checked for multicollinearity by looking at tolerance values and variance inflation factors. Analyses were done in IBM SPSS Statistics 22.
Results
Population and healthcare availability per country
In the countries studied, between 13% (Mexico) and 30% (Netherlands) of all deaths had cancer listed as the underlying cause (Table 1). The percentage of women among those who died of cancer was slightly less than 50% in all countries except Mexico; in all countries around half died between the age of 60 and 79; around one-third died at the age of 80 or over except in Mexico (19%) and South Korea (17%). Mexico was the only country where more than one-third (35%) died between the age of 0 and 59. Between 51% (US) and 65% (South Korea) of those who died of cancer had been married (Supplementary Table S2, Supplementary Material). A relatively high number of beds (more than 70 per 10 000 population) existed in hospitals in Korea, Hungary, France, Czech Republic and Belgium, while a relatively high number of long-term care beds (more than 70 per 1000 population aged 65 or over) existed in the Netherlands, New Zealand and Belgium (Supplementary Table S3, Supplementary Material).
Place of death per country
Between 12% (Korea) and 56% (Mexico) of people who died of cancer died at home. The largest proportion of home deaths was in Mexico, followed by the Netherlands (46%), Italy (45%) and USA (39%). Deaths in hospital ranged from 26% in the Netherlands and New Zealand to 87% in Korea (Table 2).
Table 2. The place of death of cancer and non-cancer deaths in 14 countries during 2008.
| Place of death | FR | IT | ES | BE | NL | CZ | HU | ENG | WAL | NZ | CA | US | MX | KR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | Home | 19.1 | 45.3 | 31.6 | 28.9 | 46.3 | 18.2 | /a | 26.1 | 26.2 | 28.5 | 16.1 | 39.0 | 57.3 | 11.8 |
| Hospital | 72.8 | 47.3 | 64.9 | 59.3 | 25.8 | 64.7 | 67.7 | 44.4 | 56.6 | 26.2 | 67.6 | 33.7 | 39.9 | 87.2 | |
| Nursing home | 5.5 | 3.9 | 3.3 | 11.2 | 19.3 | 16.6 | /a | 10.9 | 6.0 | 24.0 | 11.4 | 16.3 | /a | 0.8 | |
| PC institutions | /a | /a | /a | /a | 7.3 | /a | /a | 17.2 | 9.6 | 18.9 | /a | 5.3 | /a | /a | |
| Others | 2.7 | 3.5 | 0.2 | 0.6 | 1.4 | 0.5 | 32.3 | 1.4 | 1.6 | 2.5 | 5.0 | 5.8 | 2.7 | 0.2 | |
| Non-cancer patientsb | Home | 29.4 | 41.1 | 34.9 | 21.0 | 18.8 | 22.5 | /a | 17.3 | 17.1 | 18.8 | 13.3 | 20.8 | 45.9 | 27.9 |
| Hospital | 52.7 | 46.9 | 54.8 | 48.4 | 33.5 | 56.1 | 61.3 | 59.8 | 65.4 | 38.1 | 59.4 | 47.6 | 50.4 | 68.5 | |
| Nursing home | 13.9 | 7.6 | 9.6 | 29.1 | 42.0 | 18.9 | /a | 20.7 | 15.2 | 36.4 | 22.7 | 25.6 | /a | 2.2 | |
| PC institutions | /a | /a | /a | /a | 1.8 | /a | /a | 0.6 | 0.3 | 1.3 | /a | 2.1 | /a | /a | |
| Others | 4.1 | 4.4 | 0.7 | 1.5 | 3.9 | 2.5 | 38.7 | 1.7 | 2.1 | 5.4 | 4.5 | 3.9 | 3.7 | 1.3 | |
| Unadjusted relative risks: | Home | 0.65 | 1.10 | 0.91 | 1.38 | 2.46 | 0.81 | /a | 1.51 | 1.53 | 1.52 | 1.21 | 1.88 | 1.25 | 0.42 |
| (Cancer vs non-cancer) | Hospital | 1.38 | 1.01 | 1.18 | 1.23 | 0.77 | 1.15 | 1.10 | 0.74 | 0.87 | 0.69 | 1.14 | 0.71 | 0.79 | 1.27 |
| Nursing home | 0.40 | 0.51 | 0.34 | 0.38 | 0.46 | 0.88 | /a | 0.53 | 0.39 | 0.66 | 0.50 | 0.64 | /a | 0.36 | |
| PC institutions | /a | /a | /a | /a | 4.06 | /a | /a | 28.67 | 32.00 | 14.54 | /a | 2.52 | /a | /a | |
| Others | 0.66 | 0.80 | 0.29 | 0.40 | 0.36 | 0.20 | 0.83 | 0.82 | 0.76 | 0.46 | 1.11 | 1.49 | 0.73 | 0.15 |
Abbreviations: BE=Belgium; CA=Canada; CZ=Czech Republic; ENG=England; ES=Spain; FR=France; HU=Hungary; IT=Italy; KR=South Korea; MX=Mexico; NL=Netherland; NZ=New Zealand; PC=palliative care; US=United States; WAL=Wales.
Missing data for place of death: Korea (0.1%), USA (0.2%), Czech Republic (0.8%), Italy (4.8%) and Mexico (4.9%).
Location not recorded on death certificates.
Excludes all deaths from external causes.
As compared with persons dying from natural non-cancer conditions, those dying from cancer had a higher probability of dying at home in most countries except France, Spain, the Czech Republic and Korea (Table 2); but they also had a higher probability of dying in hospital in France, Spain, Belgium Czech Republic, Hungary, Canada and South Korea. In all countries, those who died of cancer were less likely to die in a long-term care facility than those who died of non-malignant causes. People with cancer also had a higher probability of dying in a palliative care institution (in countries where such a place was registered) than did those who died from non-cancer conditions, although this difference was much smaller in the United States (unadjusted relative risk (RR)=2.52) and the Netherlands (RR=4.06) than in England (RR=28.67), Wales (RR=32.00) and New Zealand (RR=14.54).
Multivariable analysis aimed at explaining country variation in home deaths
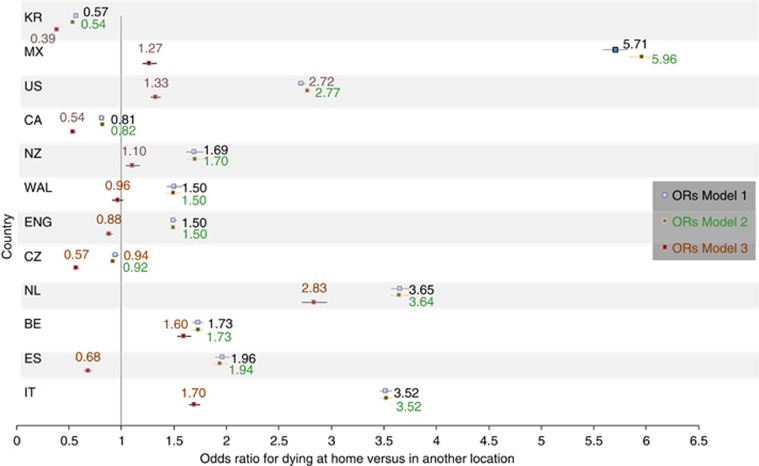
The country differences in the likelihood of dying at home vs another location (Model 1) remained largely unchanged when we controlled for differences between countries in cause of death, age and sex (Model 2; Figure 1; odds ratios (ORs) and 95% confidence intervals (CI) at each step of analysis in Supplementary Table S4, Supplementary Material). However, when the density of hospital and long-term care beds and the density of general practitioners per health region were added to the model (i.e., assuming a similar density of available healthcare resources; Model 3), a larger part of the differences was explained as the difference in the chances of dying at home between France (reference category) and several other countries was reduced. Controlling for all covariates, the chances of dying at home still remained higher in Italy, Belgium, the Netherlands, New Zealand, the United States and Mexico as compared with France.
Figure 1.
Country differences (ORs) in home death (vs other), accounting for socio-demographic factors, cause of death and healthcare supply. Hierarchical binary logistic regression analyses with home vs all other places of death as dependent variable. France is the reference category in the independent variable country. Independent variables: Model 1: country (reference category: France); Model 2: additionally sex, age, cancer site (17 categories: head and neck; stomach; colon, rectum and anus; pancreas; other gastrointestinal; trachea, bronchus and lung; other respiratory; breast; cervix uteri, corpus uteri and ovary; prostate; urinary tract; other genitourinary; central nervous system; Non-Hodgkin's lymphoma; leukaemia; and other haematologic malignancies); Model 3: additionally number of hospital beds per 1000 inhabitants, long-term care beds per 1000 inhabitants, and general practitioners per 10 000 inhabitants in the region of residence. Comparing the three models allows evaluating whether certain variables explain part of the variation between countries. ORs getting closer to each other and closer to 1 when independent variables are added to the model means that part of the variation in place of death between countries is explained by these independent variables. For many countries this is particularly the case in Model 3, which indicates that the variables entered in Model 3 explain part of the variation (more than the ones entered in Model 2). Model 3 provides the ORs for home death of the different countries as compared with France in case the density of available health resources was the same as in France. In some countries (Spain, England and Wales) the larger ORs compared with France became smaller than 1, which suggests that if these countries had the same healthcare supply as in France the home death rate could be expected to be lower than in France. However, a large part of the variation between countries remained unexplained and thus needs to be attributed to other factors.
Factors associated with death at home within each country
When the effects of socio-demographic and healthcare supply factors were adjusted for, a death from a solid tumour was in all countries more likely to occur at home than a death from a haematologic cancer, although the difference was larger in some countries than others (e.g., OR=3.17 (95% CI 2.99–3.35) in Mexico, OR=1.29 (95% CI 1.14–1.46) in South Korea; Table 3). The chances of dying at home gradually increased with age in France, Italy, Spain, Mexico and Korea but decreased with age in all other countries (except Czech Republic where no clear age pattern emerged). In all countries married people were more likely to die at home compared with the divorced, unmarried and widowed. In all European countries with educational attainment data available (Italy, Spain, Belgium and Czech Republic), those with higher educational attainment had better chances of dying at home than those with lower educational attainment. In the USA, no clear differences by educational attainment were found, and in Mexico and Korea an opposite pattern was found; those with higher educational attainment had lower chances of dying at home. A lower degree of urbanisation was related to higher chances of a cancer death at home, except in Canada and France where the opposite was true. The chances of dying at home decreased with an increasing density of hospital beds in France, Italy, Belgium, USA, Canada and Mexico, with the largest effect found in Italy. No clear association was found across countries between the chances of dying at home and the density of long-term care beds or the density of general practitioners per region of residence. The regression models explained a small proportion of the variation in dying at home vs elsewhere (R2 range from 0.02 in Czech Republic to 0.21 in Italy).
Table 3. Multivariable logistic regression modelsa per country of factors associated with home death vs other.
|
OR (95% CI) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR | IT | ES | BE | NL | CZ | ENG | WAL | NZ | CA | US | MX | KR | |
| Cases included in analysis | 152 761b | 73 042b | 13 255b | 16 059b | 40 750b | 24 321b | 119 394b | 8016b | 8454b | 50 038b | 554 917b | 61 279b | 68 130b |
|
Cancer site | |||||||||||||
| Solid (vs haematologic) | 1.39 (1.32–1.46) | 1.92 (1.84–2.00) | 1.95 (1.65–2.30) | 2.05 (1.75–2.39) | 1.84 (1.70–2.00) | 1.91 (1.61–2.25) | 1.82 (1.72–1.92) | 1.82 (1.46–2.26) | 1.44 (1.20–1.73) | 1.64 (1.49–1.81) | 1.81 (1.78–1.85) | 3.17 (2.99–3.35) | 1.29 (1.14–1.46) |
|
Sex | |||||||||||||
| Female (vs male) | ns | 0.89 (0.87–0.91) | 0.93 (0.85–1.01) | 1.09 (1.01–1.18) | 1.05 (1.00–1.09) | 1.09 (1.01–1.17) | 1.03 (1.00–1.06) | ns | ns | ns | 0.98 (0.96–0.99) | 0.96 (0.93–1.00) | 1.30 (1.23–1.38) |
|
Age | |||||||||||||
| 0–49 | 0.40 (0.37–0.44) | 0.48 (0.45–0.52) | 0.24 (0.19–0.31) | 1.62 (1.27–2.08) | 3.42 (2.98–3.93) | 0.89 (0.67–1.18) | 1.75 (1.60–1.91) | 2.02 (1.42–2.87) | 4.37 (3.14–6.06) | 1.86 (1.59–2.18) | 1.29 (1.24–1.33) | 0.33 (0.29–0.37) | 0.26 (0.21–0.31) |
| 50–59 | 0.43 (0.40–0.46) | 0.52 (0.49–0.56) | 0.26 (0.21–0.32) | 1.22 (0.98–1.53) | 2.82 (2.49–3.19) | 0.81 (0.63–1.05) | 1.78 (1.65–1.92) | 1.97 (1.45–2.66) | 3.94 (2.89–5.37) | 2.01 (1.75–2.32) | 1.34 (1.30–1.38) | 0.37 (0.33–0.42) | 0.31 (0.26–0.37) |
| 60–69 | 0.44 (0.42–0.47) | 0.57 (0.54–0.60) | 0.31 (0.25–0.38) | 1.12 (0.91–1.38) | 2.54 (2.26–2.86) | 0.75 (0.59–0.97) | 1.74 (1.63–1.87) | 1.89 (1.44–2.50) | 3.88 (2.88–5.21) | 1.77 (1.55–2.02) | 1.35 (1.31–1.38) | 0.42 (0.38–0.47) | 0.45 (0.38–0.53) |
| 70–79 | 0.48 (0.45–0.51) | 0.67 (0.63–0.70) | 0.48 (0.40–0.58) | 1.04 (0.84–1.27) | 2.04 (1.82–2.29) | 0.77 (0.61–0.99) | 1.59 (1.49–1.70) | 1.68 (1.29–2.19) | 2.80 (2.08–3.75) | 1.54 (1.35–1.75) | 1.31 (1.27–1.34) | 0.53 (0.47–0.60) | 0.66 (0.56–0.77) |
| 80–89 | 0.62 (0.59–0.66) | 0.80 (0.76–0.84) | 0.73 (0.61–0.88) | 1.05 (0.85–1.28) | 1.49 (1.33–1.68) | 0.80 (0.63–1.02) | 1.35 (1.27–1.44) | 1.43 (1.09–1.86) | 1.91 (1.41–2.57) | 1.38 (1.21–1.56) | 1.18 (1.14–1.21) | 0.70 (0.62–0.78) | 0.84 (0.71–0.99) |
| 90 and older | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
|
Marital status | |||||||||||||
| Unmarried | 1.10 (1.03–1.17) | 1.21 (1.11–1.33) | 1.00 (0.77–1.31) | 1.04 (0.86–1.26) | ref | 1.28 (1.06–1.54) | 0.79 (0.74–0.84) | 0.63 (0.48–0.83) | c | 1.13 (0.99–1.28) | 0.75 (0.73–0.77) | ns | 1.07 (0.87–1.30) |
| Married | 1.49 (1.41–1.56) | 2.07 (1.90–2.26) | 1.17 (0.92–1.49) | 1.99 (1.73–2.28) | 2.54 (2.43–2.65) | 1.86 (1.66–2.10) | 1.79 (1.71–1.88) | 1.76 (1.47–2.11) | c | 1.76 (1.60–1.94) | 1.72 (1.69–1.75) | ns | 1.39 (1.22–1.60) |
| Widowed | 1.01 (0.95–1.07) | 1.63 (1.49–1.78) | 1.07 (0.83–1.38) | 0.91 (0.78–1.07) | c | 1.46 (1.27–1.67) | 1.03 (0.97–1.08) | 1.01 (0.83–1.24) | c | 1.33 (1.19–1.48) | 1.22 (1.19–1.24) | ns | 1.09 (0.94–1.27) |
| Divorced | ref | ref | ref | ref | c | ref | ref | ref | c | ref | ref | c | ref |
|
Educational attainment | |||||||||||||
| Primary or less | c | ref | ref | ref | c | ref | c | c | c | ref | ref | ref | |
| Lower secondary | c | 1.01 (0.98–1.05) | 0.95 (0.86–1.04) | 1.22 (1.11–1.34) | c | 1.00 (0.92–1.08) | c | c | c | 0.96 (0.93–0.99) | 0.58 (0.52–0.65) | c | |
| Higher secondary | c | 1.15 (1.10–1.20) | 0.98 (0.88–1.09) | 0.93 (0.84–1.02) | c | 1.08 (0.97–1.21) | c | c | c | 0.93 (0.91–0.95) | 0.76 (0.71–0.80) | 0.73 (0.69–0.77) | |
| Higher | c | 1.28 (1.20–1.37) | 1.26 (1.06–1.50) | 1.46 (1.28–1.65) | c | 1.47 (1.24–1.74) | c | c | c | 0.98 (0.95–1.00) | 0.62 (0.59–0.65) | 0.55 (0.50–0.61) | |
|
Urbanisation | |||||||||||||
| Strong | ref | ref | ref | ref | ref | c | ref | ns | c | ref | ref | ||
| Average | 0.81 (0.79–0.84) | 1.19 (1.15–1.22) | 1.89 (1.74–2.05) | 1.40 (1.29–1.52) | 1.18 (1.11–1.24) | c | 1.24 (1.20–1.28) | ns | c | c | 0.87 (0.79–0.95) | ||
| Rural | 0.91 (0.88–0.95) | 1.35 (1.32–1.39) | 1.78 (1.52–2.08) | 1.47 (1.32–1.63) | 1.50 (1.44–1.57) | c | 1.41 (1.28–1.55) | ns | c | 0.93 (0.87–0.99) | 1.24 (1.16–1.33) | ||
|
Healthcare resources | |||||||||||||
| LTC beds per 1000, 65 plus | 0.99 (0.99–0.99) | 1.05 (1.05–1.06) | ns | ns | c | ns | ns | 0.99 (0.98–0.99) | 1.03 (1.03–1.04) | 0.998 (0.998–0.999) | c | c | |
| Hospital beds per 10 000 | 0.98 (0.98–0.98) | 0.70 (0.70–0.71) | ns | 0.99 (0.99–0.99) | 1.01 (1.00–1.01) | c | 1.01 (1.00–1.01) | ns | ns | 0.86 (0.85–0.87) | 0.99 (0.98–0.99) | 0.80 (0.79–0.82) | c |
| GPs per 10 000 | 1.28 (1.26–1.30) | 2.69 (2.53–2.86) | 1.78 (1.52–2.08) | ns | 0.88 (0.80–0.96) | c | ns | 0.94 (0.91–0.97) | 1.76 (1.66–1.87) | 0.97 (0.97–0.98) | 1.15 (1.14–1.17) | c | |
|
Model fit | |||||||||||||
| Nagelkerke R2 (%) | 4.2 | 21.1 | 8.5 | 7.6 | 12.8 | 1.7 | 4.6 | 5.0 | 5.2 | 4.6 | 3.9 | 10.4 | 5.3 |
| Hosmer–Lemeshow test | <0.01 | <0.001 | 0.037 | 0.292 | 0.070 | 0.273 | 0.339 | 0.284 | 0.020 | 0.010 | <0.001 | <0.001 | 0.029 |
| Home death predicted correctly | 0.1% | 58.2% | 18.0% | 2.7% | 62.4% | 0.0% | 0.0% | 0.0% | 0.0% | 0.1% | 9.7 | 83.6 | 0.0 |
Abbreviations: BE=Belgium; CA=Canada; CI=confidence interval; CZ=Czech Republic; ENG=England; ES=Spain; FR=France; GPs=general practitioners/primary care physicians/family physicians; IT=Italy; KR=South Korea; LTC=long-term care; MX=Mexico; NL=Netherland; ns=not significant; NZ=New Zealand; OR=Odds ratio; PC=palliative care; ref=reference category; US=United States; WAL=Wales.
Missing data for all variables were 0.0–0.6% in all countries except for educational attainment in (10.8% in ES, 41.8% in BE, 11.3% in CZ, 1.5% in US, 4% in MX and 1.9% in KR) and marital status (3.3% in ES, 2% in MX). Cases with missing data were excluded from the analysis.
To construct a parsimonious model and select the relevant variables for each country, several alternative logistic regression models were constructed and evaluated for goodness of fit and regression diagnostics, and a stepwise variable selection (forward and backward) was used with significance levels for entry and removal set at 0.10. Only covariates with P<0.05 were permitted to stay on the final logistic regression model using a backward conditional procedure. We aimed for similar regression models across countries, to enhance comparability of the effects between countries.
Sample sizes per country may not be equal to the total population due to missing data for certain independent variables. Cases with missing data were excluded from the multivariable analyses.
NZ: marital status not recorded on death certificates and hence not included in the model. In the Netherlands marital status is recorded as married or not. Urbanisation in Canada is coded as urban vs rural. There are no long-term care beds in Mexico. Educational attainment not recorded on death certificates in France, Netherlands, England, Wales and New Zealand. There is no separate category for lower secondary education for educational attainment in Korea.
Discussion
Our study found a strikingly large variation across 14 countries in the place of death of people with cancer, especially in the extent to which home (percentages ranging from 12 to 57%) or hospital (percentages ranging from 26 to 87%) was the place of death. These large country differences were explained only to a limited extent by demographic differences (age, sex), cancer site and healthcare supply (rate of hospital beds, long-term care beds and general practitioners per health region), which indicates that a large part of the variation between countries is attributable to other factors. There were also notable differences between countries in the strength and direction of associations between various factors (e.g., age) and the chances of dying at home, while some factors were consistently associated with home death in the same direction (e.g., solid vs nonsolid tumour and marital status).
To our knowledge, this is the largest cross-national study on the place of death of people who died from cancer, having examined over 1.3 million deaths in 14 countries on 4 continents. Death certificate data have the major advantage that they provide population-level data that do not suffer from potential bias inherent to samples, and ensure good statistical power to evaluate a larger number of associated factors. Similarities in death certification and cause of death coding make the pooling of the individual country data for cross-national analysis both feasible and reliable. They also entail a number of limitations. Inaccuracies in cause of death recording through death certificates have been reported (O'Sullivan, 2011). With regard to cancer, this mainly concerns site-specific accuracy rather than causes other than cancer being recorded (Lund et al, 2010). Our use of aggregated cause of death categories (solid vs haematological cancer) may have mitigated this problem. Coding of place of death is not uniform across countries (i.e., categories like hospice/palliative care institution in most countries are missing and in Hungary only hospital vs outside hospital is coded), and this reaffirms previous calls for more standardisation and completeness in place of death coding on death certificates (Cohen et al, 2007; Pivodic et al, 2013). Death certificate data do not provide all important factors known to influence place of death (Gomes and Higginson, 2006). Information needed to estimate the clinical predictability of dying in a certain care setting, such as detailed clinical events and symptoms, is not provided and death certificates do not shed light on the reasons for hospitalisation at the very end of life (Cohen et al, 2007). Differences between countries in these factors may have been able to explain an additional part of the country differences in place of death. Another limitation is that the same set of variables potentially associated with place of death is not available in all countries (e.g., hospital bed availability per healthcare region). However, an evaluation of the influence of the omission of factors in some countries indicated that these only marginally affected the ORs of the other variables in the multivariable analysis. We therefore believe that the effects of different factors within a country can be compared between countries. Conceptual equivalence is a common issue in cross-national comparative research. In our study, acute hospitals and long-term care settings can have different characteristics in different countries, and this should be kept in mind when interpreting the country differences in place of death, controlled for availability of hospital beds, long-term care beds and general practitioners.
Although caution not to overinterpret the found country differences is necessary and multiple complex reasons can underlie these differences, they do present real differences in terms of where similar patients spend their final hours. The statistical patterns of place of death across the different countries can be interpreted as empirical indications of the way in which each country manages end-of-life care. The findings of this study provide two important types of information for addressing some of the public health challenges related to end-of-life cancer care. Firstly, the findings inform decisions regarding the allocation of end-of-life cancer care resources, support and improvement strategies. In some countries, notably France, Spain, Belgium, Czech Republic, Hungary, Canada and Korea (countries with 60% or more of cancer deaths occurring in hospital), the hospital appears to have an important role in terminal cancer care. In other countries, notably Italy, the Netherlands, the USA and Mexico, the home setting has a more prominent role. Although high quality terminal care needs to be promoted and safeguarded in all care settings, the findings imply that some countries should primarily focus on evaluating and, if needed, improving resources for terminal cancer care in hospitals, while the focus in other countries should be the home setting. In countries where a high percentage of people with cancer die at home, such as Mexico, where this is likely to be due to issues of accessing (institutional) healthcare and where dying at home may imply dying without professional support (Cárdenas-Turanzas et al, 2011), guaranteeing a ‘good death' at home is a major public health challenge. Our findings also illustrate that, although long-term care facilities are often overlooked as places for cancer care (Fennell, 2009), they are important places of death for people with cancer in some countries (e.g., Netherlands and New Zealand). It is important therefore to ensure that terminal and end-of-life care in those settings meets the specific needs of those with cancer.
Secondly, our findings highlight factors that could potentially influence place of death patterns. Country variation in place of death can to a certain degree be attributed to the availability of hospital and long-term care beds or general practitioners. However, a large part of the country variation in place of death remained unexplained. A review of country-level data for contextual explanation of the differences did not show any clear association between country patterns of place of death and their health expenditure or social expenditure as a percentage of the GDP, nor the availability of palliative care resources (Supplementary Table S5, Supplementary Material); (Pastrana et al, 2012; Centeno et al, 2013; Cleary et al, 2013a, 2013b). This suggests that other more complex health or social policy differences, particularly those related to end-of-life cancer care, have a role that cannot easily be captured in a density or availability measure. Past policies could explain why in some countries (France, Spain and Korea), cancer patients were less likely than non-cancer patients to die at home, although cancer patients are often considered as having a more predictable course of disease that increases the chance of a home death (Murtagh et al, 2004). In countries such as France, Spain, Canada and Korea, end-of-life cancer care is more often provided in hospital (in palliative care units or other wards) than at home. In France, it appears that there is little support for palliative home care and this carries the risk of aggressive care in the final stages (Observatoire National de la Fin de Vie, 2013). In the Czech Republic, palliative home care is not recognised in the national health insurance law and so provision is low (Slama, 2009). Many patients are thus treated in hospitals, often without palliative care. On the other hand, countries such as the Netherlands (and to a lesser extent the USA) seem to have explicitly chosen to focus on the provision of palliative care at home, which would limit hospital use. England's End-of-life Strategy has a strong focus on primary care and previous research has suggested that it may be the reason for the increased home deaths there (Gao et al, 2013).
It needs to be acknowledged that the place of death patterns we found in people with cancer, as well as the choices in national healthcare organisation that may underlie them, are also the result of historical contingencies and cultural values or circumstances surrounding families and death and dying in the community (Brown and Colton, 2001).
Conclusions
Where cancer patients die appears to be determined by factors beyond medical necessity or patient characteristics. Countries' healthcare resources, notably availability of hospital beds and long-term care beds, explained part of the country variation in place of death. Healthcare policies specific to end-of-life care likely explain an additional part. This may suggest that choices regarding organising end-of-life cancer care within a society can influence where people receive end-of-life care and die. Further cross-national research applying a more case-oriented qualitative in-depth comparison between a number of relevant comparator countries is needed to understand the reasons behind the differences in place of death.
Acknowledgments
We thank the following agencies for the delivery of the death certificate data: Belgium: Flemish Agency for Care and Health, Brussels Health and Social Observatory, French Community of Belgium; France: Inserm-CépiDc (Centre d'épidémiologie sur les causes médicales de décès, Institut national de la santé et de la recherche médicale); Italy: Italian National Institute of Statistics (unit for cause of death statistic); Netherlands: Statistics Netherlands; Spain (Andalusia): Instituto de Estadística y Cartografía de Andalucía; Czech Republic: Institute of Health Information and Statistics of the Czech Republic; Hungary: Central Statistical Office Hungary; USA: Center for Disease Control and Prevention based on Data Use Agreement; Canada: Statistics Canada; New Zealand: New Zealand Ministry of Health; England and Wales: Office for National Statistics; Mexico: Secretaria de Salud and Sistema Nacional de Informacion en Salud; Korea: Statistics Korea. We also thank Jane Ruthven for linguistic help. This work was supported by the Research Foundation Flanders and the Willy Gepts Fund (Wetenschappelijk Fonds Willy Gepts).
The authors declare no conflicts of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Boockvar K, Fishman E, Kyriacou CK, Monias A, Gavi S, Cortes T (2014) Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med 164: 545–550. [DOI] [PubMed] [Google Scholar]
- Brown M, Colton T (2001) Dying epistemologies: an analysis of home death and its critique. Environ Plan 33: 799–821. [Google Scholar]
- Cárdenas-Turanzas M, Torres-Vigil I, Tovalín-Ahumada H, Nates JL (2011) Hospital versus home death: results from the Mexican Health and Aging Study. J Pain Symptom Manage 41: 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno C, Pons JJ, Lynch T, Donea O, Rocafort J, Clark D (2013) EAPC atlas of palliative care in Europe - Cartographic edition. EAPC Press: Milan. [Google Scholar]
- Cleary J, De Lima L, Eisenchlas J, Radbruch L, Torode J, Cherny NI (2013. a) Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Latin America and the Caribbean: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol 24(Suppl 1): 41–50. [DOI] [PubMed] [Google Scholar]
- Cleary J, Radbruch L, Torode J, Cherny NI (2013. b) Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Asia: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol 24(Suppl 1): 24–32. [DOI] [PubMed] [Google Scholar]
- Cohen J, Bilsen J, Miccinesi G, Löfmark R, Addington-Hall J, Kaasa S, Norup M, van der Wal G, Deliens L (2007) Using death certificate data to study place of death in 9 European countries: opportunities and weaknesses. BMC Public Health 7: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Houttekier D, Onwuteaka-Philipsen B, Miccinesi G, Addington-Hall J, Kaasa S, Bilsen J, Deliens L (2010) Which patients with cancer die at home? A study of six European countries using death certificate data. J Clin Oncol 28: 2267–2273. [DOI] [PubMed] [Google Scholar]
- Davies E, Higginson IJ (2004) Palliative Care: the Solid Facts. World Healh Organization: Copenhagen. [Google Scholar]
- Department of Health (2008) End of Life Care Strategy: Promoting High Quality Care for all Adults at the End of Life. London. [Google Scholar]
- Earle CC (2003) Identifying Potential Indicators of the Quality of End-of-Life Cancer Care From Administrative Data. J Clin Oncol 21: 1133–1138. [DOI] [PubMed] [Google Scholar]
- Federale Evaluatiecel Palliatieve Zorg (2008) Evaluatierapport Palliatieve Zorg. Brussels. [Google Scholar]
- Fennell ML (2009) Nursing homes and cancer care. Health Serv Res 44: 1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Ho YK, Verne J, Glickman M, Higginson IJ (2013) Changing patterns in place of cancer death in England: a population-based study. PLoS Med 10: e1001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes B, Calanzani N, Gysels M, Hall S, Higginson IJ (2013) Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes B, Higginson IJ (2006) Factors influencing death at home in terminally ill patients with cancer: systematic review. BMJ 332: 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantrais L (2008) International Comparative Research: Theory, Methods and Practice. Palgrave Macmillan: Basingstoke, Hampshire, UK. [Google Scholar]
- Higginson IJ, Sarmento VP, Calanzani N, Benalia H, Gomes B (2013) Dying at home - is it better: a narrative appraisal of the state of the science. Palliat Med 27: 918–924. [DOI] [PubMed] [Google Scholar]
- House of Commons Health Committee (2004) Palliative Care. Fourth Report of Session 2003–2004 vol. 1. [Google Scholar]
- Houttekier D, Cohen J, Bilsen J, Addington-Hall J, Onwuteaka-Philipsen BD, Deliens L (2010) Place of death of older persons with dementia. A study in five European countries. J Am Geriatr Soc 58: 751–756. [DOI] [PubMed] [Google Scholar]
- Lund JL, Harlan LC, Yabroff KR, Warren JL (2010) Should cause of death from the death certificate be used to examine cancer-specific survival? A study of patients with distant stage disease. Cancer Invest 28: 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie Curie Cancer Care (2012) Understanding the Cost of End of Life Care in Different Settings. Marie Curie Cancer Care: London, UK. [Google Scholar]
- Murtagh FEM, Preston M, Higginson I (2004) Patterns of dying: palliative care for non-malignant disease. Clin Med 4: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan EM (2011) Using epidemiological surveillance systems and routine data sets to study place of care and place of death: strengths and weaknesses. Palliat Med 25: 94–96. [DOI] [PubMed] [Google Scholar]
- Observatoire National de la Fin de Vie (2013) Vivre la fin de sa vie chez soi Observatoire National de la Fin de Vie: Paris, France. [Google Scholar]
- Pastrana T, De Lima L, Wenk R, Eisenchlas J, Monti C, Rocafort J, Centeno C (2012) Atlas of Palliative Care in Latin America. IAHPC Press: Houston. [Google Scholar]
- Pennec S, Gaymu J, Monnier A, Riou F, Aubry R, Pontone S, Cases C (2013) Le dernier mois de l'existence: les lieux de fin de vie et de décès en France. Population 68(4): 585–615. [Google Scholar]
- Pivodic L, Higginson IJ, Sarmento VP, Gomes B (2013) Health metrics: standardize records of place of death. Nature 495: 449–449. [DOI] [PubMed] [Google Scholar]
- Slama O (2009) [Palliative and hospice care in the Czech Republic and in Europe]. Klin Onkol 22: 183–185. [PubMed] [Google Scholar]
- Smith TJ, Temin S, Alesi ER, Abernethy AP, Balboni T a, Basch EM, Ferrell BR, Loscalzo M, Meier DE, Paice J a, Peppercorn JM, Somerfield M, Stovall E, Von Roenn JH (2012) American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 30: 880–887. [DOI] [PubMed] [Google Scholar]
- Van Beek K, Woitha K, Ahmed N, Menten J, Jaspers B, Engels Y, Ahmedzai SH, Vissers K, Hasselaar J (2013) Comparison of legislation, regulations and national health strategies for palliative care in seven European countries (results from the Europall research group): a descriptive study. BMC Health Serv Res 13: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M, Wood J, Lynch T, Clark D (2008) Mapping levels of palliative care development: a global view. J Pain Symptom Manage 35: 469–485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.