Abstract
Background:
Helicobacter pylori are major carcinogen of gastric cancer, but the associations among gastric cancer, H. pylori infection status, and alcohol consumption are not fully described. This study aimed to clarify how H. pylori infection status affects the association between alcohol consumption and gastric cancer risk.
Methods:
We selected 949 case–cohort participants from the 18 863 Korean Multi-center Cancer Cohort (KMCC) populations. Gastric cancer incidence inside and outside of the subcohort were 12 and 254 cases, respectively. Seropositivities for CagA, VacA, and H. pylori infection were determined by performing immunoblot assays. Weighted Cox regression models were used to calculate hazard ratios and 95% confidence intervals (CIs).
Results:
Relative to non-drinking, heavy drinking (⩾7 times a week), and binge drinking (⩾55 g alcohol intake per occasion) showed a 3.48-fold (95% CI, 1.13–10.73) and 3.27-fold (95% CI, 1.01–10.56) higher risk in subjects not previously infected by H. pylori. There was no significant association between drinking pattern and gastric cancer risk in H. pylori IgG seropositive subjects. An increased risk for gastric cancer in heavy- and binge-drinking subjects were also present in subjects not infected by CagA- or VacA-secreting H. pylori.
Conclusions:
Heavy and binge alcohol consumption is an important risk factor related to an increasing incidence of gastric cancer in a population not infected by H. pylori.
Keywords: gastric cancer, alcohol, drinking patterns, Helicobacter pylori infection, cohort, case–cohort
Gastric cancer is the fifth most common cancer and the third most common cause of cancer-associated death in the world (Ferlay et al, 2015). In South Korea, gastric cancer was the second most common cancer and the third most common cause of cancer-associated death in 2009 (Jung et al, 2014).
Gastric cancer is a multi-factorial disease (Kelley and Duggan, 2003), and Helicobacter pylori infection has been reported to be the most important aetiological factor for the development of non-cardia gastric cancer (Forman et al, 1991). However, in countries with a high prevalence of H. pylori infection, such as Korea and Japan, gastric cancer patients have H. pylori infection prevalence similar to that in control subjects (Tajima, 2002; Lunet and Barros, 2003; Shin et al, 2004). Therefore, the presence of H. pylori infection alone is not a sufficient basis for gastric cancer development (Tajima, 2002). In our previous study, an association between H. pylori infection and gastric cancer risk was not detected. However, seropositivity of the H. pylori virulence factor cytotoxin-associated gene A (CagA) immunoglobulin G (IgG) antibody was associated with an increased gastric cancer risk (Gwack et al, 2006).
Results in recent meta-analyses (Li et al, 2011; Tramacere et al, 2012) and in large cohort studies in China and Europe (Ji et al, 1996; Duell et al, 2011) indicate that alcohol consumption is a risk factor for gastric cancer. However, there is controversy over the aetiological aspects of the correlation between alcohol and gastric cancer, and the International Agency for Research on Cancer (IARC) has reported inconsistency in the association between alcohol consumption and gastric cancer risk (IARC Working Group on the Evaluation of Carcinogenic Risk to Humans, 2010). Most previous studies have not considered H. pylori infection as a confounder or potential effect modifier of gastric cancer risk. Only two studies have evaluated the association between alcohol and gastric cancer by adjusting for (Duell et al, 2011) or stratifying by (Zaridze et al, 2000) H. pylori infection status. The study based on the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort analysed heavy alcohol consumption (⩾60 g per day) in relation to gastric cancer risk adjusted for H. pylori serostatus, but the results indicated no difference between adjusted (odds ratio (OR), 1.60; 95% confidence interval (CI), 0.91–2.82) or not adjusted (hazard ratio (HR), 1.65; 95% CI, 1.06–2.58) (Duell et al, 2011). The stratified study from Russia reported that vodka consumption increased the risk of gastric cancer regardless of H. pylori infection status. Vodka consumption increased gastric cancer risk 2.0-fold (95% CI, 1.2–3.1) and 2.3-fold (95% CI, 1.4–3.7) from that in non-drinking subjects without or with H. pylori infection, respectively (Zaridze et al, 2000). However, the effects of heavy- and binge-drinking alcohol consumption patterns in relation to H. pylori were not considered in Russian study.
Korea has been reported to have the highest gastric cancer incidence in the world (Ferlay, 2013). In 2007, about 60% of the Korean population was infected by H. pylori, although the prevalence of H. pylori infection has been decreasing (Yim et al, 2007). These noteworthy conditions in Korea indicate the importance of elucidating the association between alcohol consumption and gastric cancer in relation to H. pylori infection status in the Korean population.
In this study we hypothesised that H. pylori may be an effect modifier in the relationship between alcohol drinking and gastric cancer development, or alcohol drinking may be an independent risk factor for gastric cancer regardless of H. pylori infection. We thus investigated the association between alcohol-drinking patterns and gastric cancer risk within a prospective Korean Multi-center Cancer Cohort study (KMCC).
Materials and Methods
Study participants and data collection
The study's subjects were recruited from the KMCC study, a population-based, prospective cohort study designed to investigate causes of cancer in Korea. From 1993 to 2004, the KMCC study recruited 19 688 voluntary participants from four urban and rural areas in Korea (Haman, Chungju, Uljin, and Pohang). All participants completed standardised questionnaires during personal interviews after providing informed consent. Information on individual characteristics, including lifestyle, medical history, and environmental exposure factors, was collected by using a detailed, standardised questionnaire. Biological samples (urine and blood) were also collected and stored under stable conditions (Yoo et al, 2002). Of the 19 688 participants in the KMCC study, we excluded those under 20 years old and those with no confirmed alcohol-drinking status. After those exclusions, 18 863 participants with information of alcohol consumption were identified and included in our study population. By 31 December 2006, among the study population there were 301 cases of gastric cancer, as defined by the International Statistical Classification of Diseases and Related Health Problems tenth Revision (ICD-10, C16). The cases were identified through computerised record linkage to the Korean national cancer registry and the national death certificate. The passive follow-up methods were reported to be 99% efficient, and completeness was assured (Cho et al, 2009).
Case–cohort population was derived from total KMCC participants. An 89% of total 19 688 members (N=17 522) donated blood specimens and of them we selected 4% population (N=701) by age/sex stratified random sampling. After excluding subject not having plasma or serum, 695 were selected as subcohort members, including 12 gastric cancer cases inside of subcohort. In contrast, of total 301 gastric cancer cases, 268 cases donated their blood at the enrolment, and finally 266 gastric cancer cases were included in this study, after excluding subjects not having plasma or serum specimens (N=2). In summary, our case–cohort population consisted of total 949 participants: total 695 subcohort (including 683 healthy subjects and 12 gastric cancer cases inside of subcohort) and additional 254 gastric cancer cases outside of subcohort.
The study protocol was approved by the Institutional Review Boards of the Seoul National University Hospital (H-0110-084-002) and the National Cancer Center of Korea (C-0910-049-297 and C-0907-044-286).
Exposure assessment
Patterns of alcohol consumption were determined by reviewing the KMCC questionnaire items related to alcohol-drinking status (non-drinker, past drinker, or current drinker), total years of drinking, frequency of drinking alcohol in 1 week, and the amount of alcohol consumed on one occasion. These items were surveyed separately by type of alcohol beverage, as reported in the National Alcohol Survey (Greenfield et al, 2009).
Internationally, there is no consensus on the definition of heavy and binge drinking. The Substance Abuse and Mental Health Services Administration has suggested that heavy drinking involves consuming more than five drinks in a single episode on more than 5 days within 1 month (Substance of Abuse and Mental Health Services Administration, the Center Behavioral Health Statistics and Quality, 2014). The National Institute of Alcohol Abuse and Alcoholism defines binge drinking as alcohol consumption that produces a blood alcohol concentration over 0.08 g dl−1 with this concentration corresponding to drinking more than five drinks in men or more than four drinks in women within 2 h (National Institute on Alcohol Abuse and Alcoholism, 2004). Without a standardised definition of alcohol amounts for heavy or binge drinking, we developed consumption classification criteria based on the drinking patterns in the study population. Additional classification criteria were used to define different patterns of alcohol consumption.
With regard to drinking status, the study participants were divided into three groups: never drinkers, past drinkers, and current drinkers; the latter two were denoted as ‘drinkers' in our analysis. Frequency of consumption was used to categorise the drinkers as non-drinker, drinking <4 times per week, drinking 4–6 times per week, and drinking ⩾7 times per week (Tables 1, 2, 3). Participants who drank ⩾7 times per week were defined as heavy drinkers.
Table 1. The risk for gastric cancer in relation to alcohol-drinking status in the KMCC, 1994–2004.
| Total cohort (N=18 863) |
Case–cohorta (N=949) |
|||||
|---|---|---|---|---|---|---|
| Person-years | No. of cases (N=403) | HR (95% CI)b | Person-years | No. of cases (N=266) | HR (95% CI)b | |
|
Drinking status | ||||||
| Non-drinking | 129 602 | 166 | 1 (reference) | 91 801 | 107 | 1 (reference) |
| Past | 10 143 | 52 | 1.45 (0.99–2.12) | 7851 | 35 | 1.32 (0.83–2.08) |
| Current | 75 039 | 185 | 1.21 (0.94–1.56) | 55 555 | 124 | 1.02 (0.74–1.40) |
|
Duration of alcohol drinking (year) | ||||||
| Non-drinking | 129 602 | 166 | 1 (reference) | 91 801 | 107 | 1 (reference) |
| ⩽10 | 19 585 | 23 | 1.25 (0.79–1.96) | 1583 | 10 | 1.04 (0.54–2.00) |
| 11–30 | 33 378 | 54 | 0.91 (0.64–1.31) | 2773 | 27 | 0.95 (0.48–1.88) |
| 31+ | 24 393 | 134 | 1.49 (1.11–2.01) | 2375 | 86 | 1.30 (0.93–1.83) |
|
Drinking frequency (times per week) | ||||||
| Non-drinking | 129 602 | 166 | 1 (reference) | 91 801 | 107 | 1 (reference) |
| <4 | 40 198 | 80 | 1.24 (0.92–1.68) | 28 766 | 48 | 1.50 (0.87–1.81) |
| 4–6 | 13 048 | 24 | 1.00 (0.62–1.55) | 9785 | 14 | 1.28 (0.36–5.16) |
| ⩾7 | 16 684 | 76 | 1.50 (1.08–2.07) | 13 170 | 56 | 1.35 (0.96–1.88) |
|
Average alcohol-drinking dose (g per single occasion) | ||||||
| Non-drinking | 129 602 | 166 | 1 (reference) | 91 801 | 107 | 1 (reference) |
| <25 | 39 754 | 93 | 1.33 (1.01–1.77) | 24 132 | 48 | 1.22 (0.83–1.79) |
| 25–54.9 | 19 296 | 26 | 1.14 (0.72–1.80) | 7971 | 21 | 0.95 (0.56–1.60) |
| ⩾55 | 15 768 | 51 | 1.36 (0.95–1.96) | 16 867 | 44 | 1.05 (0.70–1.58) |
Abbreviations: CI=confidence interval; HR=hazard ratio; KMCC=Korean Multi-center Cancer Cohort. The bold values indicate statistical significance at 95% confidence levels.
Case–cohort subjects had the information of H. pylori infection.
Adjusted for age, sex, body mass index (BMI), educational level, and smoking status in total cohort population; adjusted for age, sex, BMI, educational level, smoking status, and H. pylori infection in case–cohort population.
Table 2. The risk for gastric cancer in relation to alcohol-drinking status according to H. pylori antibodies in the KMCC case–cohort population with the information of H. pylori antibody, 1994–2004.
|
H. pylori (+) (N=817) |
H. pylori (−) (N=132) |
|||||
|---|---|---|---|---|---|---|
| Person-years | No. of cases (N=235) | HR (95% CI)a | Person-years | No. of cases (N=31) | HR (95% CI)a | |
|
Drinking status | ||||||
| Non-drinking | 3021 | 95 | 1 (reference) | 437 | 12 | 1 (reference) |
| Past drinker | 549 | 30 | 1.32 (0.81–2.57) | 84 | 5 | 1.36 (0.37–5.01) |
| Current drinker | 3176 | 110 | 1.01 (0.72–1.48) | 432 | 14 | 1.17 (0.44–3.12) |
|
Duration of alcohol drinking (year) | ||||||
| Non-drinking | 3021 | 95 | 1 (reference) | 437 | 12 | 1 (reference) |
| ⩽10 | 318 | 10 | 1.95 (0.52–2.09) | 28 | 0 | 0.84 (0.22–3.18)b |
| 11–30 | 766 | 23 | 1.01 (0.60–1.70) | 130 | 4 | |
| 31+ | 1761 | 75 | 1.17 (0.80–1.70) | 230 | 11 | 1.65 (0.54–5.10) |
|
Alcohol-drinking frequency (times per week) | ||||||
| Non-drinker | 3021 | 95 | 1 (reference) | 437 | 12 | 1 (reference) |
| <4 | 1136 | 45 | 1.19 (0.80–1.78) | 228 | 3 | 0.21 (0.03–1.74) |
| 4–6 | 532 | 12 | 0.60 (0.31–1.14) | 71 | 2 | 1.76 (0.31–9.96) |
| ⩾7 | 1192 | 48 | 1.17 (0.78–1.77) | 102 | 8 | 3.48 (1.13–10.73)c |
|
Average alcohol-drinking dose (g per single occasion) | ||||||
| Non-drinker | 3021 | 95 | 1 (reference) | 437 | 12 | 1 (reference) |
| <25 | 993 | 43 | 1.36 (0.92–2.02) | 213 | 4 | 0.42 (0.10–2.64) |
| 25–54.9 | 583 | 18 | 0.86 (0.49–1.51) | 74 | 3 | 1.86 (0.40–3.93) |
| ⩾55 | 1153 | 39 | 0.94 (0.61–1.46) | 93 | 6 | 3.27 (1.01–10.56)c |
Abbreviations: CI=confidence interval; H. pylori=Helicobacter pylori; HR=hazard ratio; KMCC=Korean Multi-center Cancer Cohort. The bold values indicates statistical significance at 95% confidence levels.
Adjusted for age, sex, body mass index, educational level, and smoking status in case–cohort population.
The results were combined due to few events among subpopulation.
P-value for heterogeneity between two hazard ratio (95% CIs) in infection-positive and infection-negative groups was statistically significant (P=0.047 for drinking frequency in the groups of ‘⩾7 times per week' P=0.042 for average alcohol-drinking dose in the groups of ‘⩾55 g per day').
Table 3. The risk for gastric cancer in relation to alcohol-drinking status according to CagA- or VacA-secreting H. pylori antibodies in the KMCC case–cohort population with the information of H. pylori antibody, 1994–2004.
| CagA (+) (N=772) |
CagA (−) (N=85) |
VacA (+) (N=567) |
VacA (−) (N=126) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Person-years | No. of cases (N=227) | HR (95% CI)a | Person-years | No. of cases (N=16) | HR (95% CI)a | Person-years | No. of casesa (N=171) | HR (95% CI)a | Person-years | No. of cases (N=28) | HR (95% CI)a | |
|
Drinking frequency (times per week) | ||||||||||||
| Non-drinker | 2794 | 92 | 1 (reference) | 313 | 6 | 1 (reference) | 2170 | 68 | 1 (reference) | 410 | 11 | 1 (reference) |
| <4 | 1080 | 44 | 1.19 (0.79–1.78) | 188 | 3 | 0.44 (0.05–4.19) | 837 | 34 | 1.02 (0.63–1.66) | 228 | 3 | 0.25 (0.03–2.06) |
| 4–6 | 527 | 12 | 0.59 (0.30–1.13) | 62 | 2 | 5.14 (0.53–49.95) | 386 | 10 | 0.57 (0.28–1.87) | 71 | 2 | 2.53 (0.42–15.37) |
| ⩾7 | 1110 | 46 | 1.17 (0.77–1.78) | 44 | 4 | 11.31 (1.45–87.92)b | 779 | 37 | 1.17 (0.72–1.88) | 102 | 7 | 3.76 (1.13–12.49)b |
|
Average alcohol-drinking dose (g per single occasion) | ||||||||||||
| Non-drinker | 2794 | 92 | 1 (reference) | 313 | 6 | 1 (reference) | 2170 | 68 | 1 (reference) | 410 | 11 | 1 (reference) |
| <25 | 922 | 42 | 1.39 (0.93–2.07) | 163 | 4 | 0.69 (0.11–3.87) | 742 | 31 | 1.13 (0.70–1.82) | 213 | 4 | 0.51 (0.10–2.53) |
| 25–54.9 | 572 | 18 | 0.85 (0.49–1.50) | 92 | 3 | 2.64 (0.43–16.59) | 401 | 16 | 0.95 (0.51–1.77) | 74 | 2 | 1.60 (0.31–8.33) |
| ⩾55 | 1104 | 37 | 0.90 (0.57–1.40) | 26 | 2 | 6.06 (1.04–35.32)b | 753 | 29 | 0.90 (0.54–1.51) | 93 | 6 | 4.44 (1.13–17.39)b |
Abbreviations: CagA=cytotoxin-associated gene A; CI=confidence interval; H. pylori=Helicobacter pylori; HR=hazard ratio; KMCC=Korean Multi-center Cancer Cohort; VacA=vacuolating cytotoxin A. The bold values indicates statistical significance at 95% confidence levels.
Adjusted for age, sex, body mass index, educational level, and smoking status in case–cohort population.
P-value for heterogeneity between two hazard ratio (95% CIs) in infection-positive and infection-negative groups was statistically significant (for drinking frequency in the groups of ‘⩾7 times per week', P=0.033 between CagA-positive and -negative groups and P=0.036 between VacA-positive and -negative groups; for average alcohol-drinking dose in the groups of ‘⩾55 g per day', P=0.028 between CagA-positive and -negative groups and P=0.036 between VacA-positive and -negative groups).
We also classified the drinking frequency of drinkers as non-drinker, light drinker (alcohol-drinking frequency ⩽2.5 and ⩽0.6 times a week in men and women, respectively), moderate drinker (alcohol-drinking frequency between light and heavy drinkers), and heavy drinker (alcohol-drinking frequencies >7 and >3 times a week in men and women, respectively). These categories were based on the cutoff levels for the 80th and 40th percentiles of alcohol consumption frequency for both men and women.
In addition, the participants were asked to identify the type of alcoholic beverage consumed (soju, beer, gin, rice wine, etc.) and the average amount of alcohol per drink (in cc). The amount of pure alcohol intake in the different types of alcoholic beverage was calculated from the known percentage of ethanol in each alcoholic beverage: soju, 20% beer, 4.5% gin, 40% rice wine, 6% and other alcohol beverages common in Korea, 25%. To determine average alcohol-drinking dosage, the average alcohol consumption was categorised into quartiles based on the frequency distribution among the total cohort. The alcohol doses per single occasion were then presented as non-drinker, <25 g alcohol, 25–54.9 g alcohol, and ⩾55 g alcohol (Tables 1, 2, 3). Participants whose drinking dose was ⩾55 g alcohol per drinking episode were defined as binge drinkers in total cohort population. In Supplementary Tables, the average alcohol-drinking dose was classified by sex as non-drinking, low dose (<28 g alcohol and <4 g alcohol on a single occasion in men and women, respectively), intermediate dose (alcohol dose between low and high dose), and high dose (⩾120 g alcohol and ⩾29 g alcohol on a single occasion in men and women, respectively). The high-dose category was denoted as a binge-drinking dose level.
Because U-shaped associations between alcohol-drinking dose or frequency and gastric cancer risk were observed in spline analysis and most of women in our study were non-drinkers or drank small amount of alcohol, alcohol drinking may seem protective factor of gastric cancer if we applied lower cutoff level in women. We also want to evaluate the association between absolute quantity of alcohol-drinking dose or frequency and gastric cancer, thus the same cutoff points were applied in both men and women. The participants' drinking pattern assessment was based on the year before interview, and we assumed that pattern would be maintained during follow-up time.
H. pylori infection, CagA, and VacA seropositivity
The CagA protein (cytotoxin-associated gene A), a key toxin of H. pylori, is reported to be associated with the development of gastric cancer. The toxin produces an inflammation of the gastric epithelium (Wang et al, 2013). It has also been reported that certain H. pylori genotypes produce vacuolating cytotoxin A (VacA) protein, another multi-functional virulence factor of H. pylori that has been associated with an increase in gastric cancer risk (Ogiwara et al, 2009). Thus, in addition to recording H. pylori infection status, both CagA and VacA IgG antibody seropositivities were determined in the study participants.
Seropositivities were determined by using the Helico Blot 2.1 (MP Biomedicals Asia Pacific, Singapore) immunoblot assay. H. pylori IgG seropositivity according to the immunoblot assay determined at enrolment, which H. pylori seropositivity reflects individuals' current infection as well as ever infection in the past.
Statistical analysis
Average and s.d. values of possible confounding factors including age, height, weight, and body mass index (BMI) were calculated for each alcohol-drinking status (non-, past, and current drinkers). Differences in height, weight, and BMI between groups were compared by Student's t-tests. Differences in proportions by sex, smoking status (non-, past, and current smokers), history of gastric ulcer (no/yes), and education level (lower, middle, high school, community college, or higher) between groups were tested by χ2 or Fisher's exact test.
To determine whether alcohol consumption is associated with a risk for gastric cancer, we conducted Cox regressions in the total cohort analysis and estimated HRs and 95% CIs in the models. Analyses were adjusted for age, sex, BMI, educational level, and smoking status in the total cohort. In addition, H. pylori infection status was adjusted in the case–cohort. The assumption that each predictor affected risk for gastric cancer proportionally over the entire follow-up period was examined by using graphical methods; the results indicated that the assumption was reasonable for all predictors considered in this study. The significance of the explanatory variables included in the Cox models was computed by the likelihood-ratio test. Tests for dose–response trends were assessed by fitting ordinal exposure variables as continuous terms.
We used weighted Cox proportional hazard regression models for case–cohort analysis, and each subject's weight was the inverse of their sample fraction to account for the differential sample proportion among the cases and the subcohort participants; that analysis was undertaken by using Barlow's method (Barlow et al, 1999). Gastric cancer cases were given a sample weight of 1 because they were sampled with certainty, whereas the subcohort participants were given a sample weight based on inverse selection probability.
We compared the Akaike's Information Criteria (AIC) in nonlinear spline and linear model for the relationship between alcohol consumption and gastric cancer and finally decide nonlinear cubic spline model to fit the relationship. The number and location of the knots used to fix the splines were created by following the recommendations of Harrell and Steyerberg (Harrell, 2001; Steyerberg, 2009).
Analyses were performed by using Stata version 12.1 and SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). A two-sided significance level of 0.05 was used.
Results
A summary of the general characteristics of the study population is presented in Supplementary Table 1. On average, current and past drinkers were mostly men, were older, had lower BMI, and smoked more than non-drinkers.
Table 1 shows the alcohol-drinking-related HRs (95% CIs) for gastric cancer obtained from a multivariable analysis adjusted for age, sex, BMI, educational level, and smoking status for the total cohort. The results indicate a statistically significant association between long duration of alcohol drinking (HR, 1.49; 95% CI, 1.11–2.01), drinking frequency ⩾7 times per week (HR, 1.50; 95% CI, 1.08–2.07) and gastric cancer risk in the total cohort. In the subcohort population for which there was information on H. pylori status, the multivariable model results adjusted for age, sex, BMI, educational level, smoking status, and H. pylori were similar to those for the total cohort, although the associations between alcohol-drinking patterns and gastric cancer risk were not statistically significant.
Table 2 shows the alcohol-drinking-related HRs (95% CIs) for gastric cancer in the KMCC subcohort population with H. pylori IgG antibody seropositivity. In our analysis stratified for anti H. pylori IgG antibody status, drinking alcohol ⩾7 times a week resulted in a 3.48-fold higher risk of gastric cancer (95% CI, 1.13–10.73), and drinking frequency of at least seven times per week resulted in an HR of 3.48 (95% CI, 1.13–10.73) as compared with the non-drinking, non-H. pylori-infected group. An increased risk was also detected in the groups without CagA IgG- or VacA IgG-secreting H. pylori (Table 3). In particular, the increase in gastric cancer risk associated with drinking ⩾7 times a week was greatest in the group not infected by CagA-secreting H. pylori (HR, 11.31; 95% CI, 1.45–87.92) and 3.76 times (95% CI, 1.13–12.49) in the group not infected by VacA. However, there are no heterogeneous in HR for gastric cancer between H. pylori non-infected population, CagA (or VacA)-secreting H. pylori non-infected population (P-heterogeneity >0.3). In the ever infected by H. pylori group, none of the alcohol-drinking variables were significantly associated with gastric cancer. In the not infected by H. pylori group, the risk of gastric cancer increased according to average alcohol-drinking dose, and those consuming a high alcohol dose had a 3.27-fold higher risk of gastric cancer (95% CI, 1.01–10.56) than non-drinker. An increase in gastric cancer risk associated with high-dose alcohol consumption, that is, ⩾55 g of alcohol per single occasion, was also detected in groups not infected by CagA-secreting H. pylori and VacA-secreting H. pylori (HR, 6.06; 95% CI, 1.04–35.32 for the CagA; and HR, 4.44; 95% CI, 1.13–17.39 for VacA). In the group infected by H. pylori, there were no statistically significant results for any of the different doses of alcohol consumed per day. The trend to an increased gastric cancer risk with increases in drinking frequency and average alcohol dose was persistent even when we applied different alcohol-drinking classifications (Supplementary Table 2). Although there is no gender-specific effect in the association between alcohol drinking and gastric cancer risk (P-heterogeneity of ORs between gender groups >0.1), most results were statistically significant in men group (table not shown).
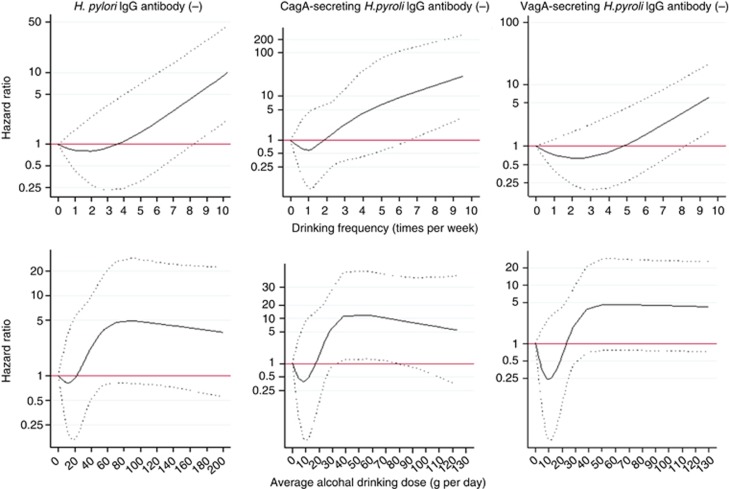
Figure 1 presents the results for the associations between alcohol consumption and gastric cancer risk in only H. pylori-negative subjects based on the spline regression analyses. The results were obtained by using three (drinking frequency) or four knots (average alcohol-drinking dose) with the exclusion of extreme consumption values (last three values in each knot) and values for non-consumers. After comparing each obtained AICspline and AICLinear value, it was observed that use of the spline model resulted in only a very slight improvement in fit over that from a standard linear regression model (data not shown).
Figure 1.
HRs (solid line) and 95% CIs for baseline alcohol consumption and gastric cancer risk assessed by using restricted cubic spline regression in KMCC cohort. The spline regression model excluded extreme consumption (top 1%, ⩾200 g per day; three cases). Weighted model (four knots) adjusted for age (1-year categories), sex, BMI, and smoking status.
Discussion
Our results showed that alcohol drinking for long periods of time (decades) and frequent drinking (daily) is associated with an increased risk of gastric cancer in the study cohort. However, this increased risk of gastric cancer by heavy or binge drinkers relative to non-drinkers was observed in only H. pylori non-infected subjects. Heavy drinking (i.e., drinking seven or more times a week) and binge drinking (i.e., alcohol consumption of ⩾55 g per occasion) were associated with 3.48-fold and 3.27-fold higher risks for gastric cancer compared with non-drinking among the H. pylori non-infected study population.
The associations between heavy or binge drinking and gastric cancer risk in the non-infection groups were not statistically heterogeneous, regardless of common or highly pathogenic H. pylori, classified by CagA or VacA. This association may be only conditioned by H. pylori non-infection. On the contrary, our finding of non-association in H. pylori-infected group may be due to any antimicrobial effect of H. pylori on high-dose alcohol-drinking condition. Our hypothesis is supported by several epidemiologic studies reported an antimicrobial effect of alcohol on H. pylori (Brenner et al, 1997, 1999; Ogihara et al, 2000). High alcohol consumption was significantly associated with a decrease in H. pylori infection. In spite of the negative relationship between alcohol intake and H. pylori infection, only two previous studies included information on H. pylori infection status when evaluating the association between alcohol drinking and gastric cancer risk (Zaridze et al, 2000; Duell et al, 2011). One of those studies presented results adjusted for H. pylori infection (Duell et al, 2011), whereas the other study, conducted in Russia, presented the results stratified by H. pylori infection status (Zaridze et al, 2000). In the Russian study, vodka consumption was associated with an increased risk of gastric cancer in both H. pylori-positive and H. pylori-negative subjects (Zaridze et al, 2000).
Our study showed dose- and frequency-dependent associations between alcohol drinking and gastric cancer risk, but only in subjects not infected by H. pylori. The difference in results between our study and the Russian study might be related to the different amounts of alcohol consumption in the two studies. Vodka drinking in the Russian study was classified as ‘never' or ‘ever', and the average intake of pure alcohol was not presented. In the absence of dose and frequency data, we were unable to compare directly the two studies results. The World Health Organization (WHO) has reported that from 2003 to 2005 the pure alcohol intake per capita for those over 15 years old was 16.1 l in Russia and 12.3 l in Korea (World Health Organization, 2014). On the basis of those WHO report, we assume that the average alcohol dose in the Russian study was higher than that in our study, which may account for the difference in results.
Supposing some peoples with H. pylori infection (or highly pathogenic H. pylori infection) has gastric symptoms due to H. pylori or highly pathogenic H. pylori, individuals with gastric symptoms possibly decide to reduce or quit drinking alcohol. However, subjects infected with H. pylori, even with CagA-positive H. pylori, are generally asymptomatic and have no difference in symptom severity even in subjects with mild gastric symptoms (Bommelaer et al, 2001). In our cohort, the proportions of heavy drinking and binge drinking among H. pylori-infected peoples (20% and 21%, respectively) were higher than those in non-infected peoples (12% and 11%, respectively). On the basis of the research showing no difference of symptoms according to virulence of H. pylori and the evidence from our cohort population, we did conclude that our finding was not biased by changing alcohol habit due to H. pylori infection and gastric symptoms. An in vitro study reported by Brenner et al showed a relationship between H. pylori prevalence and alcohol drinking and included results that considered alcohol dose. In that study moderate drinking (alcohol consumption of 75–175 g per week) was associated with a decreased OR of H. pylori infection (OR, 0.60; 95% CI, 0.38–0.94), but the antimicrobial effect of alcohol consumption was lessened at higher alcohol dose level (Brenner et al, 2001). Those results suggest a U-shaped curve relationship between alcohol consumption level and prevalence of H. pylori. On the basis of that type of relationship, we conclude that the alcohol dose level in the Russian study was excessive and that dose level would not inhibit H. pylori infection efficiently, suggesting that the H. pylori infection status was similar in non-drinkers and drinkers. Under such conditions, the independent effect of alcohol on gastric epithelium may result in an increased risk of gastric cancer compared with the level of risk in non-drinking subjects. In our study, the alcohol consumption amounts for heavy and binge drinkers may be close to the level that can inhibit the infection of H. pylori efficiently. In a H. pylori-infected group, a decreased level of H. pylori infection derived from the effects of alcohol consumption may lessen the gastric cancer risk through the independent effect of alcohol as a carcinogen. This could result in no detectable difference in gastric cancer risk between drinkers and non-drinkers in a H. pylori-infected population. However, in a population that is not infected by H. pylori, the carcinogenic effects of alcohol may increase the risk of gastric cancer.
The mechanisms responsible for the carcinogenic effect of alcohol consumption on gastric cancer have not been fully elucidated. Thus far, some reports suggest that ingested alcohol can cause direct and indirect dose-dependent mechanical damage to the gastric epithelium (Chari et al, 1993; Knoll et al, 1998). It has been reported that alcohol intake increases acid secretion from the stomach, which leads to gastric mucosal damage (Chari et al, 1993). In addition, a high concentration of ingested alcohol has been associated with the generation of reactive oxygen species (ROS; e.g., peroxide, superoxide) as well as other free radicals, inorganic arsenic, preservatives, and additives (Zimmerman et al, 1995), which can produce alterations in hormonal balance and depletion of vitamin deposits, subsequently promoting carcinogenesis of gastric cancer (Blot, 1992). The direct processes that produce mucosal damage, such as increased acid secretion and ROS generation in the stomach, can induce an inflammatory environment in the stomach. The correlation between gastric inflammation and carcinogenesis of gastric cancer has been well described (Rakoff-Nahoum, 2006). Moreover, alcohol can have indirect effects on carcinogenesis of gastric cancer by conversion of alcohol to metabolites. Alcohol is endogenously broken down into acetaldehyde, which can produce DNA strand breakage and abnormal binding to proteins, potentially leading to cancer development (Aberle et al, 2004).
Our study has two limitations. First, the number of gastric cancer cases and the number of study population members with H. pylori infection information were small, thus some groups were combined into one group for statistical analysis. Second, information on alcohol consumption was collected at enrolment and was not repeated measurements during the follow-up period. We assumed this baseline information reflected the routine alcohol-drinking pattern of study population throughout the follow-up period. Effects of changes in alcohol-drinking patterns on gastric cancer development were not evaluated. However, considering the latent period of gastric cancer is over 10 years and median follow-up time of our study was 8.4 years, the baseline information of alcohol drinking is still valid for this study.
Nevertheless, this study has some strength. First, this study is a case–cohort study and its data are derived from prospective cohort data that were collected considering the time sequence between exposure and outcome. Thus, we can use the data to assess the causality relative to other study designs and minimise the effects of recall bias. Second, the sensitivity and specificity of the immunoblot assay (Helico Blot 2.1) used to detect H. pylori, CagA IgG seropositivity, and VacA IgG seropositivity are very high (99%, 99%, 93% for sensitivity, and 98%, 90%, 88% for specificity, respectively) (Park et al, 2002). Third, our study classified the subjects' alcohol-drinking patterns according to duration, frequency, and dose of alcohol consumption. Analysis of these different aspects of alcohol consumption can indicate which of these aspects provide the best information on alcohol effects on gastric cancer risk.
In conclusion, alcohol drinking was associated with an increase in gastric cancer risk. The effects were exhibited in binge or heavy drinkers who were not previously infected by H. pylori. The associations between alcohol drinking and risk of gastric cancer were similar in subjects not infected by CagA- or VacA-secreting H. pylori. The study results can partly explain why an H. pylori not-infected group may have gastric cancer. Such results suggest that abstention from alcohol can lower the risk of gastric cancer, especially among subjects who are not infected by H. pylori.
Acknowledgments
This research was supported by the Korean Foundation for Cancer Research (KFCR-CB-2013-01).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Aberle NS, Burd L, Zhao BH, Ren J (2004) Acetaldehyde-induced cardiac contractile dysfunction may be alleviated by vitamin B1 but not by vitamins B6 or B12. Alcohol Alcohol 39(5): 450–454. [DOI] [PubMed] [Google Scholar]
- Barlow WE, Ichikawa L, Rosner D, Izumi S (1999) Analysis of case-cohort designs. J Clin Epidemiol 52(12): 1165–1172. [DOI] [PubMed] [Google Scholar]
- Blot WJ (1992) Alcohol and cancer. Cancer Res 52(7 Suppl): 2119s–2123s. [PubMed] [Google Scholar]
- Bommelaer G, Bruley Des Varannes S, Flejou JF, Matysiak T, Poynard T, Richard A, Slama A, Megraud F, Groupe d'Etude H (2001) [CagA status and virulence of Helicobacter pylori strains. Results of a French multicentric prospective study]. Gastroenterol Clin Biol 25(12): 1084–1089. [PubMed] [Google Scholar]
- Brenner H, Berg G, Lappus N, Kliebsch U, Bode G, Boeing H (1999) Alcohol consumption and Helicobacter pylori infection: results from the German National Health and Nutrition Survey. Epidemiology 10(3): 214–218. [PubMed] [Google Scholar]
- Brenner H, Bode G, Adler G, Hoffmeister A, Koenig W, Rothenbacher D (2001) Alcohol as a gastric disinfectant? The complex relationship between alcohol consumption and current Helicobacter pylori infection. Epidemiology 12(2): 209–214. [DOI] [PubMed] [Google Scholar]
- Brenner H, Rothenbacher D, Bode G, Adler G (1997) Relation of smoking and alcohol and coffee consumption to active Helicobacter pylori infection: cross sectional study. BMJ 315(7121): 1489–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari S, Teyssen S, Singer MV (1993) Alcohol and gastric-acid secretion in humans. Gut 34(6): 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho LY, Kim CS, Li L, Yang JJ, Park B, Shin A, Chang SH, Lee KS, Kim H, Yoo KY, Park SK (2009) Validation of self-reported cancer incidence at follow-up in a prospective cohort study. Ann Epidemiol 19(9): 644–646. [DOI] [PubMed] [Google Scholar]
- Duell EJ, Travier N, Lujan-Barroso L, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, Palli D, Krogh V, Panico S, Tumino R, Sacerdote C, Quiros JR, Sanchez-Cantalejo E, Navarro C, Gurrea AB, Dorronsoro M, Khaw KT, Allen NE, Key TJ, Bueno-de-Mesquita HB, Ros MM, Numans ME, Peeters PHM, Trichopoulou A, Naska A, Dilis V, Teucher B, Kaaks R, Boeing H, Schutze M, Regner S, Lindkvist B, Johansson I, Hallmans G, Overvad K, Egeberg R, Tjonneland A, Lund E, Weiderpass E, Braaten T, Romieu I, Ferrari P, Jenab M, Stenling R, Aune D, Norat T, Riboli E, Gonzalez CA (2011) Alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am J Clin Nutr 94(5): 1266–1275. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5): E359–E386. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Vol. 2015. International Agency for Research on Cancer: Lyon, France. [DOI] [PubMed] [Google Scholar]
- Forman D, Newell DG, Fullerton F, Yarnell JWG, Stacey AR, Wald N, Sitas F (1991) Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ 302(6788): 1302–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield TK, Kerr WC, Bond J, Ye Y, Stockwell T (2009) Graduated Frequencies alcohol measures for monitoring consumption patterns: Results from an Australian national survey and a US diary validity study. Contemp Drug Probl 36(3–4): 75056015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwack J, Shin A, Kim C-S, Ko K, Kim Y, Jun J, Bae J, Park S, Hong Y, Kang D (2006) CagA-producing Helicobacter pylori and increased risk of gastric cancer: a nested case–control study in Korea. Br J Cancer 95(5): 639–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE (2001) Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans (2010) Alcohol consumption and ethyl carbamate: IARC monographs on the evaluation of carcinogenic risks to humans. World Health Organization, International Agency for Research on Cancer 96: 3–1383. [PMC free article] [PubMed] [Google Scholar]
- Ji BT, Chow WH, Yang G, McLaughlin JK, Gao RN, Zheng W, Shu XO, Jin F, Fraumeni JF, Gao YT (1996) The influence of cigarette smoking, alcohol, and green tea consumption on the risk of carcinoma of the cardia and distal stomach in Shanghai, China. Cancer 77(12): 2449–2457. [DOI] [PubMed] [Google Scholar]
- Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS (2014) Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat 46(2): 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JR, Duggan JM (2003) Gastric cancer epidemiology and risk factors. J Clin Epidemiol 56(1): 1–9. [DOI] [PubMed] [Google Scholar]
- Knoll MR, Kolbel CB, Teyssen S, Singer MV (1998) Action of pure ethanol and some alcoholic beverages on the gastric mucosa in healthy humans: a descriptive endoscopic study. Endoscopy 30(3): 293–301. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang HA, Cao J (2011) Association between alcohol consumption and cancers in the Chinese population—a systematic review and meta-analysis. PLoS One 6(4): e18776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunet N, Barros H (2003) Helicobacter pylori infection and gastric cancer: facing the enigmas. Int J Cancer 106(6): 953–960. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (2004) NIAAA Council Approves Definiton of Binge Drinking. NIAAA Newsletter 3: 3.
- Ogihara A, Kikuchi S, Hasegawa A, Kurosawa M, Miki K, Kaneko E, Mizukoshi H (2000) Relationship between Helicobacter pylori infection and smoking and drinking habits. J Gastroenterol Hepatol 15(3): 271–276. [DOI] [PubMed] [Google Scholar]
- Ogiwara H, Sugimoto M, Ohno T, Vilaichone RK, Mahachai V, Graham DY, Yamaoka Y (2009) Role of deletion located between the intermediate and middle regions of the Helicobacter pylori vacA gene in cases of gastroduodenal diseases. J Clin Microbiol 47(11): 3493–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Cho YK, Kodama T, El-Zimaity HM, Osato MS, Graham DY, Yamaoka Y (2002) New serological assay for detection of putative Helicobacter pylori virulence factors. J Clin Microbiol 40(12): 4753–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S (2006) Why cancer and inflammation? Yale J Biol Med 79(3-4): 123–130. [PMC free article] [PubMed] [Google Scholar]
- Shin A, Yoo K-Y, Kang D, Park SK, Yang M, Kim C-S, Shin H-R (2004) A nested case-control study on the association of Helicobacter pylori infection with gastric cancer in the KMCC cohort. Proc Am Assoc Cancer Res 2004(1): 109. [Google Scholar]
- Steyerberg EW (2009) Clinical prediction models. Springer. [Google Scholar]
- Substance of Abuse and Mental Health Services Administration, the Center Behavioral Health Statistics and Quality (2014) Substance Use and Mental Health Estimates from the 2013 National Survey on Drug Use and Health: Overview of Findings. The NSDUH Report. [PubMed]
- Tajima K (2002) Challenging epidemiological strategy for paradoxical evidence on the risk of gastric cancer from Helicobacter pylori infection. Jpn J Clin Oncol 32(8): 275–276. [DOI] [PubMed] [Google Scholar]
- Tramacere I, Negri E, Pelucchi C, Bagnardi V, Rota M, Scotti L, Islami F, Corrao G, La Vecchia C, Boffetta P (2012) A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol 23(1): 28–36. [DOI] [PubMed] [Google Scholar]
- Wang HP, Zhu YL, Shao W (2013) Role of Helicobacter pylori virulence factor cytotoxin-associated gene A in gastric mucosa-associated lymphoid tissue lymphoma. World J Gastroenterol 19(45): 8219–8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2014) Global status report on alcohol and health 2014. World Health Organization: Geneva, Switzerland. [Google Scholar]
- Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, Seo GS, Kim HU, Baik GH, Sin CS, Cho SH, Oh BH (2007) Seroprevalence of Helicobacter pylori in South Korea. Helicobacter 12(4): 333–340. [DOI] [PubMed] [Google Scholar]
- Yoo KY, Shin HR, Chang SH, Lee KS, Park SK, Kang D, Lee DH (2002) Korean Multi-center Cancer Cohort Study including a Biological Materials Bank (KMCC-I). Asian Pac J Cancer Prev 3(1): 85–92. [PubMed] [Google Scholar]
- Zaridze D, Borisova E, Maximovitch D, Chkhikvadze V (2000) Alcohol consumption, smoking and risk of gastric cancer: case-control study from Moscow, Russia. Cancer Causes Control 11(4): 363–371. [DOI] [PubMed] [Google Scholar]
- Zimmerman BT, Crawford GD, Dahl R, Simon FR, Mapoles JE (1995) Mechanisms of acetaldehyde-mediated growth-inhibition—delayed cell-cycle progression and induction of apoptosis. Alcohol Clin Exp Res 19(2): 434–440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.