Abstract
Background:
Testicular embryonal carcinoma (EC) is a major subtype of non-seminomatous germ cell tumours in males. Embryonal carcinomas are pluripotent, undifferentiated germ cell tumours believed to originate from primordial germ cells. Epigenetic changes during testicular EC tumorigenesis require better elucidation.
Methods:
To identify epigenetic changes during testicular neoplastic transformation, we profiled DNA methylation of six ECs. These samples represent different stages (stage I and stage III) of divergent invasiveness. Non-cancerous testicular tissues were included. Expression of a number of hypermethylated genes were examined by quantitative RT-PCR and immunohistochemistry (IHC).
Results:
A total of 1167 tumour-hypermethylated differentially methylated regions (DMRs) were identified across the genome. Among them, 40 genes/ncRNAs were found to have hypermethylated promoters. Quantitative RT-PCR confirmed downregulation of 8 out of 9 of the genes. Among the confirmed genes, five were sex-linked genes, including X-linked genes STAG2, SPANXD/E and MIR1184, and Y-linked genes RBMY1A1/1B/1D and FAM197Y2P. RBMY1A is a testis-specific gene for spermatogenesis. RNF168 and USP13 are potential tumour suppressors. Expression of RBMY1A was lost in EC and seminoma as documented in the Protein Atlas. We confirmed downregulation of USP13 in EC by IHC.
Conclusions:
Our genome-wide analysis of testicular EC identified methylation changes in several previously unknown genes. This may provide insight of crosstalk between normal germ cell development and carcinogenesis.
Keywords: Embryonal carcinoma, DNA methylation, sex-linked genes, USP13, RBMY1A
Testicular tumour is the most common malignancy in young men between ages of 20 and 39 years. Over 90% are testicular germ cell tumours (TGCTs) that originate from germ cells. Testicular germ cell tumours (TGCTs) are heterogeneous neoplasms that arise relatively earlier than other carcinomas (Bahrami et al, 2007; McIver et al, 2013; Looijenga, 2014).
In clinical practice, TGCTs are classified into two histopathological categories, namely seminoma and non-seminoma. Non-seminoma consists of mixed types of embryonal carcinoma (EC), teratoma, yolk sac tumour, choriocarcinoma and sometimes seminoma (Crundwell, 2004). Among them, EC is the most common non-seminomatous germ cell tumour (NSGCT). It is present in ∼77% of mixed NSGCTs. Pure forms of NSGCT are relatively uncommon, and ∼72% of pure NSGCT are EC (Bahrami et al, 2007).
Different from seminoma, EC arises at an earlier age, ∼10 years earlier than the average for seminoma. Comparison of the histological types shows a higher incidence of inguinal metastasis for NSGCT (4.9%) than seminoma (0.5% Daugaard et al, 2006). As EC is an aggressive tumour, vascular invasion to parenchymal vessels and rete testis invasion are frequently detected in stage I and II NSGCT. About two-third of cases develop retroperitoneal lymph node or distant metastases (Hamid and Umbas, 2009; Yilmaz et al, 2013). Because of these features, our current focus is on pure EC only, whereas seminoma and other subtypes in mixed NSGCT are excluded. It is critical to use relatively pure form of EC for methylation analysis, as the epigenetic variation among heterogeneous NSGCTs complicates the interpretation of experimental data.
Many risk factors have been well documented to increase the chance of having TGCT. Cryptorchidism increases the risk of developing TGCT due to failure of normal descent of testes (Lip et al, 2013). Patients with congenital disorders such as Klinefelter's syndrome or Down syndrome have a higher risk of TGCT because of the abnormal sexual development (Dieckmann et al, 1997; Swerdlow et al, 2005). Positive family history is also a risk factor, indicative of genetic causes of TGCT (Nordsborg et al, 2011). Data from molecular profiling and marker expression suggest many similarities between TGCT development and normal embryogenesis (for examples, the expression of pluripotency marker OCT3/4) (Jones et al, 2004; Hatada et al, 2008; Almstrup et al, 2010; Kristensen et al, 2013). Recent methylation studies indicated that epigenetic factors may have an essential role in the genesis of germ cell neoplasia (Cheung et al, 2010; Mirabello et al, 2012; Chen et al, 2014). In addition, environmental factors may contribute to risk independent of genetic susceptibility (Smiraglia et al, 2002; Kristensen et al, 2008). However, the responsible epigenetic changes for development of germ cell neoplasms remain to be elucidated.
To identify epigenetic alterations during testicular tumorigenesis, we profiled the DNA methylome of six ECs and two non-cancerous normal testes. The neoplastic samples represent stage I and stage III and exhibited different degrees of invasiveness. A set of hypermethylated genes was found in aggressive ECs. These genes, including several sex-linked genes, may have a central role in the regulation of energy metabolism and spermatogenesis and tumour suppression.
Materials and methods
Tumour specimens
We obtained genomic DNA directly extracted from pathologically confirmed ECs provided by Oncomatrix (San Marcos, CA, USA). Non-cancerous testicular tissues from healthy donors were obtained from Biochain (Hayward, CA, USA). Matched RNA samples from the same specimen, if available, were also obtained. Additional normal/tumour RNA samples were included in the real-time quantitative PCR (qPCR) analysis. Tumour staging was based on the pathologic report for testicular tumour by TNM classification. Tissue arrays for testicular cancer progression were purchased from US Biomax (TE2081; Rockville, MD, USA).
MeDIP and DNA-tiling hybridisation
Methylated DNA immunoprecipitation (MeDIP) was performed as we previously described (Cheung et al, 2010). Genomic DNA (5 μg) of tumour/control samples were sonicated on ice to generate random fragments of 100–500 bp in size. Fragmented DNA was subsequently heated at 95 °C, snap-cooled on ice and then incubated with mouse anti-5-methylcytidine monoclonal antibody (anti-5mC, Eurogenetec, Seraing, Belgium) in IP buffer containing 10 mM sodium phosphate (pH 7.0), 140 mM sodium chloride and 0.05% Triton X-100 for 2 h at 4 °C with gentle shaking. Magnetic beads conjugated with sheep anti-mouse IgG (Invitrogen, Grand Island, NY, USA) were added to the IP mixture and incubated for another 2 h. After incubation, the beads were washed three times in IP buffer and then digested by 80 μg of proteinase-K for 3 h at 50 °C. DNA was extracted by phenol/chloroform and ethanol precipitation. Following MeDIP, DNA was amplified using Whole Genome Amplification Kit (Sigma-Aldrich, St Louis, MO, USA), biotin-labelled and hybridised to Human Tiling Array 2.0R Chips (Affymetrix, Santa Clara, CA, USA) according to the manufacturer's instruction. After overnight hybridisation at 45 °C, chips were washed and stained on the Affymetrix Fluidic Station 450 and scanned on GeneChip Scanner GCS3000 (Affymetrix). Technical replicate of MeDIP-chip procedure was performed using the same DNA sample.
Analysis of microarray data
Raw data of tiling array (.CEL files) were submitted for quantile normalisation using the Tiling Analysis Software (TAS; Affymetrix). Pairwise comparisons were performed to compare tumour samples of each stage with normal testicular tissues in TAS. Differentially methylated regions (DMRs) were generated using parameters as we previously described (Cheung et al, 2010). To identify differentially methylated genes, promoter annotations (+500 to −1500 bp relative to TSS) were retrieved from Refseq in UCSC Genome Browser, and mapped to DMRs using web-based tool TileMapper (Cheung et al, 2013). To visualise genomic features of differentially expressed genes, CEL and BED (which contains DMR information) files were loaded in Integrated Genome Browser. Array data were deposited at GEO (Series GSE66784).
Real-time qPCR analysis
Total RNA samples were converted to cDNA using SuperScript III (Invitrogen). Diluted cDNA was mixed with gene-specific primers and SYBR Green master mix for qPCR analysis in Applied Biosystems 7500 Fast Real-Time PCR System (Life Technologies, Grand Island, NY, USA). Relative gene expression level was normalised by 18S rRNA. All RT-qPCR experiments were replicated three times and represented as mean±s.d. The list of the primer sequences is shown in Supplementary Table 2.
Immunohistochemistry
Tissue array was deparaffinized in xylene (3 min, 2 ×) and rehydrated in serial ethanol (100–50%). Antigen retrieval was performed by heating the slides in sodium citrate solution (10 mM, pH 6.0, 0.05% Tween-20) using a microwave oven. Endogenous peroxidase was blocked by Dako REAL Peroxidase-Blocking Solution (Dako North America, Carpinteria, CA, USA). Slides were blocked with 10% goat serum before incubation with antibodies. Anti-USP13 (1 : 100 dilution) antibody was purchased from Sigma-Aldrich (HPA004827) and incubated with tissues at 4 °C overnight. HRP-labelled secondary antibody conjugated in polymer (Dako North America) was incubated with tissues at room temperature for 1 h. After thorough washing, slides were stained with DAB substrates and counter-stained with haematoxylin and viewed under light microscope (Leica, Wetzlar, Germany). The staining intensity was averaged by two duplicated tissue spots and scored as negative (<10%), weak (10–25%), moderate (25–75%) and strong (>75%), according to the common guidelines (Gremel et al, 2014).
Statistics
One-way ANOVA was used to compare the difference in gene expression between normal testicular tissues and ECs. Fisher's exact test was used to compare the difference between normal and different subtypes of TGCT. P<0.05 is considered statistically significant.
Results
Hypermethylation in ECs
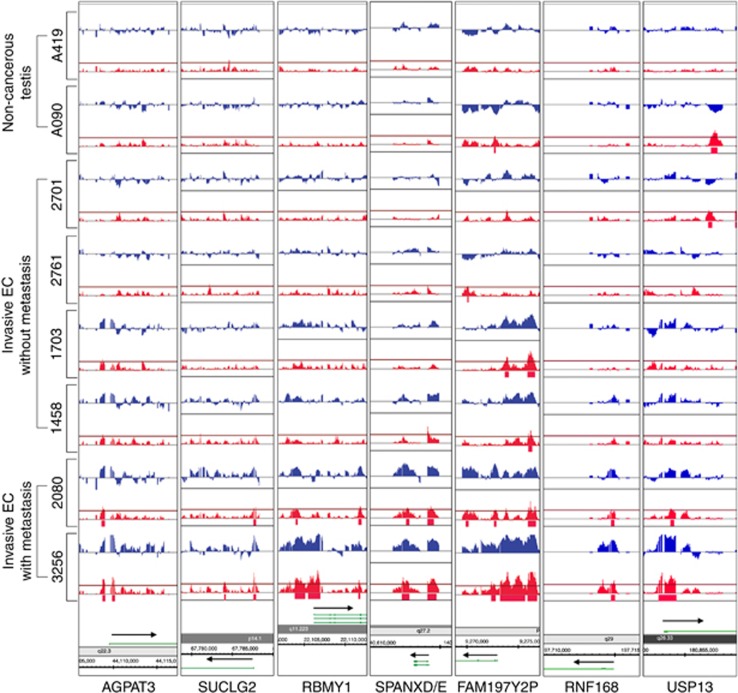
We attempted to identify epigenetic alterations that occurred during TGCT tumorigenesis. The current focus is on EC, a subtype of the most common germ cell tumour in both mixed and pure NSGCTs. Furthermore, ECs are highly aggressive in nature, with both local and distant metastasis. To examine genomic ‘hotspots' associated with tumour progression, we analysed the global DNA methylation of six ECs representing non-metastatic tumours without local invasion (stage I, pT1), non-metastatic tumours with vascular/lymphatic invasion (stage I, pT2) and tumours with distant metastasis and vascular/lymphatic invasion (stage III, pT2). Non-cancerous testicular tissues were included as controls (Supplementary Table 1). Genome-wide methylation analysis revealed distinct methylation profiles between cancerous versus non-cancerous tissues. We were specifically interested in the DMRs identified from ECs. The majority of these DMRs are hypermethylation. A total of 1167 hypermethylated DMRs were enriched in the ECs. To gain knowledge of the regulatory relationship of the DMRs on genes (including non-coding RNAs), we mapped the hypermethylated DMRs to Refseq promoters (Figure 1). A total of 40 genes/ncRNAs were identified (Table 1). The other DMRs (∼97%) were mapped to gene bodies or intergenic regions, consistent with our previous finding in other testicular cancer cells (Cheung et al, 2010). The function of such DMRs remains to be elucidated. For the 40 genes/ncRNAs with hypermethylated promoters, 10 were found in pathways of metabolism including TCA cycle and mitochondrial protein import (by REACTOME). Altered energy metabolism in cancer cells is well established. Although only 40 genes/ncRNAs were identified in our study, 27 (67.5%) were implicated in cancer, as identified by Ingenuity Pathway Analysis (Table 2; Supplementary Figure 1).
Figure 1.
Genomic representation of differentially methylated genes. Hypermethylation is observed at the promoters of seven representative genes (AGPAT3, SUCLG2, RBMY1, SPANXD/E, FAM197Y2P, RNF168 and USP13) in metastatic and non-metastatic ECs. Orientation of transcription for each gene is indicated by arrows. Blue peaks represent probe signal, whereas red peaks represent P-value (cutoff=0.01).
Table 1. Hypermethylated genes in EC.
| Chr | Genes |
|---|---|
| chr1 | OR2T8 |
| chr2 | TRIM43B, INO80B |
| chr3 | FAM157A, SUCLG2, USP13, NPHS2, ASB3, CACNA2D3, C1orf182, RNF168, ADCY5, LOC729375, LOC100128640 |
| chr4 | INPP1, SMEK2, UGT2B28, C1orf87 |
| chr7 | AOAH, SPACA1, RNF144B, LOC285733, MIR592, CYP3A5 |
| chr17 | MAPK7, CDK5RAP3 |
| chr19 | PIP5K1C, BTBD2, ZNF699, |
| chr21 | DSCR3, AGPAT3, ABCG1 |
| chr22 | ACO2, PHF5A, SAMM50 |
| chrX | STAG2, SPANXD/SPANXE, MIR1184-1,2,3 |
| chrY | RBMY1B/RBMY1A1/RBMY1D, FAM197Y2P |
Table 2. Gene ontology analysis of the hypermethylated genes on diseases and biofunctions.
| Categories | # of genes | P-value |
|---|---|---|
| Cancer | 27 | 2.06E−02 |
| Cancer, gastrointestinal disease, hepatic system disease | 15 | 1.38E−02 |
| Hereditary disorder, neurological disease, psychological disorders, skeletal and muscular disorders | 4 | 2.38E−02 |
| Infectious disease | 4 | 2.52E−02 |
| Cancer, neurological disease | 3 | 3.58E−02 |
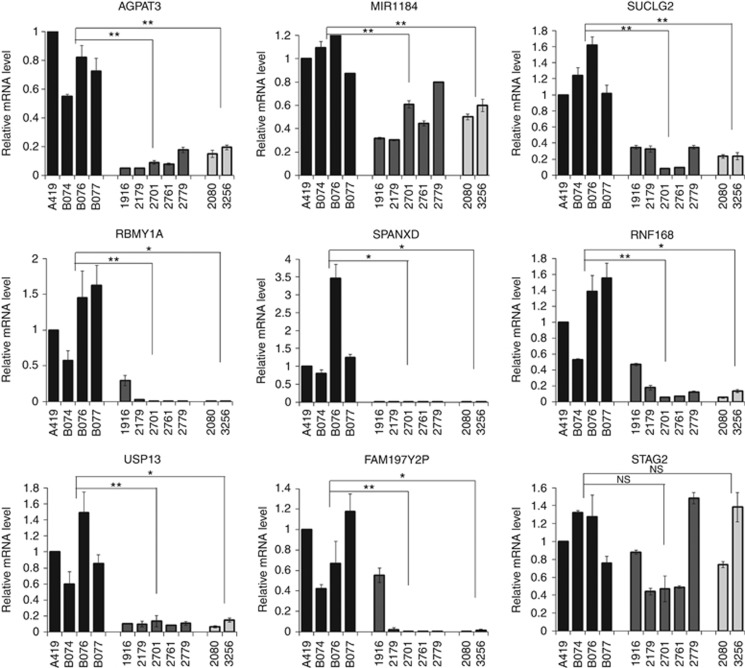
DNA hypermethylation at promoters is usually associated with transcriptional repression. To investigate whether the identified hypermethylated genes/ncRNAs are associated with reduced transcription, the relative mRNA expression of nine randomly selected genes/ncRNAs (AGPAT3, MIR1184, SUCLG2, RBMY1, SPANXD, RNF168, USP13, FAM197Y2P and STAG2) was examined by RT-qPCR. Although we had limited sample size, 8 out of 9 genes (except STAG2) showed reduced expression in tumours of both metastatic and non-metastatic ECs (Figure 2), suggesting promoter methylation may hinder transcription of these genes.
Figure 2.
Relative expression of nine hypermethylated genes/ncRNAs. Gene expression is measured by quantitative RT-PCR and normalised by 18S rRNA. Values represent mean±s.d. Non-cancerous testicular tissues: A419, B074, B076 and B077; non-metastatic EC: 1916, 2179, 2701, 2761 and 2779; metastatic EC: 2080 and 3256. *P<0.05, **P<0.01; NS=not significant (one-way ANOVA).
Epigenetic changes in sex-linked genes
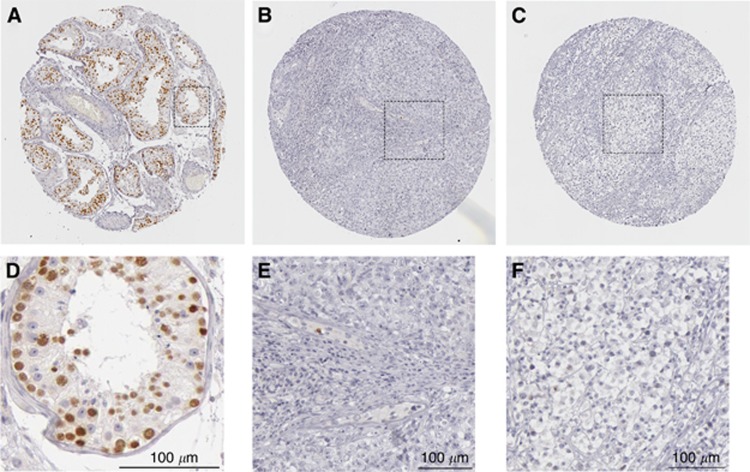
From the list of hypermethylated genes, we noticed several sex-linked genes/ncRNAs that were epigenetically changed, including X-linked genes STAG2, SPANXD/E and MIR1184, and Y-linked genes RBMY1A1/1B/1D and FAM197Y2P (Table 1). Interestingly, the X-linked SPANXD/E is localised to a previously identified susceptibility locus on chromosome Xq27 for TGCT and prostate cancer (Rapley et al, 2000; Kouprina et al, 2007). Consistent with this, the SPANXD transcript was expressed in normal testis but not detected in our EC samples (Figure 2). Although it is sex linked, SPANXD is not testis specific. Another sex-linked gene, RBMY1A1 is a testis-specific RNA-binding protein and is important for spermatogenesis. Like SPANXD, the RBMY1A1 transcript is lost in both metastatic and non-metastatic EC, as measured by RT-qPCR (Figure 2). As the result obtained from RT-qPCR represents the average mRNA level in the whole testis, we asked whether RBMY1A1 is restricted to germ cells. Immunohistochemistry (IHC) data from Protein Atlas indicated strong expression of RBMY1A1 protein in spermatogonia, spermatocytes and spermatids, but not in Sertoli cells or Leydig cells of somatic origin, confirming the germ cell specificity of this gene (Figure 3A and D). Antibody detected very few cells expressing RBMY1A1 in ECs and seminomas (Figure 3B, C, E and F). The IHC data confirmed the downregulation of RBMY1A1 in ECs and seminomas (Figure 3; Uhlen et al, 2005). As the majority of type-2 TGCTs originate from germ cells, it suggests that epigenetic silencing of sex-linked genes might have a crucial role in suppressing tumour initiation or its progression. The identification of DNA methylation of these sex-linked genes suggests that epigenetic alterations associated with testicular transformation may involve genes critical for germ cell development.
Figure 3.
Immunohistochemistry of RBMY1A on testicular tissue, EC and seminoma. RBMY1A protein is detected by a rabbit polyclonal antibody (Sigma-Aldrich, HPA001534). (A) Non-cancerous testicular tubules. (B) EC. (C) Seminoma. (D–F) Magnified view from A–C. Pictures were adopted from The Human Protein Atlas (http://www.proteinatlas.org). Scale bar, 100 μm.
Downregulation of USP13 in EC
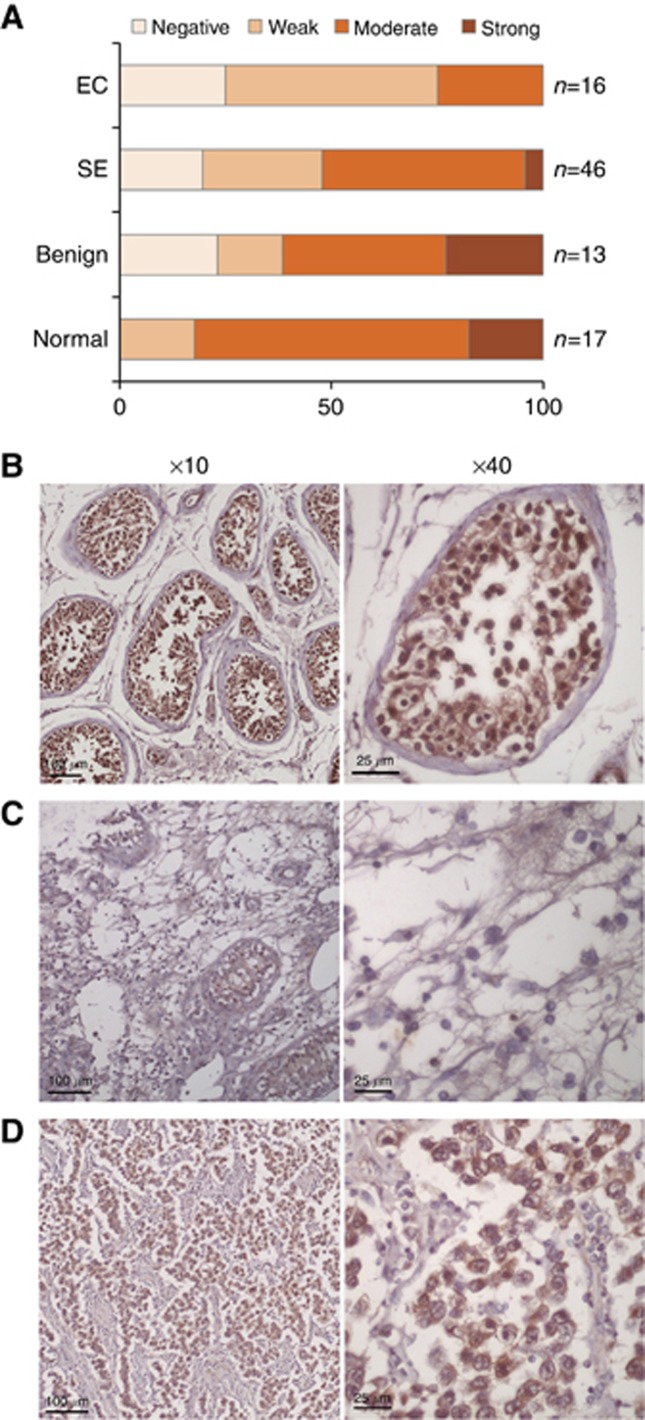
USP13 is another downregulated candidate gene as confirmed by RT-qPCR. Unlike RBMY1A, USP13 is not testis specific. It may act as a tumour suppressor by stabilizing PTEN (Zhang et al, 2013). To further confirm our result in an independent cohort of TGCT patients, we performed IHC using tissue microarray. The array covers 46 seminomas, 8 yolk sac tumours, 16 ECs, 13 benign tumours (mature teratoma, tuberculosis and atrophy), 13 adjacent normal tissues and 4 normal tissues (Table 3). Each case is duplicated. To increase statistical power, we grouped the adjacent normal tissues (n=13) and normal tissues (n=4) together. The result indicated that USP13 was expressed in normal testis including germ cells, Sertoli cells and Leydig cells (Figure 4B). On the basis of the staining intensity, 82.4% of normal cases (14 out of 17) expressed moderate to strong USP13 protein, compared with only 25% of ECs (4 out of 16) stained at a moderate level (P=0.004, Fisher's exact test; Figure 4A–C). For seminoma, USP13 expression was also downregulated but to a less extent than ECs—52% cases of seminomas were stained at moderate or strong level (P=0.052, Fisher's exact test). Interestingly, we observed a subpopulation of cells in seminoma stained with USP13 at moderate to strong level (Figure 4D). The present data cannot distinguish any pathological difference between these USP13-strong and USP13-weak carcinomas. In conclusion, by our genomic approach, we identified several epigenetically changed genes and these were confirmed by expression in ECs.
Table 3. Expression of USP13 in TGCTs.
| Negative | Weak | Moderate | Strong | n | aP-value | |
|---|---|---|---|---|---|---|
| Normal | 0 | 3 | 11 | 3 | 17 | |
| Benign | 3 | 2 | 5 | 3 | 13 | 0.223 |
| SE | 9 | 13 | 22 | 2 | 46 | 0.052 |
| YST | 3 | 2 | 3 | 0 | 8 | 0.038 |
| EC | 4 | 8 | 4 | 0 | 16 | 0.004 |
Abbreviations: EC=embryonal carcinoma; SE=seminoma; TGCS=testicular germ cell tumour.
P-value is calculated by Fisher's exact test by comparing each group with the normal.
Figure 4.
Immunohistochemistry of USP13 on testicular tissue, EC and seminoma. (A) Expression of USP13 protein in different subtypes of TGCT. Representative pictures of USP13 staining were shown in (B) for normal testis, (C) for EC and (D) for seminoma. EC=embryonal carcinoma; SE=seminoma. Scale bar, 100 μm (× 10) or 25 μm (× 40).
Discussion
In this report, we identified 40 hypermethylated genes in EC through DNA methylation profiling. Many of the genes (27 out of 40) are related to cancer biology. Notably, one-forth are metabolism-related genes including the TCA cycle. The data suggest that an epigenetic mechanism leads to altered metabolic pathways associated with TGCT.
We also identified new candidate genes that were not previously linked to TGCT. For instance, RNF168 is an E3 ubiquitin ligase involved in repair of DNA double-strand break. Mutation of this gene gives rise to Riddle syndrome (Stewart et al, 2009). In a mouse model of Riddle syndrome, Rnf168 deficiency causes defective spermatogenesis and increased cancer susceptibility due to genomic instability and immunodeficiency. Such data support Rnf168 as a tumour suppressor by cooperation with p53 (Bohgaki et al, 2011). In EC, diminished expression of RNF168 is concordant with hypermethylation. It is possible that impaired spermatogenesis may be accompanied with testicular germ cell tumorigenesis. Genes that govern tumour suppression could be regulated by epigenetic changes. From this study, we also found another gene, USP13 to be epigenetically changed. USP13 is a newly identified tumour suppressor protein that functions through deubiquitylation and stabilisation of PTEN protein (Zhang et al, 2013). USP13 expression was found significantly decreased in ECs, but to a less extent in seminomas. We also observed a subpopulation of seminoma expressed USP13. As seminoma is relatively pure, we do not know the function of USP13 in seminoma. The role of USP13 in suppression of TGCT remains to be determined. It may add an avenue to the well-established role of PTEN in the transition of benign carcinoma in situ (CIS) to invasive TGCT (Kimura et al, 2003; Di Vizio et al, 2005; Hennenlotter et al, 2011).
Sex-linked genes encoded in X- or Y-chromosome have been known to regulate sex determination and inheritance of sex-linked traits. Some directly govern spermatogenesis and male infertility, such as genes located within the azoospermia factor region in Y-chromosome (Sadeghi-Nejad and Farrokhi, 2007). From this study, we identified several hypermethylated sex-linked genes. These include three X-linked genes/ncRNA (STAG2, SPANXD/E and MIR1184-1/2/3) and two Y-linked genes (RBMY1A1/1B/1D and FAM197Y2P). One of the X-linked genes, SPANXD/E, belongs to the cancer/testis-associated SPANX gene family. Together with other testis-specific genes, they are required for initiating the molecular and morphological changes in male germ cells necessary for the development of mature spermatozoa (Westbrook et al, 2006). SPANXD/E encodes sperm proteins localised to the nucleus with a suggested role in spermatogenesis. However, the specific functions have not been well understood, although it is reported to be silenced in tumours (Zendman et al, 2003). Testis-specific, Y-linked genes have been proposed to have a pivotal role in the pathogenesis of TGCT, for instance, the TSPY gene (Li et al, 2007a, 2007b; Akimoto et al, 2010). One of the Y-linked genes, RBMY1A, has been reported to be regulated by DNA methylation in urological prostate cancer (Dasari et al, 2002). RBMY1A encodes a testis-specific RNA-binding protein with unknown function in spermatogenesis and TGCT (Zeng et al, 2011; Alikhani et al, 2013). From the Protein Atlas, IHC analysis on normal testis, EC and seminoma tissues documented downregulation of this protein (Uhlen et al, 2005). Expression of RBMY1A is restricted to male germ cells, and disappears in ECs and seminomas. These data are consistent with those obtained in our qPCR analysis and implicate the regulatory role of methylation on RBMY1A, whereas the function of this protein and the potential utilisation as a marker in testicular tumorigenesis need to be further elucidated.
A limitation of the current study is the small number of pure EC samples available. Although mixed NSGCT represents 35–55% of all TGCTs, pure EC is relatively uncommon. A larger cohort of methylation profiling with bigger sample size should better define the critical genes dysregulated by epigenetic changes. Second, the difficulty to obtain fetal or even adult CIS impedes our ability to monitor the gene expression and epigenetic changes during the course of TGCT development. The spatiotemporal changes for the candidate genes identified through this study remain to be examined. Last, we did not observe a significant DNA methylation change for the three previously identified genes (APOLD1, PCDH10 and RGAG1; Cheung et al, 2010). One possible reason is the heterogeneity of TGCT among different patients. A second reason is the limitation of ChIP-based method in detecting DNA methylation especially for whole-genome profiling. Bisulfite sequencing-based method may improve the accuracy and resolution of 5mC epigenome. In summary, our genome-wide analysis identified methylation changes in several previously unknown genes for testicular ECs.
Acknowledgments
This work was supported by funds provided by the Chinese University of Hong Kong and the CUHK-Shandong University Joint Laboratory.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Akimoto C, Ueda T, Inoue K, Yamaoka I, Sakari M, Obara W, Fujioka T, Nagahara A, Nonomura N, Tsutsumi S, Aburatani H, Miki T, Matsumoto T, Kitagawa H, Kato S (2010) Testis-specific protein on Y chromosome (TSPY) represses the activity of the androgen receptor in androgen-dependent testicular germ-cell tumors. Proc Natl Acad Sci USA 107(46): 19891–19896. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Alikhani M, Sharifi Tabar M, Mirshahvaladi S, Kheimeh A, Sadighi Gilani MA, Sabbaghian M (2013) Expression analysis of RNA-binding motif gene on Y chromosome (RBMY) protein isoforms in testis tissue and a testicular germ cell cancer-derived cell line (NT2). Iran Biomed J 17(2): 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almstrup K, Nielsen JE, Mlynarska O, Jansen MT, Jorgensen A, Skakkebaek NE, Rajpert-De Meyts E (2010) Carcinoma in situ testis displays permissive chromatin modifications similar to immature foetal germ cells. Br J Cancer 103(8): 1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami A, Ro JY, Ayala AG (2007) An overview of testicular germ cell tumors. Arch Pathol Lab Med 131(8): 1267–1280. [DOI] [PubMed] [Google Scholar]
- Bohgaki T, Bohgaki M, Cardoso R, Panier S, Zeegers D, Li L, Stewart GS, Sanchez O, Hande MP, Durocher D, Hakem A, Hakem R (2011) Genomic instability, defective spermatogenesis, immunodeficiency, and cancer in a mouse model of the RIDDLE syndrome. PLoS Genet 7(4): e1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BF, Gu S, Suen YK, Li L, Chan WY (2014) microRNA-199a-3p, DNMT3A, and aberrant DNA methylation in testicular cancer. Epigenetics 9(1): 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung HH, Claus J, Singh S, Sastry C, Rennert OM, Chan WY, Lee TL (2013) Mapping genomic features of tiling microarray data by TileMapper. Methods Mol Biol 1067: 225–233. [DOI] [PubMed] [Google Scholar]
- Cheung HH, Lee TL, Davis AJ, Taft DH, Rennert OM, Chan WY (2010) Genome-wide DNA methylation profiling reveals novel epigenetically regulated genes and non-coding RNAs in human testicular cancer. Br J Cancer 102(2): 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crundwell M (2004) Pathology and genetics of tumours of the urinary system and male genital organs. In BJU International, JN Elbe, GS, JI Epstein, IA Sesterheim, (eds) Vol. 94, pp 675. Oxford University Press: Oxford, UK. [Google Scholar]
- Dasari VK, Deng D, Perinchery G, Yeh CC, Dahiya R (2002) DNA methylation regulates the expression of Y chromosome specific genes in prostate cancer. J Urol 167(1): 335–338. [PubMed] [Google Scholar]
- Daugaard G, Karas V, Sommer P (2006) Inguinal metastases from testicular cancer. BJU Int 97(4): 724–726. [DOI] [PubMed] [Google Scholar]
- Di Vizio D, Cito L, Boccia A, Chieffi P, Insabato L, Pettinato G, Motti ML, Schepis F, D'Amico W, Fabiani F, Tavernise B, Venuta S, Fusco A, Viglietto G (2005) Loss of the tumor suppressor gene PTEN marks the transition from intratubular germ cell neoplasias (ITGCN) to invasive germ cell tumors. Oncogene 24(11): 1882–1894. [DOI] [PubMed] [Google Scholar]
- Dieckmann KP, Rube C, Henke RP (1997) Association of Down's syndrome and testicular cancer. J Urol 157(5): 1701–1704. [PubMed] [Google Scholar]
- Gremel G, Bergman J, Djureinovic D, Edqvist PH, Maindad V, Bharambe BM, Khan WA, Navani S, Elebro J, Jirstrom K, Hellberg D, Uhlen M, Micke P, Ponten F (2014) A systematic analysis of commonly used antibodies in cancer diagnostics. Histopathology 64(2): 293–305. [DOI] [PubMed] [Google Scholar]
- Hamid AR, Umbas R (2009) Metastasis of testicular carcinoma in the inguinal region. Acta Med Indones 41(1): 25–29. [PubMed] [Google Scholar]
- Hatada I, Morita S, Kimura M, Horii T, Yamashita R, Nakai K (2008) Genome-wide demethylation during neural differentiation of P19 embryonal carcinoma cells. J Hum Genet 53(2): 185–191. [DOI] [PubMed] [Google Scholar]
- Hennenlotter J, Amend B, Vogel U, Renninger M, Springer C, Kuehs U, Stenzl A, Bedke J (2011) Differential Akt signalling in non-seminomatous testicular germ cell tumors. Anticancer Res 31(11): 3783–3788. [PubMed] [Google Scholar]
- Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L (2004) OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol 28(7): 935–940. [DOI] [PubMed] [Google Scholar]
- Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T (2003) Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development 130(8): 1691–1700. [DOI] [PubMed] [Google Scholar]
- Kouprina N, Noskov VN, Solomon G, Otstot J, Isaacs W, Xu J, Schleutker J, Larionov V (2007) Mutational analysis of SPANX genes in families with X-linked prostate cancer. Prostate 67(8): 820–828. [DOI] [PubMed] [Google Scholar]
- Kristensen DG, Skakkebaek NE, Rajpert-De Meyts E, Almstrup K (2013) Epigenetic features of testicular germ cell tumours in relation to epigenetic characteristics of foetal germ cells. Int J Dev Biol 57(2-4): 309–317. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Sonne SB, Ottesen AM, Perrett RM, Nielsen JE, Almstrup K, Skakkebaek NE, Leffers H, Rajpert-De Meyts E (2008) Origin of pluripotent germ cell tumours: the role of microenvironment during embryonic development. Mol Cell Endocrinol 288(1-2): 111–118. [DOI] [PubMed] [Google Scholar]
- Li Y, Tabatabai ZL, Lee TL, Hatakeyama S, Ohyama C, Chan WY, Looijenga LH, Lau YF (2007. a) The Y-encoded TSPY protein: a significant marker potentially plays a role in the pathogenesis of testicular germ cell tumors. Hum Pathol 38(10): 1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Vilain E, Conte F, Rajpert-De Meyts E, Lau YF (2007. b) Testis-specific protein Y-encoded gene is expressed in early and late stages of gonadoblastoma and testicular carcinoma in situ. Urol Oncol 25(2): 141–146. [DOI] [PubMed] [Google Scholar]
- Lip SZ, Murchison LE, Cullis PS, Govan L, Carachi R (2013) A meta-analysis of the risk of boys with isolated cryptorchidism developing testicular cancer in later life. Arch Dis Child 98(1): 20–26. [DOI] [PubMed] [Google Scholar]
- Looijenga LH (2014) Testicular germ cell tumors. Pediatr Endocrinol Rev 11 Suppl 2: 251–262. [PubMed] [Google Scholar]
- McIver SC, Roman SD, Nixon B, Loveland KL, McLaughlin EA (2013) The rise of testicular germ cell tumours: the search for causes, risk factors and novel therapeutic targets. F1000Res 2: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabello L, Kratz CP, Savage SA, Greene MH (2012) Promoter methylation of candidate genes associated with familial testicular cancer. Int J Mol Epidemiol Genet 3(3): 213–227. [PMC free article] [PubMed] [Google Scholar]
- Nordsborg RB, Meliker JR, Wohlfahrt J, Melbye M, Raaschou-Nielsen O (2011) Cancer in first-degree relatives and risk of testicular cancer in Denmark. Int J Cancer 129(10): 2485–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapley EA, Crockford GP, Teare D, Biggs P, Seal S, Barfoot R, Edwards S, Hamoudi R, Heimdal K, Fossa SD, Tucker K, Donald J, Collins F, Friedlander M, Hogg D, Goss P, Heidenreich A, Ormiston W, Daly PA, Forman D, Oliver TD, Leahy M, Huddart R, Cooper CS, Bodmer JG, Easton DF, Stratton MR, Bishop DT (2000) Localization to Xq27 of a susceptibility gene for testicular germ-cell tumours. Nat Genet 24(2): 197–200. [DOI] [PubMed] [Google Scholar]
- Sadeghi-Nejad H, Farrokhi F (2007) Genetics of azoospermia: current knowledge, clinical implications, and future directions. Part II: Y chromosome microdeletions. Urol J 4(4): 192–206. [PubMed] [Google Scholar]
- Smiraglia DJ, Szymanska J, Kraggerud SM, Lothe RA, Peltomaki P, Plass C (2002) Distinct epigenetic phenotypes in seminomatous and nonseminomatous testicular germ cell tumors. Oncogene 21(24): 3909–3916. [DOI] [PubMed] [Google Scholar]
- Stewart GS, Panier S, Townsend K, Al-Hakim AK, Kolas NK, Miller ES, Nakada S, Ylanko J, Olivarius S, Mendez M, Oldreive C, Wildenhain J, Tagliaferro A, Pelletier L, Taubenheim N, Durandy A, Byrd PJ, Stankovic T, Taylor AM, Durocher D (2009) The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell 136(3): 420–434. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Schoemaker MJ, Higgins CD, Wright AF, Jacobs PA (2005) Cancer incidence and mortality in men with Klinefelter syndrome: a cohort study. J Natl Cancer Inst 97(16): 1204–1210. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Bjorling E, Agaton C, Szigyarto CA, Amini B, Andersen E, Andersson AC, Angelidou P, Asplund A, Asplund C, Berglund L, Bergstrom K, Brumer H, Cerjan D, Ekstrom M, Elobeid A, Eriksson C, Fagerberg L, Falk R, Fall J, Forsberg M, Bjorklund MG, Gumbel K, Halimi A, Hallin I, Hamsten C, Hansson M, Hedhammar M, Hercules G, Kampf C, Larsson K, Lindskog M, Lodewyckx W, Lund J, Lundeberg J, Magnusson K, Malm E, Nilsson P, Odling J, Oksvold P, Olsson I, Oster E, Ottosson J, Paavilainen L, Persson A, Rimini R, Rockberg J, Runeson M, Sivertsson A, Skollermo A, Steen J, Stenvall M, Sterky F, Stromberg S, Sundberg M, Tegel H, Tourle S, Wahlund E, Walden A, Wan J, Wernerus H, Westberg J, Wester K, Wrethagen U, Xu LL, Hober S, Ponten F (2005) A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics 4(12): 1920–1932. [DOI] [PubMed] [Google Scholar]
- Westbrook VA, Schoppee PD, Vanage GR, Klotz KL, Diekman AB, Flickinger CJ, Coppola MA, Herr JC (2006) Hominoid-specific SPANXA/D genes demonstrate differential expression in individuals and protein localization to a distinct nuclear envelope domain during spermatid morphogenesis. Mol Hum Reprod 12(11): 703–716. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Cheng T, Zhang J, Trpkov K (2013) Testicular hilum and vascular invasion predict advanced clinical stage in nonseminomatous germ cell tumors. Mod Pathol 26(4): 579–586. [DOI] [PubMed] [Google Scholar]
- Zendman AJ, Zschocke J, van Kraats AA, de Wit NJ, Kurpisz M, Weidle UH, Ruiter DJ, Weiss EH, van Muijen GN (2003) The human SPANX multigene family: genomic organization, alignment and expression in male germ cells and tumor cell lines. Gene 309(2): 125–133. [DOI] [PubMed] [Google Scholar]
- Zeng M, Liang S, Zhao S, Liu Y, Sun H, Zhang S, Ma Y (2011) Identifying mRNAs bound by human RBMY protein in the testis. J Reprod Dev 57(1): 107–112. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang P, Wei Y, Piao HL, Wang W, Maddika S, Wang M, Chen D, Sun Y, Hung MC, Chen J, Ma L (2013) Deubiquitylation and stabilization of PTEN by USP13. Nat Cell Biol 15(12): 1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.