Abstract
Background:
Over the last decade, the approach to the management of brain tumours and the understanding of glioblastoma tumour biology has advanced and a number of therapeutic interventions have evolved, some of which have shown statistically significant effects on overall survival (OS) and progression-free survival in glioblastoma. The aim of this study is to compare survival in glioblastoma patients over a 10-year period (1999–2000 and 2009–2010).
Methods:
A retrospective cohort study was performed. Identification of all histologically confirmed glioblastoma in a single centre in years 1999, 2000, 2009 and 2010, and production of survival analysis comparing 1999–2000 and 2009–2010 were achieved.
Results:
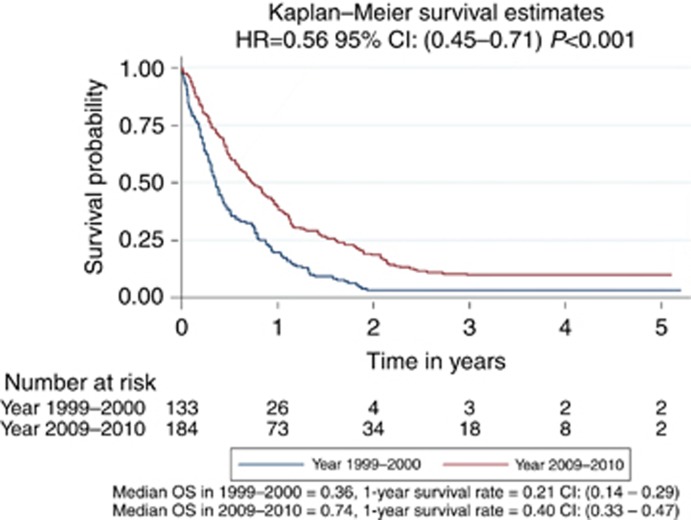
A total of 317 patients were included in the analysis (133 in year 1999–2000, and 184 in year 2009–2010). Cox regression analysis showed that the survival was significantly longer in patients in years 2009–2010 than those in 1999–2000 at P<0.001 with HR=0.56, confidence interval (CI) (0.45–0.71). The 1- and 3-year survival rates were 20.7% and 4.4%, respectively, for patients in 1999–2000, improving to 40.0% and 10.3%, respectively, for patients in 2009–2010. The comparisons between the two groups in survival at 1, 2 and 3 years are all statistically significant at P<0.001, respectively. The median OS was 0.36 and 0.74 in 1999–2000 and 2009–2010 groups, respectively.
Conclusions:
Over this period, OS from glioblastoma has increased significantly in our unit. We believe this is due to the institution of evidence-based surgical and oncological strategies practised in a multidisciplinary setting.
Keywords: glioblastoma, survival, time, neuro-oncology
The prognosis of glioblastoma is one of the most dismal of all cancers and survival is typically reported as less than a year after diagnosis. Over the last decade, the overall approach to the management of glioblastoma has evolved into a formal multidisciplinary structure that involves neurosurgeons, neurologists, neuro-oncologists, neuropathologists, neuroradiologists, palliative care physicians, specialist nurses and therapists. This aims to provide timely, tailored and evidence-based treatment for each patient, as well as awareness of potential for participation in clinical trials. In addition, the understanding of glioblastoma tumour biology and treatment strategies has evolved, with some therapies leading to increased survival. Therapeutic advances and prognostic information is however tempered by the knowledge that glioblastoma is a tumour with considerable molecular, immunohistochemical and genetic heterogeneity, where no ‘final common pathway' can yet be exploited for therapeutic purposes (Bonavia et al, 2011). The aim of this study is to evaluate overall survival (OS) from glioblastoma in a single centre from 1999–2000 compared with a decade later, 2009–2010. We are not aiming to report the effect of individual patient, tumour or treatment-related factors on survival as we believe any such analysis will require a multicentre approach to sufficiently power the study.
Materials and methods
We identified all glioblastomas diagnosed at our centre in the years 1999, 2000, 2009 and 2010 from neuropathology central records' database. We then identified the date of death for these individuals from hospital and general practice records. The survival time was calculated from the date of histological diagnosis to the date of death or the date of the last clinic visit for those patients still alive at the time of data collection. We constructed a Kaplan–Meier survival curve for the 1999–2000 cohort and a curve for the 2009–2010 cohort. The reason for choosing this time interval was to allow studying the many changes that had occurred during this time both in terms of delivery of neuro-oncology care and advances in clinical management of patients with glioblastoma (Allahdini et al, 2010). The year 2010 was selected as the final year of data collection to maximise the chance of obtaining complete survival data. This study was approved by the King's College Hospital NHS trust audit board.
Results
There were 500 new referrals to the neuro-oncology multidisciplinary team (MDT) for all brain tumours (excluding skull base and pituitary) in 2009 and 747 new referrals in 2010. An audit of neuro-oncology MDT referrals from 2008 and 2009 demonstrated that just under half of all referrals go on to have diagnostic and therapeutic interventions with the rest deemed palliative at presentation or not requiring treatment. In 1999 and 2000, there was no MDT format and no record of neuro-oncology referrals was therefore kept. The cases then were dealt with by the individual consultant they were referred to with no central register. There were a total of three patients with no data available on survival (two patients in year 1999–2000 and one patient in year 2009–2010). After excluding these three patients, there were a total of 317 patients remaining in the survival analysis (133 in year 1999–2000 and 184 in year 2009–2010, demographics in Table 1). Cox regression analysis (Figure 1) showed that the survival was significantly longer in patients in year 2009–2010 than those in 1999–2000 at P<0.001 with hazard ratio (HR)=0.56, confidence interval (CI; 0.45–0.71). Of particular interest, the ‘longer survival group' was significantly larger in the 2009–2010 cohort compared with the 1999–2000 cohort. Thus 1- and 3-year survival rates were 20.7% and 4.4%, respectively, for patients in 1999–2000, improving to 40.0% and 10.3%, respectively, for patients in 2009–2010. The comparisons between the two groups in survival at 1, 2 and 3 years are all statistically significant at P<0.001, respectively. The median OS was 0.36 and 0.74 in 1999–2000 and 2009–2010 groups, respectively. Comparing 1999 with 2000, HR was 1.00 with 95% CI (0.71–1.41) at P=0.986. Comparing 2009 with 2010, HR was 0.91 with 95% CI (0.67–1.22) at P=0.522. The data therefore suggest a gradual trend towards better survival, rather than a sudden change, over the years. In 2009, 1 patient had carmustine wafers (Gliadel, Archimedes Pharma, UK) and none had a 5-aminolevulinic acid (5-ALA)-assisted resection. In 2010, 7 had carmustine wafers (10% of resections) and 27 (39% of resections) had a 5-ALA-assisted procedures. The Stupp protocol was not practiced in 1999–2000 period. In 2009–2010 cohorts, 48% of patients were treated with the Stupp protocol.
Table 1. Demographics of glioblastoma cohort diagnosed in 2000 and 2010.
| 1999–2000 group | 2009–2010 group | |
|---|---|---|
| Total | 135 | 185 |
| Gender | 52 (38.5%) female | 69 (36.5%) female |
| 83 (61.5%) male | 120 (63.5%) male | |
| M : F=1.6 : 1 | M : F=1.7 : 1 | |
| Mean age | 61 | 59 |
| Tumour location | 10 deep seated, 125 lobar | 8 deep seated, 177 lobar |
Figure 1.
Cox regression analysis of survival in glioblastoma patients diagnosed in 1999–2000 compared with patients diagnosed in 2009–2010.
Discussion
The key finding from our study is that OS from glioblastoma has increased significantly between 1999–2000 and 2009–2010. In particular, the percentage of patients reaching the ‘longer survivor' end of the curve has increased most markedly.
The last decade has seen major changes in the management of patients with glioblastoma in the United Kingdom. In our unit in line with many others, we have introduced the use of concomitant temozolamide chemoradiation, intraoperative 5-ALA, carmustine wafers (Gliadel), advanced structural and metabolic imaging and molecular neuropathology. Of equal relevance, the initial assessment, management and follow-up of our patients are now performed in a specialist neuro-oncology MDT environment.
The MDT approach has become central to the practice of neuro-oncology since the 2006 National Institute for Clinical Excellence (NICE) guidance on formal referral of all brain tumour patients to a dedicated neuro-oncology MDT (NICE, 2006). This removes the vast majority of neuro-oncology patients from emergency ‘on-call' decision-making processes, allows the case to be managed electively, ensures the patients receive the most appropriate treatment for their tumour, meet national cancer targets, have access to clinical trials and are optimised peri-operatively to maximise performance status. The MDT process has reduced hospital stay and costs (Guilfoyle et al, 2011) without incurring delay in time to surgery (Rittman et al, 2012). In our unit, in addition to the weekly MDT where new referrals and post-treatment patients are discussed, all patients needing treatment are also seen in a specialist MDT clinic involving neurosurgeons, neuroradiologists, neuropathologists, oncologists, neurologists, therapists, specialist nurses and research staff. Involvement in a trial is a factor that is alone associated with improved survival as it is likely to reflect the aggressive approach to glioblastoma taken in that unit (Field et al, 2013).
In the recent times, there has also been a change in terms of surgical management philosophy for patients with glioblastoma, with a move away from limited surgery or a biopsy towards maximal resection where safe and possible. Trials showing that maximal resection is associated with improved survival are not level 1 evidence as the majority are retrospective. However, the studies that are available, do suggest that maximal resection is associated with improved survival (McGirt et al, 2009; Allahdini et al, 2010; Sanai et al, 2011). Maximal resection is also important as it is cytoreductive and has been shown to facilitate delivery of adjuvant therapy (Ng et al, 2007); remove the hypoxic, therapy-resistant tumour core; sensitise cells to chemotherapy by pushing them into the G1 and G2 checkpoints of the cell cycle; obtain large specimen to reduce sampling error for neuropathology; and reduce the need for steroids in patients. Recent American Association of Neurological Surgeons level 2 guidelines recommend that patients with recurrent malignant glioma who have had previous surgical resection are considered for repeat cytoreductive surgery, taking into account performance status, tumour location and size (Ryken et al, 2014). We have been routinely practising this in our unit in the recent years.
In patients for whom only a biopsy is possible, surgical and radiological techniques to obtain representative tumour samples are crucial given the intra-tumoural heterogeneity at the cellular, genetic and molecular levels (Bonavia et al, 2011). The portions demonstrating contrast enhancement on magnetic resonance imaging (MRI) studies are often chosen for sampling as they are thought to represent the most aggressive part of the tumour, ultimately defining the prognosis (Roberts et al, 2011). In tumours with no contrast enhancement, advanced imaging techniques such as perfusion MRI imaging and positron-emission tomography imaging may help choosing a target for biopsy (Pirotte et al, 2009; Floeth et al, 2011). Newer therapies, especially those used in the clinical trial setting, including vaccines and anti-angiogenic agents may alter MRI findings and enhancement patterns, making correlation with other forms of imaging more useful (Huang et al, 2015).
Where possible, in our unit maximal resection is aimed for. Intraoperative image guidance and 5-ALA (Gliolan, Medac, Germany)-assisted surgery facilitates this. 5-Aminolevulinic acid has been shown to maximise surgical resection and prolong progression-free survival by 6 months in patients with malignant glioma (Stummer et al, 2006). Aggressive resections with 5-ALA do risk temporary neurological deficit but are overall safe (Stummer et al, 2011). In our unit we introduced the use of 5-ALA-assisted surgery in 2010, using the technique in 27 (39%) of resections performed that year. Since, the use has increased further, currently practiced in over 60% of resections. The interim results of an ongoing randomised trial on intraoperative MRI in glioblastoma resection do not show any advantage vs conventional neuronavigation although more studies are needed (Kubben et al, 2014). Preservation of eloquent white matter tracts by use of intraoperative diffusion tensor imaging has shown some clinical benefits in preventing deficits (Vassal et al, 2013).
The delivery of the adjuvant therapy has also changed in the past decade. The European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada (EORTC–NCIC) trial followed 573 patients with glioblastoma over 5 years after randomisation to temozolamide (an alkylating agent) and radiotherapy or to radiotherapy alone (Stupp et al, 2009). The addition of temozolamide generated a significant survival advantage at 2 years and also at 5-year follow-up (Stupp et al, 2005, 2009), including in patients up to 70 years (Stupp et al, 2009). We use temozolamide and radiotherapy on all patients who fit the Stupp criteria, even if 90% tumour resection was not possible. In 2009–2010 period, 48% of our patients received the Stupp protocol treatment. The effectiveness of temozolamide is more pronounced in patients who demonstrate silencing of the O6-methylguanin-DNA-methyltransferase (MGMT) repair gene via promoter region methylation (Hegi et al, 2005; Malmström et al, 2012). Dose-dense temozolamide has shown no OS benefit in newly diagnosed glioblastoma (Gilbert et al, 2013). Its role in recurrent glioblastoma remains under investigation (Norden et al, 2013).
Local chemotherapy has been investigated. Intraoperative carmustine wafers (Gliadel) implanted into the tumour bed followed by radiotherapy (vs surgery and radiotherapy) have demonstrated a survival benefit in a multicentre trial of 207 patients (Westphal et al, 2006). A NICE technology appraisal in 2007 advocated the use of carmustine wafers and adjuvant temozolamide (NICE, 2007), although no comment on combining the two was made. The 2007 NICE technology appraisal recommends that temozolamide is suitable for patients with newly diagnosed glioblastoma, selected by a specialist neuro-oncology MDT, with performance status 0 or 1, over 90% of the tumour resected using neuronavigation and the tumour type verified by intraoperative neuropathology (NICE, 2007). Gliadel has been reported in a Cochrane review of three randomised trials to increase OS but not progression-free survival in glioblastoma (Hart et al, 2011). The potential side effects of Gliadel have been a concern in the neuro-oncological community. A phase 3 trial of Gliadel vs placebo in 240 patients with high-grade glioma reported a 5% incidence of CSF leak (0.8% with control) and a 9.1% incidence of intracranial hypertension (1.7% in control; Westphal et al, 2003). We introduced the use of Gliadel into our unit in 2009 and by 2010, 10% of patients undergoing resection were receiving Gliadel. During our study period, we did not encounter any significant adverse effects from the use of Gliadel such as brain swelling and infection. Of note, we used this drug in accordance with the NICE guidelines, for first operations in patients with more than 90% tumour resected. We stress meticulous dural and wound closure, 5 days of antibiotics and 2 weeks of high-dose dexamethasone. Of particular interest, there were no attributable complications in patients receiving both Gliadel and temozolamide chemotherapy. In line with our experience, a review of 19 retrospective and prospective studies combining carmustine wafers and temozolamide showed an improvement in survival, and no major safety concerns (Gutenberg et al, 2013).
In addition to therapeutic interventions, a greater understanding of glioblastoma tumour biology has aided in prognostication. Key markers are isocitrate dehydrogenase (IDH) 1 and 2, 1p19q codeletion, MGMT and epidermal growth factor receptor (EGFR) gene amplification. In our unit over the years, we have introduced analysis of gliomas for IDH1 and 2, 1p19q codeletion, MGMT and EGFR amplification. IDH mutations are found in low-grade glioma and secondary glioblastoma, and are rare in primary glioblastoma (Yan et al, 2009). IDH 1 and 2 mutations are associated with better prognosis than wild-type IDH (Sanson et al, 2009). MGMT promoter methylation has been shown to predict a favourable response to alkylating agents (Hegi et al, 2005; Esteller et al, 2000). The 1p19q codeletion in gliomas has been found to be associated with a favourable response to treatment (Wick et al, 2009). Amplification of EGFR is associated with increased invasiveness in glioblastoma (Friedman and Bigner, 2005). The interactions between cell-signalling pathways, molecular markers and the effect of therapy on each are myriad, complex and evolving (Weller et al, 2012).
Whilst the combined effect of these strategies has been to improve OS, the prognosis still remains dismal for glioblastoma patients. In this study, by definition, all patients included were well enough to at least have had a biopsy to achieve histological diagnosis. Not all, however, would have completed adjuvant therapy. It is of interest to note that perhaps the greatest impact over the years has been on the patients in the more favourable end of the survival curve, predominantly those able to undergo and tolerate their therapy. Thus, the percentage of the patients living beyond 1 and 3 years is now significantly increasing whilst those with the most aggressive tumours continue to have very poor outcomes. Interestingly, similar increase in survival in high-grade glioma has also been reported in other recent studies, with patients over 60 having significant survival benefit (Asklund et al, 2013, 2015).
The challenge in the management of gliomas may reflect molecular heterogeneity and constant evolution of the tumour, making it difficult to discover and therapeutically target a consistently present, reliable final common pathway. Cellular, cytogenetic and molecular heterogeneity is not unique to glioblastoma but is especially pronounced in this tumour type, which may account for its resistance to standard therapies (Inda et al, 2010). No single mechanism for tumour heterogeneity has been identified. Possible mechanisms include clonal expansion of single cells, cancer stem cell subpopulations within the tumour and interactions between neighbouring cells (Nishikawa et al, 2004). To cope with this inter- and intra-tumour diversity new treatment strategies are needed. Immunotherapy has been considered as one potential avenue to follow given its high degree of specificity and adoptability. In line with this, in our unit we are currently recruiting for a phase 3 trial of dendritic cell immunotherapy vaccine for patients with newly diagnosed glioblastoma (Polyzoidis and Ashkan, 2014). The impact of this therapy on the survival of our patients will be of major interest to report in the future.
In conclusion, within the limitations of a retrospective study, we have demonstrated that OS of patients with glioblastoma in our unit has improved significantly over 10 years. We believe this to be due to advances in glioblastoma diagnostics and therapeutics. Key advances are molecular subtyping, tailored oncological treatments including Stupp regime and carmustine wafers, image-guided surgery and Gliolan-assisted resections. The application of these advances in an MDT setting facilitates organised delivery of care, adherence to national guidelines and access to clinical trials.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Allahdini F, Amirjamshidi A, Reza-Zarei M, Abdollahi M (2010) Evaluating the prognostic factors effective on the outcome of patients with glioblastoma multiformis: does maximal resection of the tumour lengthen the median survival? World Neurosurg 73(2): 128–134. [DOI] [PubMed] [Google Scholar]
- Asklund T, Malmström A, Björ O, Blomquist E, Henriksson R (2013) Considerable improvement in survival for patients aged 60-84 years with high grade malignant gliomas – data from the Swedish Brain Tumour Population-based Registry. Acta Oncol 52(5): 1041–1043. [DOI] [PubMed] [Google Scholar]
- Asklund T, Malmström A, Bergqvist M, Björ O, Henriksson R (2015) Brain tumors in Sweden: data from a population-based registry 1999-2012. Acta Oncol 54(3): 377–384. [DOI] [PubMed] [Google Scholar]
- Bonavia R, Inda MM, Cavenee WK, Furnari FB (2011) Heterogeneity maintenance in glioblastoma: a social network. Cancer Res 71(12): 4055–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG (2000) Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343: 1350–1354. [DOI] [PubMed] [Google Scholar]
- Field KM, Drummond KJ, Yilmaz M, Tacey M, Compston D, Gibbs P, Rosenthal MA (2013) Clinical trial participation and outcome for patients with glioblastoma: multivariate analysis from a comprehensive dataset. J Clin Neurosci 20(6): 783–789. [DOI] [PubMed] [Google Scholar]
- Floeth FW, Sabel M, Ewelt C, Stummer W, Felsberg J, Reifenberger G, Steiger HJ, Stoffels G, Coenen HH, Langen KJ (2011) Comparison of (18)F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur J Nucl Med Mol Imaging 38(4): 731–741. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Bigner DD (2005) Glioblastoma multiforme and the epidermal growth factor receptor. N Engl J Med 353: 1997–1999. [DOI] [PubMed] [Google Scholar]
- Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, Mahajan A, Schultz CJ, Erridge S, Baumert B, Hopkins KI, Tzuk-Shina T, Brown PD, Chakravarti A, Curran WJ Jr, Mehta MP (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31(32): 4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle MR, Weerakkody RA, Oswal A, Oberg I, Jeffery C, Haynes K, Kullar PJ, Greenberg D, Jefferies SJ, Harris F, Price SJ, Thomson S, Watts C (2011) Implementation of neuro-oncology service reconfiguration in accordance with NICE guidance provides enhanced clinical care for patients with glioblastoma multiforme. Br J Cancer 104(12): 1810–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutenberg A, Bock HC, Brück W, Doerner L, Mehdorn HM, Roggendorf W, Westphal M, Felsberg J, Reifenberger G, Giese A (2013) MGMT promoter methylation status and prognosis of patients with primary or recurrent glioblastoma treated with carmustine wafers. Br J Neurosurg 27(6): 772–778. [DOI] [PubMed] [Google Scholar]
- Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K (2011) Chemotherapy wafers for high grade glioma. Cochrane Database Syst Rev 16(3): CD007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and response to temozolomide in glioblastoma. N Engl J Med 352: 997–1003. [DOI] [PubMed] [Google Scholar]
- Huang RY, Neagu MR, Reardon DA, Wen PY (2015) Pitfalls in the neuroimaging of glioblastoma in the era of antiangiogenic and immuno/targeted therapy – detecting illusive disease, defining response. Front Neurol 6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MM, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S, Brennan C, Johns TG, Bachoo R, Hadwiger P, Tan P, Depinho RA, Cavenee W, Furnari F (2010) Tumour heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev 24(16): 1731–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubben PL, Scholtes F, Schijns OE, Ter Laak-Poort MP, Teernstra OP, Kessels AG, van Overbeeke JJ, Martin DH, van Santbrink H (2014) Intraoperative magnetic resonance imaging versus standard neuronavigation for the neurosurgical treatment of glioblastoma: a randomized controlled trial. Surg Neurol Int 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME, Rosell J, Henriksson R Nordic Clinical Brain Tumour Study Group (NCBTSG) (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy for patients aged over 60 years with glioblastoma: the Nordic randomized phase 3 trial. Lancet Oncol 13: 916–926. [DOI] [PubMed] [Google Scholar]
- McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, Quiñones-Hinojosa AR (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110(1): 156–162. [DOI] [PubMed] [Google Scholar]
- Ng WH, Wan GQ, Too HP (2007) Higher glioblastoma tumour burden reduces efficacy of chemotherapeutic agents: in vitro evidence. J Clin Neurosci 14(3): 261–266. [DOI] [PubMed] [Google Scholar]
- NICE (2006) Pathways: brain cancers overview. Availabe at http://pathways.nice.org.uk/pathways/brain-cancers/brain-cancers-overview.pdf accessed June 2015.
- NICE (2007) Technology appraisals [TA121]: carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma. Availabe at http://www.nice.org.uk/guidance/ta121/chapter/4-Evidence-and-interpretation accessed June 2015.
- Nishikawa R, Sugiyama T, Narita Y, Furnari F, Cavenee WK, Matsutani M (2004) Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumour Pathol 21(2): 53–56. [DOI] [PubMed] [Google Scholar]
- Norden AD, Lesser GJ, Drappatz J, Ligon KL, Hammond SN, Lee EQ, Reardon DR, Fadul CE, Plotkin SR, Batchelor TT, Zhu JJ, Beroukhim R, Muzikansky A, Doherty L, Lafrankie D, Smith K, Tafoya V, Lis R, Stack EC, Rosenfeld MR, Wen PY (2013) Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol 15(7): 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotte BJ, Levivier M, Goldman S, Massager N, Wikler D, Dewitte O, Bruneau M, Rorive S, David P, Brotchi J (2009) Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery 64(3): 471–481. [DOI] [PubMed] [Google Scholar]
- Polyzoidis S, Ashkan K (2014) Dendritic cell immunotherapy for glioblastoma. Expert Rev Anticancer Ther 14(7): 761–763. [DOI] [PubMed] [Google Scholar]
- Rittman T, Corns R, Kumar A, Bhangoo R, Ashkan K (2012) Is referral to the neuro-oncology MDT safe? Br J Neurosurg 26(3): 321–324. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Valdés PA, Harris BT, Fontaine KM, Hartov A, Fan X, Ji S, Lollis SS, Pogue BW, Leblond F, Tosteson TD, Wilson BC, Paulsen KD (2011) Coregistered fluorescence-enhanced tumour resection of malignant glioma: relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg 114(3): 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryken TC, Kalkanis SN, Buatti JM, Olson JJ AANS/CNS Joint Guidelines Committee (2014) The role of cytoreductive surgery in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol 118(3): 479–488. [DOI] [PubMed] [Google Scholar]
- Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115(1): 3–8. [DOI] [PubMed] [Google Scholar]
- Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27(25): 4150–4154. [DOI] [PubMed] [Google Scholar]
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ ALA-Glioma Study Group (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7(5): 392–401. [DOI] [PubMed] [Google Scholar]
- Stummer W, Tonn JC, Mehdorn HM, Nestler U, Franz K, Goetz C, Bink A, Pichlmeier U ALA-Glioma Study Group (2011) Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J Neurosurg 114(3): 613–623. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy GroupsNational Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10): 987–996. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology GroupsNational Cancer Institute of Canada Clinical Trials Group (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5): 459–466. [DOI] [PubMed] [Google Scholar]
- Vassal F, Schneider F, Sontheimer A, Lemaire JJ, Nuti C (2013) Intraoperative visualisation of language fascicles by diffusion tensor imaging-based tractography in glioma surgery. Acta Neurochir (Wien) 155(3): 437–448. [DOI] [PubMed] [Google Scholar]
- Weller M, Stupp R, Hegi ME, van den Bent M, Tonn JC, Sanson M, Wick W, Reifenberger G (2012) Personalized care in neuro-oncology coming of age: why we needMGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro Oncol 14(Suppl 4): iv100–iv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J, Ram Z (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 5(2): 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M, Ram Z, Riddle V, Hilt D, Bortey E Executive Committee of the Gliadel Study Group (2006) Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 148(3): 269–275. [DOI] [PubMed] [Google Scholar]
- Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel MC, Koeppen S, Ketter R, Meyermann R, Rapp M, Meisner C, Kortmann RD, Pietsch T, Wiestler OD, Ernemann U, Bamberg M, Reifenberger G, von Deimling A, Weller M (2009) NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. J Clin Oncol 27: 5874–5880. [DOI] [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360(8): 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]