Abstract
Background:
We sought to determine which parsimonious combination of complete blood count (CBC)-based biomarkers most efficiently predicts oncologic outcomes in patients undergoing radical cystectomy (RC) for bladder cancer (BC).
Methods:
Using our institutional RC database (1992–2012), nine CBC-based markers (including both absolute cell counts and ratios) were evaluated based on pre-treatment measurements. The outcome measures were recurrence-free survival (RFS), cancer-specific survival (CSS), and overall survival (OS). Time-dependent receiver-operating characteristics curves were used to characterise each biomarker. The CBC-based biomarkers, along with several clinical predictors, were then considered for inclusion in predictive multivariable Cox models based on the Akaike Information Criterion.
Results:
Our cohort included 418 patients. Neutrophil–lymphocyte ratio (NLR) was the only biomarker satisfying criteria for inclusion into all models, independently predicting RFS (HR per 1-log unit=1.52, 95% CI=1.17–1.98, P=0.002), CSS (HR=1.47, 95% CI=1.20–1.80, P<0.001), and OS (HR=1.56, 95% CI=1.16–2.10, P=0.004). Haemoglobin was also independently predictive of CSS (HR per 1 g/dl=0.91, 95% CI=0.86–0.95, P<0.001) and OS (HR=0.90, 95% CI=0.88–0.93, P<0.001), but not RFS.
Conclusions:
Among CBC biomarkers studied, NLR was the most efficient marker for predicting RFS, whereas NLR and haemoglobin were most efficient in predicting CSS and OS. NLR and haemoglobin are promising, cost-effective, independent biomarkers for predicting oncologic BC outcomes following RC.
Condensed abstract:
Various CBC-based biomarkers have separately been shown to be predictive of oncologic outcomes in patients undergoing cystectomy for BC. Our study evaluated these biomarkers, and determined that NLR is the best CBC-based biomarker for predicting RFS, whereas NLR and haemoglobin are most efficient for predicting CSS and OS.
Keywords: urinary bladder neoplasms, biological markers, prognosis, treatment outcome, cystectomy, inflammation
Radical cystectomy (RC) with pelvic lymphadenectomy remains the standard treatment for patients with localised muscle-invasive bladder cancer (MIBC) and for BCG-refractory non-muscle invasive bladder cancer (NMIBC; Clark et al, 2013). Unfortunately, 10-year cancer-specific survival (CSS) rates are only ∼70% (Cookson et al, 1997) and 40–60% (Stein et al, 2001; Shariat et al, 2006) for high-risk NMIBC and for MIBC, respectively. More aggressive approaches can be used to improve oncological outcomes, namely neo-adjuvant or adjuvant chemotherapy for patients with MIBC (Grossman et al, 2003), and early RC for patients with high-risk NMIBC (Kulkarni et al, 2010). However, the non-selective use of these approaches carries the risk of overtreatment, particularly among patients with favourable prognoses. Treatment decision-making is oftentimes challenging, particularly because risk stratification based on clinical and pathologic parameters is insufficient (Shariat et al, 2007; Canter et al, 2011).
Peripheral neutrophil–lymphocyte ratio (NLR) is a novel prognostic biomarker in bladder cancer (BC). Elevated NLR has been shown to be predictive of adverse oncologic outcomes in patients with NMIBC (Can et al, 2012; Kaynar et al, 2014; Mano et al, 2015) and MIBC (Gondo et al, 2012; Krane et al, 2013; Hermanns et al, 2014; Potretzke et al, 2014; Viers et al, 2014). Several other complete blood count (CBC)-based prognostic biomarkers have also been described for BC, including absolute platelet count (Can et al, 2012; Todenhofer et al, 2012; Moschini et al, 2014), haemoglobin, (Gondo et al, 2012; Gierth et al, 2015), and lymphocyte–monocyte ratio (LMR; Temraz et al, 2014). In addition, platelet–lymphocyte ratio (PLR) has also shown to predict oncologic outcomes for other cancer sites (Azab et al, 2013; Feng et al, 2014), but studies of its prognostic value in BC have yet to be reported.
There is likely some degree of overlap between these CBC-based biomarkers, and some may be redundant. For future studies and potential clinical use, a parsimonious combination of these biomarkers is required. Our objective was thus to determine which combination of these biomarkers most efficiently predicts oncologic outcomes in patients undergoing RC for BC.
Materials and Methods
Patients and data collection
We performed a retrospective cohort study of patients undergoing RC for urothelial carcinoma of the bladder identified using our institutional database (1992–2012), which has been described in detail in prior reports (Bhindi et al, 2014; Hermanns et al, 2014). Institutional research ethics approval was obtained. Clinical and pathologic data were obtained using chart review. Mortality data were obtained using the Princess Margaret Hospital Cancer Registry. Exclusion criteria were unavailable CBCs and differentials (n=20), history of conditions that could potentially affect blood cell lines (leukemia: n=2, human immunodeficiency virus infection: n=1, malignant lymphoma: n=3, connective tissue disease: n=4) and RC done for salvage following failed chemo-radiation (n=20).
Primary study exposure
The date of treatment initiation was defined as the date of RC or date of initiation of neo-adjuvant chemotherapy, as applicable. Complete blood count collections were performed usually a week before treatment initiation (median of 6 days, IQR=2–10 days) as part of the routine pre-treatment clinical assessment. Clinical notes were reviewed to rule out any symptoms or signs of infection around the time of CBCs.
Candidate biomarkers considered in our study included haemoglobin, individual cell counts (absolute neutrophil, lymphocyte, monocyte, and platelet counts), and the cell count ratios NLR, monocyte–lymphocyte ratio, LMR, and PLR.
Clinical follow-up and outcomes
Patients were generally seen in follow-up ∼6–8 weeks following discharge, and every 3–6 months thereafter for physical examination and imaging to assess for hydronephrosis and cancer recurrence. Visits were further spaced apart over time based on individual physician's practice patterns and clinical suspicion. The study outcomes were recurrence-free (RFS), CSS, and overall survival (OS) from time of treatment initiation. Patients were censored if event-free at their most recent clinic visit, up to 31 October 2013.
Statistical analysis
Descriptive statistics were used to summarise cohort characteristics and distributions of CBC-based predictors. Complete blood count-based predictors with skewed distributions were log-transformed to improve model fit.
An initial assessment of CBC-based predictors was performed using univariate time-dependent receiver operating characteristics (ROC) curves for each outcome measure at 12-month intervals for 5 years (Heagerty and Zheng, 2005). To avoid issues with multi-collinearity in ensuing analytic steps, similar predictors were compared and only those with superior areas under the ROC curves were retained for further evaluation.
For each of the retained predictors, Kaplan–Meier analyses with the log-rank test and univariate Cox regression analyses were performed in order to confirm the predictive value seen in other studies (Can et al, 2012; Gondo et al, 2012; Todenhofer et al, 2012; Azab et al, 2013; Krane et al, 2013; Feng et al, 2014; Hermanns et al, 2014; Moschini et al, 2014; Potretzke et al, 2014; Temraz et al, 2014; Viers et al, 2014; Gierth et al, 2015; Mano et al, 2015).
Next, we created multivariable Cox proportional hazards models considering all possible combinations of the retained CBC-based predictors and clinical parameters (age, gender, heavy smoking status defined as ⩾30 pack-years, Charlson co-morbidity index, hydronephrosis, concurrent carcinoma in situ, pathological T-stage, and pathological N-stage, lymphovascular invasion (LVI) and positive surgical margin status). A robust sandwich covariance matrix estimator was used to account for clustering of outcomes by surgeon (Lin and Wei, 1989). Optimal models were selected as those that minimised the Akaike Information Criterion (AIC), representing parsimonious models that offered the best fit to the data with the fewest number of predictors. The partial log-likelihood test was used to compare final predictive models with and without the selected CBC-based predictors to determine if these predictors significantly improved model fit.
Statistical analyses were performed using SAS v9.3 (SAS Institute Inc, Cary, NC, USA). All tests were two-sided with P-values <0.05 considered statistically significant.
Results
The final cohort included 418 patients with a median age of 70 years (IQR=61–76 years). Pathological extra-vesical extension was present in 191 patients (46%), and nodal metastases were present in 115 patients (27%). Cohort characteristics are further described in Table 1. During a median follow-up of 40 months (IQR=14–72 months), there were 136 cancer recurrences, 107 BC-related deaths, and 177 deaths overall.
Table 1. Cohort characteristics.
| Cohort characteristics | Total n=418 |
|---|---|
|
Patient characteristics | |
| Age in years, median (IQR) | 70 (61–76) |
| Female sex, n (%) | 96 (23.0) |
| Heavy smoking (⩾30pack-years), n (%) | 133 (32) |
| BMI (kg/m2), median (IQR) | 27 (24–30) |
| Charlson co-morbidity index, median (IQR) | 1 (0–2) |
|
Disease characteristics | |
| Hydronephrosis, n (%) | 117 (28) |
| Concurrent CIS, n (%) | 188 (45) |
| T-stage, n (%) | |
| pT0 | 25 (6) |
| pTa/Tis/T1/T2 | 202 (48) |
| pT3/T4 | 191 (46) |
| N-stage, n (%) | |
| pN0 | 282 (68) |
| pN+ | 115 (27) |
| pNx | 21 (5) |
| Lymphovascular invasion, n (%) | 141 (34) |
| Positive surgical margina, n (%) | 33 (8) |
|
Other treatment characteristics | |
| Year of surgery, n (%) | |
| 1992–2005 | 208 (50) |
| 2006–2012 | 210 (50) |
| Continent diversion, n (%) | 89 (21) |
| Node total, median (IQR) | 12 (7–19) |
| Neo-adjuvant chemotherapy, n (%) | 28 (7) |
| Adjuvant chemotherapy, n (%) | 87 (20) |
| Salvage chemotherapy, n (%) | 54 (13) |
|
CBC-based parameters | |
| Haemoglobin (g/dl), median (IQR) | 13.2 (11.6–14.5) |
| WBC (× 109/l), median (IQR) | 7.6 (6.0–9.1) |
| Neutrophils (× 109/l), median (IQR) | 4.8 (3.8–6.5) |
| Monocytes (× 109/l), median (IQR) | 0.6 (0.5–0.7) |
| Lymphocytes (× 109/l), median (IQR) | 1.7 (1.3–2.1) |
| Platelets (× 109/l), median (IQR) | 253 (204–303) |
| NLR, median (IQR) | 2.9 (2.1–4.3) |
| MLR, median (IQR) | 0.36 (0.26–0.50) |
| LMR, median (IQR) | 2.8 (2.0–3.8) |
| PLR, median (IQR) | 150 (103–202) |
Abbreviations: BMI=body mass index; CBC=complete blood count; CIS=carcinoma in situ; IQR=interquartile range; LMR=lymphocyte monocyte ratio; MLR=monocyte lymphocyte ratio; NLR=neutrophil lymphocyte ratio; PLR=platelet lymphocyte ratio; WBC=white blood cell count.
Positive surgical margins rates are based on permanent sections (paraffin-embedded) and exclude ureteral carcinoma in-situ.
The AUCs of the time-dependent ROC curve analyses, used to identify the best predictors among those that are similar to each other, are shown in Supplementary Table 1. The AUCs for NLR were superior to those for absolute neutrophil and lymphocyte counts, whereas the AUCs for PLR were superior to those for absolute platelet and lymphocyte counts. The AUCs for LMR were superior to those for monocyte–lymphocyte ratio, absolute monocyte count and absolute lymphocyte count. There were no predictors similar to haemoglobin. Thus, in subsequent analyses, only haemoglobin, NLR, LMR, and PLR were retained, whereas those predictors deemed similar and inferior were eliminated in order to avoid collinearity.
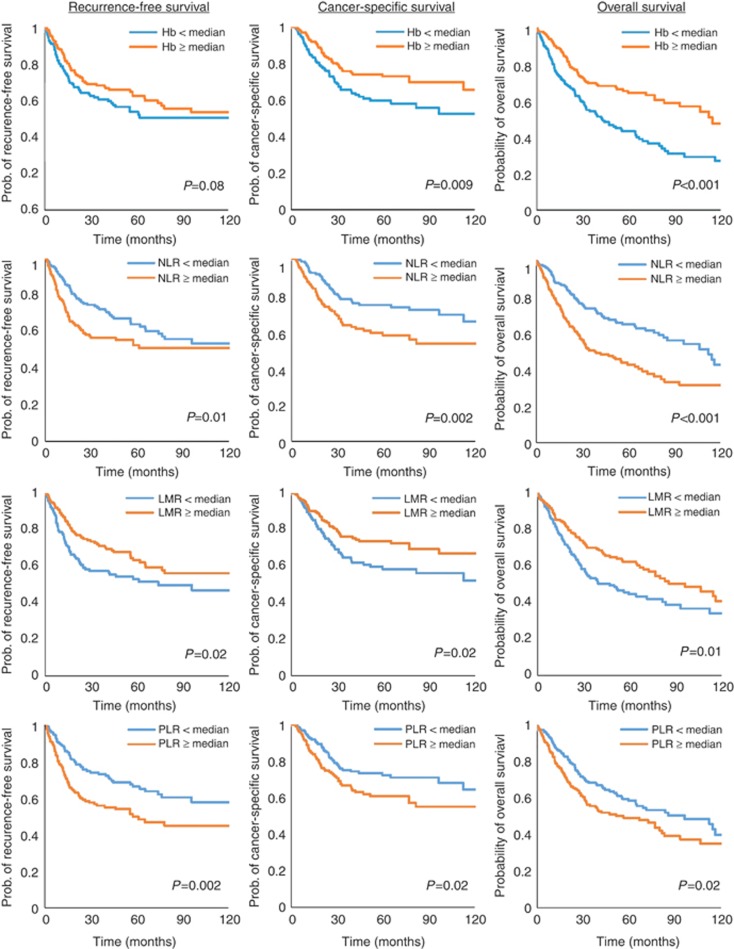
In Kaplan–Meier analyses, higher NLR and PLR values (above median), and lower haemoglobin and LMR values (below median) were shown to be associated with worse RFS, CSS, and OS (all P<0.05, except for the association between haemoglobin and RFS where P=0.08; see Figure 1). These findings were replicated in the univariate Cox-regression analyses (Table 2). Increasing NLR was associated with worse RFS (HR per 1-log unit=1.49, 95% CI=1.22–1.81, P<0.001), CSS (HR per 1-log unit=1.58, 95% CI=1.32–1.90, P<0.001), and OS (HR per 1-log unit=1.74, 95% CI=1.43–2.11, P<0.001). Increasing PLR was also associated with worse RFS, CSS, and OS. Increasing haemoglobin level was associated with improved RFS (HR per 1 g/dl=0.90, 95% CI=0.86–0.94, P<0.001), CSS (HR per 1 g/dl=0.87, 95%CI=0.82–0.91, P<0.001), and OS (HR per 1 g/dl=0.84, 95% CI=0.82–0.87, P<0.001). Increasing LMR was also associated with better RFS, CSS, and OS.
Figure 1.
Kaplan–Meier analyses of associations between CBC-based predictors (above vs below median for haemoglobin (row 1), neutrophil–lymphocyte ratio (row 2), lymphocyte–monocyte ratio (row 3), and platelet–lymphocyte ratio (row 4)) and oncologic outcomes.
Table 2. Univariate associations between predictors and oncologic outcomes following radical cystectomy.
|
Recurrence-free survival |
Cancer-specific survival |
Overall survival |
||||
|---|---|---|---|---|---|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Haemoglobin, per 1 g/dl | 0.90 (0.86–0.94) | <0.001 | 0.87 (0.82–0.91) | <0.001 | 0.84 (0.82–0.87) | <0.001 |
| Neutrophil–lymphocyte ratio, per 1-log unita | 1.49 (1.22–1.81) | <0.001 | 1.58 (1.32–1.90) | <0.001 | 1.74 (1.43–2.11) | <0.001 |
| Lymphocyte–monocyte ratio, per 1-log unita | 0.66 (0.55–0.79) | <0.001 | 0.69 (0.53–0.91) | 0.009 | 0.70 (0.55–0.88) | 0.002 |
| Platelet–lymphocyte ratio, per 100 units | 1.22 (1.08–1.38) | 0.002 | 1.21 (1.05–1.41) | 0.01 | 1.16 (1.02–1.33) | 0.03 |
Abbreviations: CI=confidence interval; HR=hazard ratio.
Log-transformed.
Final parsimonious multivariable models, created based on minimising AIC values, are shown in Table 3. NLR (HR per 1-log unit=1.52, 95% CI=1.17–1.98, P=0.002) was the only CBC-based predictor retained for inclusion in the model for RFS, along with T-stage, N-stage, LVI, and positive surgical margins. NLR (HR per 1-log unit=1.47, 95% CI=1.20–1.80, P<0.001) and haemoglobin (HR per 1 g/dl=0.91, 95% CI=0.86–0.95, P<0.001) were the only CBC-based predictors retained in the model for CSS, along with T-stage, N-stage, LVI, and positive surgical margins. Last, NLR (HR per 1-log unit HR=1.56, 95% CI=1.16–2.10, P=0.004) and haemoglobin (HR per 1 g/dl=0.90, 95% CI=0.88–0.93, P<0.001) were the only CBC-based predictors retained in the model for OS, along with age, Charlson co-morbidity index, T-stage, N-stage, LVI, and positive surgical margins.
Table 3. Final parsimonious multivariable models for recurrence-free, cancer-specific, and overall survival.
| Parameter | HR (95% CI) | P-value |
|---|---|---|
|
Model for recurrence-free survivala | ||
| T-stage, pT3-4 vs pT0-2 | 1.58 (1.03–2.42) | 0.03 |
| N-stage, N+ vs N0 | 2.15 (2.82–2.53) | <0.0001 |
| Lymphovascular invasion | 1.72 (1.04–2.86) | 0.03 |
| Positive surgical margin | 2.16 (1.42–3.28) | <0.001 |
| Neutrophil–lymphocyte ratio, per 1-unit increaseb | 1.52 (1.17–1.98) | 0.002 |
|
Model for cancer-specific survivalc | ||
| T-stage, pT3-4 vs pT0-2 | 1.67 (1.07–2.62) | 0.02 |
| N-stage, N+ vs N0 | 2.13 (1.27–3.57) | 0.004 |
| Lymphovascular invasion | 1.75 (0.94–3.28) | 0.08 |
| Positive surgical margin | 1.82 (0.88–3.79) | 0.11 |
| Haemoglobin (per 1 g/l increase) | 0.91 (0.86–0.95) | <0.001 |
| Neutrophil–lymphocyte ratio, per 1-unit increaseb | 1.47 (1.20–1.80) | <0.001 |
|
Model for overall survivald | ||
| Age, per 1 year increase | 1.03 (1.01–1.04) | 0.008 |
| Charlson co-morbidity index, per 1-point increase | 1.16 (1.03–1.32) | 0.01 |
| T-stage, pT3-4 vs pT0-2 | 1.42 (0.83–2.45) | 0.20 |
| N-stage, N+ vs N0 | 1.55 (1.12–2.14) | 0.008 |
| Lymphovascular invasion | 1.74 (1.03–2.93) | 0.04 |
| Positive surgical margin | 1.86 (0.90–3.82) | 0.09 |
| Haemoglobin, per 1 g/dl increase | 0.90 (0.88–0.93) | <0.001 |
| Neutrophil–lymphocyte ratio, per 1-unit increaseb | 1.56 (1.16–2.10) | 0.004 |
Abbreviations: AIC=Akaike Information Criterion; CI=confidence interval; HR=hazard ratio.
Likelihood ratio omnibus test: χ2=84.8, dF=5, P<0.001; AIC=1407.0.
Variable was log-transformed, and therefore hazard ratios represent effect per 1 log-unit.
Likelihood ratio omnibus test: χ2=68.9, dF=6, P<0.001; AIC=1101.6.
Likelihood ratio omnibus test: χ2=111.0, dF=8, P<0.001; AIC=1780.7.
Upon comparing final models with and without the selected CBC-based predictors, it was found that the inclusion of NLR significantly improved the goodness-of-fit of the model for RFS (P=0.014), whereas the inclusion of NLR and haemoglobin significantly improved the goodness-of-fit of the models for CSS (P=0.008) and OS (P<0.001), compared with respective models with clinical and pathologic parameters only (see Supplementary Table 2 for details).
Discussion
Although the potential role of inflammation in cancer was originally proposed by Rudolph Virchow in the nineteenth century, it is only during the past 10–15 years that a deeper understanding has emerged of the impact of inflammation in carcinogenesis and cancer progression (Grivennikov et al, 2010; Hanahan and Weinberg, 2011). Recently, there has been growing interest in using CBC-based measures as BC biomarkers, with numerous studies separately reporting on the impact of individual components of the CBC on RC outcomes (Can et al, 2012; Gondo et al, 2012; Todenhofer et al, 2012; Azab et al, 2013; Krane et al, 2013; Feng et al, 2014; Hermanns et al, 2014; Moschini et al, 2014; Potretzke et al, 2014; Temraz et al, 2014; Viers et al, 2014; Gierth et al, 2015). With growing data supporting the prognostic value of various CBC-based biomarkers, we sought to elucidate which of these variables would ultimately possess the greatest potential in the RC population.
In our study, NLR was the sole CBC-derived biomarker to be independently predictive of RFS, CSS, and OS. This is consistent with existing literature, with NLR being the most frequently reported CBC-derived biomarker in BC (Can et al, 2012; Gondo et al, 2012; Krane et al, 2013; Hermanns et al, 2014; Kaynar et al, 2014; Potretzke et al, 2014; Viers et al, 2014; Mano et al, 2015). NLR has been shown to predict muscle-invasion upon transurethral resection (Can et al, 2012; Kaynar et al, 2014), recurrence, and progression for NMIBC (Mano et al, 2015), upstaging at the time of RC (Krane et al, 2013; Hermanns et al, 2014; Potretzke et al, 2014; Viers et al, 2014), and worse oncologic outcomes following RC (Gondo et al, 2012; Krane et al, 2013; Hermanns et al, 2014; Viers et al, 2014).
NLR also has a strong biological rationale, in the context of the role of immunity and inflammation in cancer development and progression (Grivennikov et al, 2010; Hanahan and Weinberg, 2011). Conceptually, NLR represents the ratio of the innate immune response (i.e., neutrophils) to the adaptive immune response (i.e., lymphocytes). Neutrophils assemble at the margins of pre-malignant lesions and promote carcinogenesis through various mechanisms, including: (i) producing reactive oxygen species capable of inducing DNA damage and genomic instability, (ii) promoting the secretion of various growth factors that enhance the proliferation of mutated cells, (iii) promoting angiogenesis, and (iv) promoting metastasis (Grivennikov et al, 2010). Meanwhile, T and B lymphocytes are capable of recognising tumour antigens and may aid in an anti-tumour response through cell-mediated and antibody-dependent cytotoxicity, respectively, although in the majority of cases, this response alone is insufficient to restrain tumour growth (Grivennikov et al, 2010). In our study, circulating NLR had greater predictive value than either absolute neutrophil or lymphocyte counts alone, suggesting that both counts are important and that the ratio is the most informative.
Haemoglobin level also added predictive value for CSS and OS, independently of NLR and other clinic-pathologic predictors. Anaemia has been shown to be associated with worse RFS, CSS, and OS following RC (Gierth et al, 2015). Anaemia is likely multifactorial in this population. It may be in part due to blood loss from tumour-related bleeding. There may also be a component of anaemia of chronic disease (also known as anemia of chronic inflammation), which is driven by acute and/or chronic immune system activation (Weiss and Goodnough, 2005), and in turn may be a systemic effect of BC or of other co-morbid diseases.
Secondary thrombocytosis (also called reactive thrombocytosis) is most often the result of acute or chronic inflammatory conditions, infections, or malignancies. This is mediated by pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-11, and tumour necrosis factor-α, among others (Bleeker and Hogan, 2011). In patients undergoing RC, platelet count was associated with more advanced disease (Moschini et al, 2014) and worse CSS (Todenhofer et al, 2012). In our study, PLR, compared with absolute platelet counts, showed greater discrimination for oncologic outcomes following RC. Moreover, we demonstrated for the first time, the potential ability of PLR as a prognostic indicator for RC. However, PLR did not add value to multivariable models when NLR, haemoglobin level, and clinical predictors were included.
Similar to neutrophils, macrophages (differentiated monocytes) can support tumour-related angiogenesis and cell migration (Temraz et al, 2014). Lower lymphocyte–monocyte ratio (LMR) was demonstrated in one study (Temraz et al, 2014) to be associated with reduced disease-free survival and OS. The Kaplan–Meier analyses in our study are consistent with this finding. However, as was the case for PLR, LMR was not retained as an independent predictor of oncologic outcomes once other variable were considered.
To our knowledge, the present study is the first to compare various CBC-based biomarkers in order to determine which parsimonious combination most efficiently predicts oncologic outcomes in BC. Now that it is more clear which predictors are most efficient, it is possible to formally evaluate how NLR and haemoglobin can best be employed to guide clinical decision-making.
Data supporting a potential clinical role for CBC-based predictors, particularly NLR, are emerging. For example, NLR can risk-stratify patients with clinical NMIBC into a group with excellent outcomes upon early cystectomy (>90% 5-years CSS) and a group with similar oncologic outcomes to patients with clinical MIBC (∼60% 5-year CSS; Hermanns et al, 2014). This may be helpful in guiding the use of early cystectomy. Also, change in NLR during neo-adjuvant chemotherapy can be helpful in predicting pathological response (Seah et al, 2015). Last, NLR can also further risk-stratify patients beyond pathological stage, which may be helpful in guiding the use of adjuvant chemotherapy (Hermanns et al, 2014).
Our study is not without limitations. First, this is a single-institution retrospective observational study. Second, we did not routinely measure CBCs at regular intervals post-RC, and therefore cannot comment on whether changes in CBC-based biomarkers have predictive value. Given the myriad factors that can affect CBC levels postoperatively, it is unlikely that immediate post-RC CBC components will yield prognostic value. Third, our cohort included very few patients receiving neo-adjuvant chemotherapy, and therefore further studies are needed to evaluate how CBC-based biomarkers are affected by neo-adjuvant chemotherapy, and whether CBC-based biomarkers can predict response to neo-adjuvant chemotherapy. Fourth, data C-reactive protein-levels were not available in our retrospective cohort. Future studies are warranted to evaluate if C-reactive protein provides additional prognostic value to NLR and haemoglobin levels. Last, it is unclear whether our findings in patients undergoing RC are generalisable to all BC patients. Further studies are warranted in patients with low-intermediate risk NMIBC.
Conclusion
Several CBC-based biomarkers are reported to have prognostic value in BC. They are cost-effective and widely accessible. Our study has identified NLR as the most efficient marker for predicting RFS, whereas NLR and haemoglobin together were found to be the most efficient for predicting CSS and OS. These predictors can be helpful along with other known predictors such as clinical and pathologic stage and grade. It is not practical for clinicians to use redundant biomarkers, and parsimony is essential. This study hopes to guide the focus of future research on CBC-based predictors. Further studies should be directed at how NLR and haemoglobin can help guide clinical decision-making in this complex patient population.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, Habeshy A, Picon A, Bloom S (2013) Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol 30(1): 432. [DOI] [PubMed] [Google Scholar]
- Bhindi B, Yu J, Kuk C, Sridhar SS, Hamilton RJ, Finelli A, Jewett MA, Evans A, Fleshner NE, Zlotta AR, Kulkarni GS (2014) The importance of surgeon characteristics on impacting oncologic outcomes for patients undergoing radical cystectomy. J Urol 192(3): 714–720. [DOI] [PubMed] [Google Scholar]
- Bleeker JS, Hogan WJ (2011) Thrombocytosis: diagnostic evaluation, thrombotic risk stratification, and risk-based management strategies. Thrombosis 2011: 536062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can C, Baseskioglu B, Yilmaz M, Colak E, Ozen A, Yenilmez A (2012) Pretreatment parameters obtained from peripheral blood sample predicts invasiveness of bladder carcinoma. Urol Int 89(4): 468–472. [DOI] [PubMed] [Google Scholar]
- Canter D, Long C, Kutikov A, Plimack E, Saad I, Oblaczynski M, Zhu F, Viterbo R, Chen DY, Uzzo RG, Greenberg RE, Boorjian SA (2011) Clinicopathological outcomes after radical cystectomy for clinical T2 urothelial carcinoma: further evidence to support the use of neoadjuvant chemotherapy. BJU Int 107(1): 58–62. [DOI] [PubMed] [Google Scholar]
- Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, Inman BA, Kuban DA, Kuzel TM, Lele SM, Michalski J, Pagliaro LC, Pal SK, Patterson A, Plimack ER, Pohar KS, Porter MP, Richie JP, Sexton WJ, Shipley WU, Small EJ, Spiess PE, Trump DL, Wile G, Wilson TG, Dwyer M, Ho M National Comprehensive Cancer N (2013) Bladder cancer. J Natl Compr Cancer Netw 11(4): 446–475. [DOI] [PubMed] [Google Scholar]
- Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR (1997) The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 158(1): 62–67. [DOI] [PubMed] [Google Scholar]
- Feng JF, Huang Y, Chen QX (2014) Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 12: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierth M, Mayr R, Aziz A, Krieger S, Wullich B, Pycha A, Lodde M, Salvadori U, Brundl J, Fritsche HM, Hofstadter F, Pawlik MT, Otto W, May M, Burger M, Denzinger S (2015) Preoperative anemia is associated with adverse outcome in patients with urothelial carcinoma of the bladder following radical cystectomy. J Cancer Res Clin Oncol 141: 1819–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, Yoshioka K, Ohori M, Hatano T, Tachibana M (2012) Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology 79(5): 1085–1091. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140(6): 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP Jr., Raghavan D, Crawford ED (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349(9): 859–866. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5): 646–674. [DOI] [PubMed] [Google Scholar]
- Heagerty PJ, Zheng Y (2005) Survival model predictive accuracy and ROC curves. Biometrics 61(1): 92–105. [DOI] [PubMed] [Google Scholar]
- Hermanns T, Bhindi B, Wei Y, Yu J, Noon AP, Richard PO, Bhatt JR, Almatar A, Jewett MA, Fleshner NE, Zlotta AR, Templeton AJ, Kulkarni GS (2014) Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br J Cancer 111(3): 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaynar M, Yildirim ME, Badem H, Cavis M, Tekinarslan E, Istanbulluoglu MO, Karatas OF, Cimentepe E (2014) Bladder cancer invasion predictability based on preoperative neutrophil-lymphocyte ratio. Tumour Biol 35(7): 6601–6605. [DOI] [PubMed] [Google Scholar]
- Krane LS, Richards KA, Kader AK, Davis R, Balaji KC, Hemal AK (2013) Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J Endourol 27(8): 1046–1050. [DOI] [PubMed] [Google Scholar]
- Kulkarni GS, Hakenberg OW, Gschwend JE, Thalmann G, Kassouf W, Kamat A, Zlotta A (2010) An updated critical analysis of the treatment strategy for newly diagnosed high-grade T1 (previously T1G3) bladder cancer. Eur Urol 57(1): 60–70. [DOI] [PubMed] [Google Scholar]
- Lin D, Wei L-J (1989) The robust inference for the Cox proportional hazards model. J Am Stat Assoc 84(408): 1074–1078. [Google Scholar]
- Mano R, Baniel J, Shoshany O, Margel D, Bar-On T, Nativ O, Rubinstein J, Halachmi S (2015) Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol 33(2): 67 e1–67 e7. [DOI] [PubMed] [Google Scholar]
- Moschini M, Suardi N, Pellucchi F, Rocchini L, La Croce G, Capitanio U, Briganti A, Damiano R, Montorsi F, Colombo R (2014) Impact of preoperative thrombocytosis on pathological outcomes and survival in patients treated with radical cystectomy for bladder carcinoma. Anticancer Res 34(6): 3225–3230. [PubMed] [Google Scholar]
- Potretzke A, Hillman L, Wong K, Shi F, Brower R, Mai S, Cetnar JP, Abel EJ, Downs TM (2014) NLR is predictive of upstaging at the time of radical cystectomy for patients with urothelial carcinoma of the bladder. Urol Oncol 32(5): 631–636. [DOI] [PubMed] [Google Scholar]
- Seah JA, Leibowitz-Amit R, Atenafu EG, Alimohamed N, Knox JJ, Joshua AM, Sridhar SS (2015) Neutrophil-lymphocyte ratio and pathological response to neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer. Clin Genitourin Cancer 13(4): e229–e233. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ, Sagalowsky AI, Schoenberg MP, Lerner SP (2006) Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 176(6 Pt 1): 2414–2422, ; discussion 2422. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Palapattu GS, Karakiewicz PI, Rogers CG, Vazina A, Bastian PJ, Schoenberg MP, Lerner SP, Sagalowsky AI, Lotan Y (2007) Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol 51(1): 137–149, ; discussion 149-51. [DOI] [PubMed] [Google Scholar]
- Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG (2001) Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1054 patients. J Clin Oncol 19(3): 666–675. [DOI] [PubMed] [Google Scholar]
- Temraz S, Mukherji D, Farhat ZA, Nasr R, Charafeddine M, Shahait M, Wehbe MR, Ghaida RA, Gheida IA, Shamseddine A (2014) Preoperative lymphocyte-to-monocyte ratio predicts clinical outcome in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: a retrospective analysis. BMC Urol 14: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todenhofer T, Renninger M, Schwentner C, Stenzl A, Gakis G (2012) A new prognostic model for cancer-specific survival after radical cystectomy including pretreatment thrombocytosis and standard pathological risk factors. BJU Int 110(11 Pt B): E533–E540. [DOI] [PubMed] [Google Scholar]
- Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, Thompson RH, Tollefson MK (2014) Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol 66(6): 1157–1164. [DOI] [PubMed] [Google Scholar]
- Weiss G, Goodnough LT (2005) Anemia of chronic disease. N Engl J Med 352(10): 1011–1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.