Abstract
Very little is known about parasitic diseases of European pond turtles (Emys orbicularis) in Iran. The objective of this study is to examine parasitic fauna of European pond turtles collected from Fars province, southwest Iran. Carcasses of turtles (n = 52) which died during dredging procedure are collected from earthen fishery basins in Zarghan region. They have been died earlier during dredging procedure in different farms. Three species of helminths in total were found in gastrointestinal tract, including two nematodes (Serpinema microcephalus and Falcaustra araxiana) and one digenean trematod (Telorchis assula). Large intestines of all examined turtles were infected by F. araxiana (100 %, Mean intensity = 18) and this nematode were also found in gastric nodules. Nine turtles (17.3 %, 3 male, 6 female, Mean intensity = 3) were infected with Serpinema microcephalus. T. assula were found in 25 turtles (48.07 %, 5 male, 20 female, mean intensity = 5). Helminths were not found in any examined organs and no ectoparasite found eighter. F. araxiana is the most prevalent nematode in European pond turtles. Detection of Serpinema.microcephalus is in agreement with the fact which this parasite is common parasite of turtles in all over the world. T. assula might be transmitted between variety of reptiles so presence of the trematod should be considered as a risk factor for other reptiles.
Keywords: Emys orbicularis, Serpinema microcephalus, Falcaustra araxiana, Telorchis assula, European pond turtle
Introduction
The reptilian class is host to a wide variety of protozoan and metazoan parasites (Segade et al. 2006; McAllister et al. 2008; Zelmer and Platt, 2008). Turtles belong to the reptilian class and three species of freshwater turtles belonging to the families Emydidae and Trionychidae are found in Iran (Kami et al. 2006).
European pond turtle E. orbicularis belongs to the family Emydidae and is described as a one of Palearctic vertebrates. It has been reported from Western North Africa, over most of southern, central, and Eastern Europe to Asia Minor and the Caspian and Aral Seas in the east (Fritz and Havas 2007).
The freshwater turtles are parasitized by some different species (Segade et al. 2006; McAllister et al. 2008; Zelmer and Platt, 2008). The foremost parasite species of the E. orbicularis are: Haemogregarina stepanovi (Siddall and Desser 1992; Lee et al. 2002), Eimeria delagei, Epistylis sp., Spironucleus emydis, Polystomoides ocellatum, Proteromonas sp., Spirhapalum polesianum (Ejsmont 1927; Platt 2000; Mihalca et al. 2003), Serpinema microcephalus (Baker 1979), Spiroxys contortus (Baker 1987). In Iran, Cammallanus sp., Anisakis sp., and Hysterothylacium have been reported from digestive tracts of E. orbicularis, caught from pond culture of sturgeon fingerling in Gorgan (Pazouki and Aghaeei Moghadam 2004).
To our best knowledge, there are no reports of helminthes in E. orbicularis in southwest of Iran. The objective of this study is to examine parasitic fauna of European pond turtles collected from Fars province, southwest Iran.
Materials and methods
Carcasses of Fifty-two E. orbicularis (14 males, 38 females) are collected from earthen fishery basins culture of carp in Zarghan region (29° 46′ 25″ N, 52° 43′ 14″ E) located in Fars Province of Iran. They died during dredging procedure in different farms. Each body cavity was opened, and the digestive tracts were removed. The organs were separated, washed in a 150-μm sieve, and the resulting content was observed by a stereomicroscope in search of helminthes. The cavity was also washed and its content examined. Nematodes were killed in hot saline solution, fixed in 70 % ethanol, cleared by lactophenol and mounted in Glycerin–gelatin. Digeneans were fixed in 70 % ethanol, slightly pressed between two flat glasses. They were stained with carmine and then mounted in Canada balsam® as permanent slides. A systematic position of the recovered parasites was determined by analyzing the morphometric characteristics. Helminthes identification was based on keys given by Yamaguti (1961), Baker (1979).
Results
Three species of helminths are found (Table 1): Two nematodes (Serpinema microcephalus from Small intestine and Falcaustra araxiana from large intestine and gastric nodules), one digenean (Telorchis assula from small intestine).
Table 1.
Sex and number of infected turtles
| Male (percent of males) | Female (percent of females) | Total percent | Mean intensity | |
|---|---|---|---|---|
| Serpinema microcephalus | 3 (21) | 5 (13.15) | 17.3 | 3 |
| Falcaustra araxiana | 14 (100) | 38 (100) | 100 | 18 |
| Telorchis assula | 5 (35.71) | 20 (52.63) | 48.07 | 5 |
Identification of helminths
Serpinema microcephalus
The body is large and 17.2 mm in length. In the female worms, vulva flap is presented and located at a distance of 7.4 mm from the anterior opening and 0.11 mm in size. Valve of the buccal cavity was thick and there were two groups of five long ridges (Fig 1).
Fig. 1.
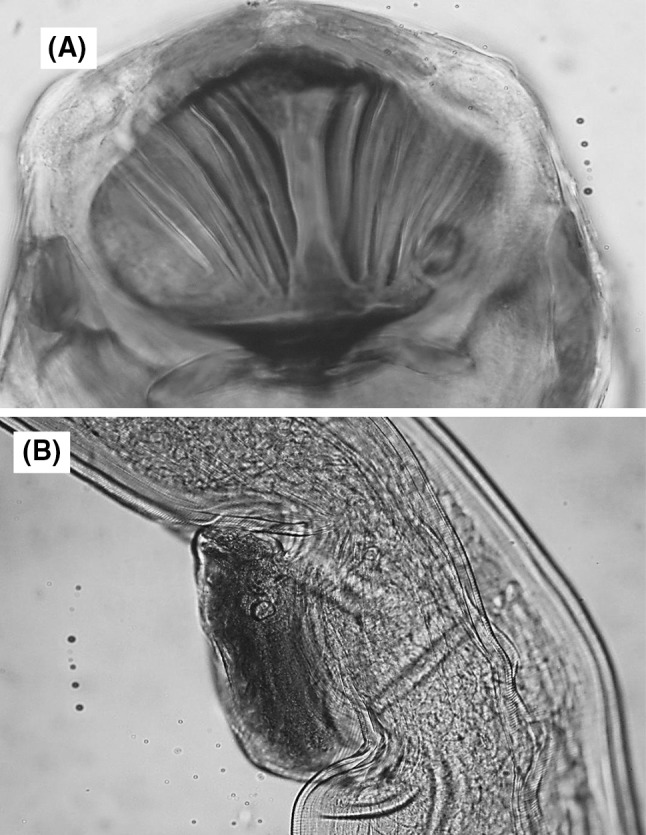
Serpinema microcephalus a cephalic extremity, lateral view (note the valve of the buccal cavity) (×40), b vulvar flap (×40)
Falcaustra araxiana
Cylindrical body tapering posteriorly, truncate anteriorly. Cuticle has fine, regular, transverse striations. The mouth opens triangularly and bounded by three large vesiculated cephalic lips, each with medial V-shaped indentation giving a hexagonal appearance to oral opening in apical view. Esophagus has posterior spherical bulb and anteriorly positioned isthmus. Tail slender, finely pointed in both sex. In males, pairs of caudal papillae are presented. Papillae pattern is 6–6–10 + 1. There are four pseudosucker in posterior end of body (Fig 2).
Fig. 2.
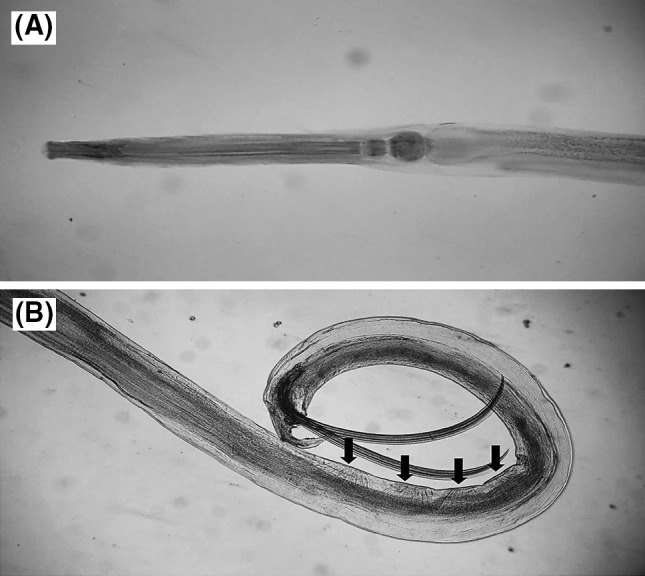
Falcaustra araxiana a anterior end (×4), b Posterior end (×4), note the pseudosuckers (arrows)
Telorchis assula
Body was elongated with parallel margins appears lingual shape. Prepharynex were observed in all of specimens. Furthermore, oesophagus of trematoda appeared moderately long. In addition, caeca seemed slender and got was looked a little thicker at the end of body. Testes were observed oval, tandem and also contiguous. Concerning cirrus-sac, appeared elongate, large, either straight or sinuous, mainly was appeared in posterior to ventral sucker and contains sacculate seminal vesicle, elongate ejaculatory duct lined with prostatic cells. Cirrus often was seen protruded. Genital pore was seen in median part. Uterus shown descending and ascending symmetrical that firmly packed transverse coils between ovary and anterior testis. In middle of body, vitellarium forms contiguous lateral fields of small follicules (Fig 3).
Fig. 3.
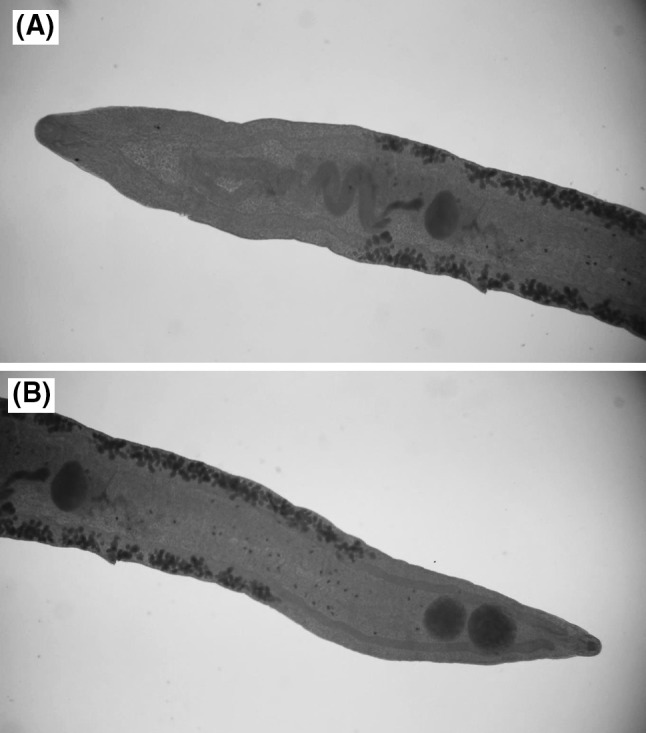
Telorchis assula a anterior end (×4), b posterior end (×4), note the tandem testis in posterior end
Prevalence of helminthes in examined turtles
Large intestines of all examined turtles are infected by F. araxiana (100 %, Mean intensity = 18) and also found in gastric nodules. Nine turtles (17.3 %, 3male, 6 female, Mean intensity = 3) were infected with Serpinema microcephalus. T. assula were found in 25 turtles (48.07 %, 5 male, 20 female, mean intensity = 5) (See Table 1).
Discussion
Reptiles have increasingly become common domestic pets. While several reptile species sold as pet animals are bred in captivity, most of them are either taken from the wild or the offspring of wild-caught parents (Rataj et al. 2011). Exotic or native species, originating from legal or illegal trade, are kept as pets, and people often look for way to get rid of them after some time mostly release them into non-native regions (Velo-Anton et al. 2011). Since very few reptilian parasites cause pathognomonic signs or lesions, a clinical diagnosis of parasitism can only be proved by identifying the disease-causing parasite or its ova (Ippen and Zwart 1996). A clinical diagnosis of parasitism can only be made by identifying the disease-causing parasite or its ova because very few reptilian parasites cause pathognomonic signs or lesions. In captivity, where animals are confined in small spaces, the concentration of parasites may be much higher (Klingenberg, 1993). Some of them are harmless but others, especially in association with poor sanitary conditions and stress from poor husbandry, can be dangerous, causing severe diseases and even death if left untreated (Marcus 1981).
Investigations of fauna helminth can provide important information about diet studies and the biology of the hosts (Mascarenhas et al. 2013) therefore knowledge of diversity of helminths associated with European pond turtle can be used to identify host habitats and their diet in the fishery industries or when they kept as pet animal.
Cammallanus sp., Anisakis sp., and Hysterothylacium have been reported from digestive tracts of Emys orbicularis, caught from pond culture of sturgeon fingerling in Gorgan, Iran (Pazouki and Aghaeei Moghadam 2004). Our turtles were caught from earthen fishery basins culture of carp and our results were completely different. It seems that species of fishes, type of ecosystem and geographical region could affect on parasite diversity.
In the recent years, (Yildirimhan and Shahin 2005) found 4 helminth species in Emys orbicularis including Patagium lazarewi (Digenea), Serpinema microcephalus, Spironoura armenica and Spiroxys contortus (Nematoda) in Turkey. Five nematodes species were found in Mauremys leprosa and Emys orbicularis from Spain including Serpinema microcephalus, Falcaustra donanaensis, Falcaustra sp. Aplectana sp., and Physaloptera sp. (Hidalgo-Vila et al. 2009).
Serpinema is morphologically most similar to Camallanus. Currently, many species of turtles nematodes, previously reported as Camallanus have been re-assigned to genus Serpinema (Baker 1979; Moravec and Vargas-Vazquez 1998). Serpinema microcephalus (Dujardin 1845), which is still reported as Camallanus microcephalum in the literatures, occurs only in turtles of the western palearctic. World geographical distribution ranges of Serpinema microcephulus remain unclear but it is common in turtles of Western Europe. It has also been reported in western U.S.S.R. 1476, North Africa (Baker 1979), in Turkey isolated from Emys orbicularis (Yildirimhan and Shahin 2005), in Spain from Mauremys leprosa as well as Emys orbicularis (Hidalgo-Vila et al. 2009), and in Japan from red-eared slider turtle (Trachemys scripta elegans) (Oia et al. 2012).
Falcaustra araxiana Massino, 1924 is one of the species which is reported from European pond turtle in Armenia (Bursey and Rivera 2009). European pond turtle is the only known host for F.araxiana (Yamaguti 1961). The results show that F. araxiana is prevalent species in Fars province and this region should be considered as a new geographical locality for the parasite.
To our best knowledge, there is no information about life cycle and geographical distribution of this species. Presence of the parasite in gastric nodules may be is the part of its life cycle. Histopathological changes of the gastric nodules are being investigated and the results will be published as soon as possible.
Four species of Telorchis are reported from freshwater turtles in Japan, T. geoclemmydis, T. konoi and T. megacotyle and T. clemmydis (Oia et al. 2012). We found another species, Telorchis assula. It has been reported from different snake and turtle hosts (Halajian et al. 2013; Youssefi et al. Yousesfi et al. 2013). In Iran, T.assula has been reported from Caspian turtle or striped-neck terrapin Mauremys caspica caspica, grass snake Natrix natrix and dice snake Natrix tessellate (Halajian et al. 2013; Yousesfi et al. 2013). With our Findings, Emys orbicularis are considered as a new host for this parasite in this country.
In this study, F. araxiana is the most prevalent nematode in European pond turtles. Detection of Serpinema microcephalus is in agreement with the fact which the parasite is common parasite of turtles in all over the world. T. assula could be transmitted between variety of reptiles and presence of the trematod should be considered as risk factor for other reptiles.
Conflict of interest
The authors declare no conflict of interest
References
- Baker MR. Serpinema spp. (Nematoda: camallanidae) from turtles of North America and Europe. Can J Zool. 1979;57:934–939. doi: 10.1139/z79-114. [DOI] [Google Scholar]
- Baker MR. Synopsis of the nematoda parasitic in amphibians and reptile. Meml Univ Nfld Occas Pap Biol. 1987;11:1–325. [Google Scholar]
- Bursey CR, Rivera S (2009) New species of Falcaustra (Nematoda: Ascaridida: Kathlaniidae) in the impressed Tortoise, Manouria impressa (Testudines: Testudinidae). Comp Parasitol 76:141–148
- Ejsmont L. Spirhapalum polesianum n.g., n.sp. trématode du sang d’Emys orbicularis L. Ann Parasitol. 1927;5:220–235. [Google Scholar]
- Fritz U, Havas P. Checklist of chelonians of the world. Vertebr Zool. 2007;57:149–368. [Google Scholar]
- Halajian A, Bursey CR, Goldberg SR, Mohammad Ali Gol S. Helminth parasites of the European glass lizard, Pseudopus apodus (Squamata: anguidae), and European grass snake, Natrix natrix (Serpentes: colubridae), from Iran. Comp Parasitol. 2013;80:151–156. doi: 10.1654/4588.1. [DOI] [Google Scholar]
- Hidalgo-Vila J, Díaz-Paniagua JC, Ribas A, Florecio M, Pérez-Santigosa N, Casanova JC. Helminth communities of the exotic introduced turtle, Trachemys scripta elegans in southwestern Spain: transmission from native turtles. Res Vet Sci. 2009;86:463–465. doi: 10.1016/j.rvsc.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Ippen R, Zwart P. Infectious and parasitic disease of captive reptiles and amphibians, with special emphasis on husbandry practices which prevent or promote diseases. Rev Sci Tech. 1996;15:43–54. doi: 10.20506/rst.15.1.909. [DOI] [PubMed] [Google Scholar]
- Kami HG, Hojati V, Pashaee Rad S, Sheidaee M. A biological study of the European pond turtle, Emys orbicularis persica, and the Caspian pond turtle, Mauremys caspica caspica, in the Golestan and Mazandaran provinces of Iran. Zool Middle East. 2006;37:21–28. doi: 10.1080/09397140.2006.10638145. [DOI] [Google Scholar]
- Klingenberg R. Understanding reptile parasites: a basic manual for Herpetoculturists & Veterinarians (Herpetocultural Library) USA: Advanced Vivarium Systems; 1993. [Google Scholar]
- Lee JJ, Leedale GF, Bradbury P. Illustrated Guide to Protozoa. 2. Lawrence: Society of Protozoologists; 2002. [Google Scholar]
- Marcus LC (1981) Veterinary Biology and Medicine of Captive Amphibians and Reptiles Lea & Febiger, Philadelphia
- Mascarenhas CS, Souza JD, Coimbra MAA, Müller G. Nematode parasites of Chelidae (Testudines) from Southern Brazil. Parasitol Res. 2013;112:3365–3368. doi: 10.1007/s00436-013-3503-3. [DOI] [PubMed] [Google Scholar]
- McAllister CT, Barger MA, Stuart JN. Neoechinorhinchus emyditoides Fisher, 1960 (Acanthocephala: neoechinorhynchidae) from the Mexican plateau slider parasitemia in different aquatic turtle species. J Parasitol. 2008;63:580–581. [Google Scholar]
- Mihalca AD, Gherman C, AchelăriŃei D, Popescu P (2003) Epidemiology of parasitic infections in Emys orbicularis in Romania. 4th international conference for PhD students, University of Miskolc in Hungary, 11–17 August 2003, Natural science section, p 75-79
- Moravec F, Vargas-Vazquez J. Some endohelminths from the freshwater turtle Trachemys scripta from Yucatán, México. J Nat Hist. 1998;32:455–468. doi: 10.1080/00222939800770241. [DOI] [Google Scholar]
- Oia M, Araki J, Matsumoto J, Nogami S. Helminth fauna of a turtle species introduced in Japan, the red-eared slider turtle (Trachemys scripta elegans) Res Vet Sci. 2012;93:826–830. doi: 10.1016/j.rvsc.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Pazouki J, Aghaeei Moghadam AA. Parasitic worms of digestive tracts of Emys orbicularis in pond culture of sturgeon fingerling in Gorgan province Iran. Iran J Fish Sci. 2004;13:25–36. [Google Scholar]
- Platt TR. Helminth parasites of the western painted turtle, Chrysemys picta belii (Gray), including Neopolystoma elizabethae n. sp. (Monogenea: polystomatidae), a parasite of the conjunctival sac. J Parasitol. 2000;86:815–818. doi: 10.1645/0022-3395(2000)086[0815:HPOTWP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rataj AV, Lindtner-Knific R, Vlahović K, Mavri U, Dovč A. Parasites in pet reptiles. Acta Vet Scand. 2011;53:33. doi: 10.1186/1751-0147-53-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segade P, Crespo C, Ayres C, Cordero A, Arias MC, Garcia-Esteves JM, Blanco RI. Eimeria species from the European pond turtle, Emys orbicularis (Reptilia: testudines) in Galicia (NW Spain) with description of two new species. J Parasitol. 2006;92:69–72. doi: 10.1645/GE-3491.1. [DOI] [PubMed] [Google Scholar]
- Siddall ME, Desser SS. Prevalence and intensity of Haemogregarina balli (Apicomplexa: adeleina: Haemogregarinidae) in three turtle species from Ontario, with observation on intraerytrocytic development. Can J Zool. 1992;70:123–128. doi: 10.1139/z92-018. [DOI] [Google Scholar]
- Velo-Anton G, Wink M, Schneeweiß N, Fritz U. Native or not? Tracing the origin of wild-caught and captive freshwater turtles in a threatened and widely distributed species (Emys orbicularis) Conserv Genet. 2011;12:583–588. doi: 10.1007/s10592-010-0141-5. [DOI] [Google Scholar]
- Yamaguti S. Systema Helminthum. vol. 3, The Nematodes of Vertebrates. New York: Wiley; 1961. [Google Scholar]
- Yildirimhan HS, Shahin R. The helminth fauna of Emys orbicularis (European pond turtle) (Linnaeus, 1758) living in freshwater. Turk Parazitol Dern. 2005;29:56–62. [PubMed] [Google Scholar]
- Yousesfi MR, Mousapour AR, Nikzad R, Mobedi I, Rahimi MT. First report of Telorchis assula (Digenea: telorchiidae) in three reptile species from North of Iran. World J Zool. 2013;8:243–244. [Google Scholar]
- Zelmer DA, Platt TR. Structure and similarity of helminth communities of six species of Australian turtles. J Parasitol. 2008;94:781–787. doi: 10.1645/GE-1487.1. [DOI] [PubMed] [Google Scholar]


