Abstract
Enterobius vermicularis is one of the most common intestinal parasite in human. The main objective of this study is to determine the role of E. vermicularis in appendicitis through histopathological examination. A cross sectional study included 200 patients who had appendectomy from three hospitals in Gaza strip. The inflamed appendix was the cause of attending the hospital. Histopathological examination for each appendix was carried out. A questionnaire was designed (interview with patients who underwent appendectomy), and information were obtained from patient and analyzed by using SPSS. The study showed that 30 (15.0 %) of 200 appendices had E. vermicularis in histopathological examination. It was found that ages of patients with histologically proven E. vermicularis in appendices less than 18 years old was found to be (18.2 %). Regarding sex, (16.5 %) of females, (14.0 %) of males patients had E. vermicularis in appendices. Patients who had the highest infection with E. vermicularis were students (17.3 %). In conclusion E. vermicularis occurs more frequently inflamed appendices than in normal. From these results we can conclude that E. vermicularis could be associated to cause of appendicitis in Gaza strip.
Keywords: Enterobius vermicularis, Appendicitis, Gaza strip, Histopathology
Introduction
E. vermicularis has a worldwide distribution and is one of the most common childhood helminthes infections in the developed world (Cook and Zumla 2003). It was estimated that 400 million persons are infected by pinworm worldwide (Stephan et al. 2006). Intestinal parasite incidence were reported by many studies in Gaza strip, including Giardialamblia, Entamoeba histolytica/dispar, Ascaris lumbricoides, Trichuris trichiura, Enterobius vermicularis and Strongyloidesstercoralis (Shubair et al. 2000; Al-Hindi 2002). The prevalence rate of intestinal parasites in Palestine was ranged from 27.6–32.3 % in Gaza strip, and 22.2 % in West Bank, the most common types were Giardia lamnblia and Entamoeba histolytica/dispar (Shtayeh et al. 1989; Yassin et al. 1999; Al-Hindi, 2009; Hussein 2011; Al-Hindi and Al-Louh, 2013). The prevalence of E. vermicularis among preschool children in nursery setting in Gaza strip reached to 46.3 % (Al-Hindi et al. 2013).
Appendicitis is the most common acute surgical condition of the abdomen emergency in the western world occurring in 7–12 % of the general population (Baert 1999).
In a study of 382 appendectomies patients who underwent either laparoscopic or open pediatric appendectomy for diagnosis of acute appendicitis and their consequent histology examined in Midwestern Regional Hospital, Limerick, Ireland were carried out and twelve cases of histologically proven Enterobius vermicularis including seven males and five females were seen. Five cases were associated with acute appendicitis, while four were associated with a normal appendix (Akhigbe et al., 2013). In Nepal, a total of 624 surgically removed appendices received were examined. E. vermicularis was identified in nine (1.62 %) appendices from the patients with a clinical diagnosis of appendicitis. E. vermicularis was found more frequently in uninflamed and histologically normal appendices (8.45 %) than those which were inflamed with histopathologic changes of acute appendicitis (0.56 %) (Sah and Bhadani, 2006). Another study in Iran included of 5048 specimens was reviewed. E. vermicularis was found in 144 (2.9 %) appendix patients (Ramezani and Dehghani, 2007).
In The United Kingdom, an evaluation was made of the histological material obtained from all 1,529 appendices removed during the last 5 years at Southmead Hospital, Bristol. E. vermicularis was identified in 2.7 % of patients with clinical appendicitis and was most commonly seen in appendices with either chronic inflammation or where the appendix was histologically normal. E. vermicularis was rarely associated with histological changes of acute appendicitis (Budd and Armstrong, 1987).
The simple presence of E. vermicularis in the appendix usually produces symptoms which resemble acute appendicitis although the mechanism for this does not involve mucosal invasion by the parasite (Sah and Bhadani 2006). While Gutierres (Gutiérrez 2000) maintains that there exists a consensus that pinworms do not produce the inflammatory reaction. Cook (1994) stated that it is controversial whether pinworms are causatively related to acute appendicitis, and (Burkhart and Burkhart 2005) reported that pinworm infection causes symptoms of appendicitis to surface.
Enterobius vermicularis prevalence is very high in Gaza strip. Appendicitis is also one of the common conditions among Gaza population, according to MOH (Ministry of Health 2014) 466 cases of appendectomy in year 2007 and 502 in year 2008. According to the knowledge of the authors, no studies have been carried out on such topic in Gaza strip. In Gaza strip there is no determination or identification of the reasons of appendicitis in case of appendectomy, as usual the patients complain from abdominal pain and other related symptoms considered as suspected with appendicitis and there was an observation and diagnostic methods for each patient before surgical remove.
General objectives
The main objective of this study is to determine the role of E. vermicularis in appendicitis through histopathological examination.
Subjects and methods
Settings and sample size
A cross sectional study included 200 patients who had appendectomy where Two hundred appendix specimens were collected from those patients in three hospitals (Kamal Edwan, Al Shifa, European) in Gaza strip in the period from Sep, 2011 till Jan, 2013.
Ethical considerations
An ethical approval from the Ministry of Health and Helsinki committee was obtained in 4-4-2011 and Dec, 2011 respectively. A consent form from each patient participated in the study was obtained before the starting of the study.
Methods
Sampling
The researchers made the essential arrangements with each surgical operation department in the three hospitals to collect appendices. Where the appendix was obtained after the surgical operation. Where the researcher obtained the appendix after the surgical operation.
Collection of appendices and histological examination
In the morning or night after appendectomy each appendix was preserved in 10 % formalin in a clean container labeled and transported to private laboratory (Specialized Medical Center in Gaza). Fixation of tissues prevents their autolysis. Each fresh tissue was cut out from the examined organ immediately from patient after the surgical operation.
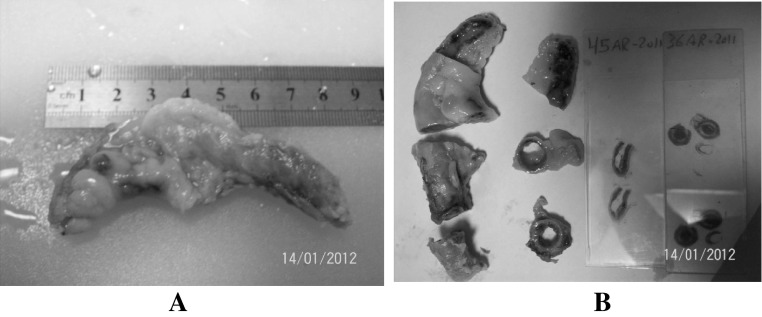
Gross examination: The appendix was examined by naked eye, then measurement was taken for each one (length and greatest diameter) (Fig. 1a)
Fig. 1.
Histological processing of appendix (from a to b). a length and greatest diameter of appendix, b staining
Each appendix was divided in two by cutting a cross Sect. 2 cm from tip, cut cross section of proximal fragment at 5 mm intervals, divide distal fragment in two by a longitudinal cut (Fig. 1b). The fixed tissue was washed in running tap water to remove the fixative.
Histological preparation
Each appendix tissue was done according to the known standard procedures used in histopathology.
Photography
Each appendix section was scanned and photographed using iScan Coreo. Ventana medical system, Inc. Sunnyvale California, USA.
Questionnaire
Each patient was interviewed to obtain the required information. The questionnaire included: Personal characters (sex of the patient, age, education, residence, occupation).
Complains of the patients of appendicitis (abdominal distension, pain degree, vomiting, nausea, frequent urination, low grade fever, wake up at night, gets worse when moving, inability to pass gas, insomnia, defecation will relive discomfort).
Clinical description of appendix by the surgeon (form of appendix, presence of abscess, diagnosis).
Statistical analysis
Data were entered to computer and analyzed using SPSS/PC (Statistical package for social science inc. Chicago, Illinois USA, version 13.0).
Chi square (χ2) was used to identify the significance of the relations and associations among various variables. The results in the mentioned procedures were accepted as statistically significant when the p value was less than 5 % (p < 0.05
Results
Personal characters of the patients
Patient’s age was between (8–54 years old), males were 121 (60.5 %) and females were 79 (39.5 %). All other personal characters are presented in Table 1.
Table 1.
Personal characters of the patients (n = 200)
| Variable | Frequency | % |
|---|---|---|
| Age(years) | ||
| <18 | 99 | 49.5 |
| >19 | 101 | 50.5 |
| Sex | ||
| Males | 121 | 60.5 |
| Females | 79 | 39.5 |
| Education(years) | ||
| >12 | 121 | 60.5 |
| <12 | 79 | 39.5 |
| Residence | ||
| North governorate | 85 | 42.5 |
| Gaza governorate | 52 | 26.0 |
| South governorate | 63 | 31.5 |
| Occupation | ||
| Employee | 22 | 11.0 |
| Non employee | 12 | 6.0 |
| House wife | 27 | 13.5 |
| Student | 139 | 69.5 |
Complains of the patients with appendicitis
The present study showed that patients with appendicitis had some complains; abdominal distension 46.3 %, vomiting 49 %, nausea 61.5 %, inability to pass gas 39.5 %, insomnia 62 %, and defecation will relieve discomfort 68 %. Patients who had getting worse when moving followed the abdominal pain were (72.5 %), both diarrhea and constipation had similar prevalence (15.0 %).
Clinical description of appendices by the surgeon
The form of appendix for each patient was reported by the surgeon. There was 13 (6.5 %) of normal looking appendices and 187 (93.5 %) were inflamed. The results showed that high prevalence of acute appendicitis was found 170 (85.0 %), while suspected were 17 (8.5 %).
Personal characters associated with E. vermicularis
From Table 2. it was found that patients with appendicitis with age >18 years were the highest group for E. vermicularis infection but no significant difference was found (χ2 = 1.557, p = 0.147). It was found that 13 (16.5 %) female, 17 (14.0 %) male patients with E. vermicularis in appendices, but no significant difference was found (χ2 = 0.217, p = 0.393). It was found that patients from south governorate had the highest infection with E. vermicularis (19.0 %) compared to north governorate and Gaza governorate, but no significant difference (χ2 = 1.350, p = 0.509). The present study showed that patients who had the highest infection with E. vermicularis are students (17.3 %) followed by house wives (11.1 %), while the other occupation had low prevalence’s (χ2 = 1.901, p = 0.593).
Table 2.
Personal characters associated with E. vermicularis (n = 200)
| Variable | With E. vermicularis no % | Without E.vermicularis no % | χ2, p value |
|---|---|---|---|
| Sex of the patient | |||
| Males | 17 (14.0) | 104 (86.0) | 0.217, 0.393 |
| Females | 13 (16.5) | 66 (83.5) | |
| Age (years) | |||
| <18 | 18 (18.2) | 81 (81.8) | 1.557, 0.147 |
| >19 | 11 (12.9) | 89 (88.1) | |
| Education (years) | |||
| >12 | 18 (14.9) | 103 (85.1) | 0.004, 0.552 |
| <12 | 12 (15.2) | 67 (84.8) | |
| Residence | |||
| North governorate | 12 (14.1) | 73 (85.9) | 1.350, 0.509 |
| Gaza governorate | 6 (11.5) | 46 (88.5) | |
| South governorate | 12 (19.0) | 51 (81.0) | |
| Occupation | |||
| Employee | 2 (9.1) | 20 (90.9) | 1.901, 0.593 |
| Non employee | 1 (8.3) | 11 (91.7) | |
| House wife | 3 (11.1) | 24 (88.9) | |
| Students | 24 (17.3) | 115 (82.7) | |
Symptoms associated with E. vermicularis
It was found that 4 (13.3 %) of symptomatic patients were infected with E. vermicularis while 26 (86.7 %) have E. vermicularis without symptoms (χ2 = 0.127, p = 0.485), with no significant difference. Also, who suffered for constipation had E. vermicularis 3 (10.0 %), while 13 (17.6 %) without E. vermicularis (χ2 = 192, p = 0.442) no significant difference. Patients who had itching in anal area and infected by E. vermicularis were 3 (10.0 %). On the other hand, patients who did not have itching in anal area and infected by E. vermicularis were 27 (90.0 %). In case of the complain of abdominal pain, loss of appetite, diarrhea and weight loss patients without E. vermicularis infection had high level of these symptoms compared to patients with E. vermicularis as shown in Table 3.
Table 3.
Symptoms associated with E. vermicularis (n = 200)
| Variable | With E. vermicularis no % | Without E. vermicularis no % | χ2, p value |
|---|---|---|---|
| With symptoms | 4 (13.3) | 27 (15.9) | |
| Without symptoms | 26 (86.7) | 143 (84.1) | 0.127, 0.485 |
| Had constipation | |||
| Yes | 3 (10.0) | 13 (7.6) | 0.192, 0.442 |
| No | 27 (90.0) | 157 (92.4) | |
| Had itching in the anal area | |||
| Yes | 3 (10.0) | 11 (6.5) | 0.488. 0.351 |
| No | 27 (90.0) | 159 (93.5) | |
| Had abdominal pain | |||
| Yes | 4 (13.3) | 29 (17.1) | 0.257, 0.421 |
| No | 26 (86.7) | 141 (82.9) | |
| Loss of appetite | |||
| Yes | 4 (13.3) | 25 (14.7) | 0.039, 0.552 |
| No | 26 (86.7) | 145 (85.3) | |
| Had diarrhea | |||
| Yes | 1 (3.3) | 8 (4.7) | 0.112, 0.598 |
| No | 29 (96.7) | 162 (95.3) | |
| Weight loss | |||
| Yes | 2 (6.7) | 12 (7.1) | 0.006, 0.649 |
| No | 28 (93.3) | 158 (92.9) | |
Complains of the patients with appendicitis who positive for E. vermicularis
Patients had nausea 73 %, followed by that defecation relieve discomfort, gets worse when moving 60 %, then insomnia 56.7 % were the most symptoms highly associated with the presence of E. vermicularis as shown in Table 4.
Table 4.
Complains of the patients with appendicitis who positive for E. vermicularis (n = 200)
| Variable | With E. vermicularis no % | Without E. vermicularis no % | χ2, p value |
|---|---|---|---|
| Abdominal dentition | |||
| Yes | 14 (46.7) | 79 (46.5) | 0.000, 0.569 |
| No | 16 (53.3) | 91 (53.5) | |
| When the pain began | |||
| Before one day | 10 (11.4) | 78 (88.6) | 3.782, 0.151 |
| Before three days | 6 (12.2) | 43 (87.8) | |
| Before more than three days | 14 (22.2) | 49 (77.8) | |
| Vomiting | |||
| Yes | 14 (46.7) | 84 (49.4) | 0.077, 0.469 |
| No | 16 (53.3) | 86 (50.6) | |
| Nausea | |||
| Yes | 22 (73.3) | 101 (59.4) | 2.087, 0.106 |
| No | 8 (26.7) | 69 (40.6) | |
| Frequent urination | |||
| Yes | 12 (40.0) | 55 (32.4) | 0.669, 0.268 |
| No | 18 (60.0) | 115 (67.6) | |
| Low grade fever | |||
| Yes | 15 (15.6) | 81 (84.6) | 0.057, 0.483 |
| No | 15 (14.4) | 89 (85.6) | |
| Wake up at night | |||
| Yes | 12 (40.0) | 57 (33.5) | 0.472, 0.312 |
| No | 18 (60.0) | 113 (66.5) | |
| Gets worse when moving | |||
| Yes | 18 (60.0) | 127 (74.7) | 2.766, 0.077 |
| No | 12 (40.0) | 43 (25.3) | |
| Inability to pass gas | |||
| Yes | 13 (43.3) | 66 (36.8) | 0.217, 0.393 |
| No | 17 (56.7) | 104 (61.2) | |
| Insomnia | |||
| Yes | 17 (56.7) | 107 (62.9) | 0.426, 0.324 |
| No | 13 (43.3) | 63 (37.1) | |
| Defecation will relieve discomfort | |||
| Yes | 20 (66.7) | 116 (68.2) | 0.29, 0.510 |
| No | 10 (33.3) | 54 (31.8) | |
| Emergency | |||
| Yes | 30 (15.4) | 156 (84.6) | 0.905, 0.440 |
| No | 0 | 5 (100) | |
The form of appendix associated with personal characters of the patients
Table 5 Shows us that males have a high range of inflamed appendix (92.6) comparing with those with normal ones (7.4 %). Female have a high range of inflamed appendix (94.9 %) comparing with those with normal ones (5.1 %) Patients less than 18 years old have a higher range of inflamed appendix (93.9 %) than those who are over 19 years old (93.1 %). Patients over 19 years old have a higher range (6.9 %) of normal appendix than those who are less than 18 years old (6.1 %).
Table 5.
The form of appendix associated with personal characters of the patients (n = 200)
| Variable | Normal no % | Inflamed no % | χ2, p value |
|---|---|---|---|
| Sex of the patient | |||
| Males | 9 (7.4) | 112 (92.6) | 0.443, 0.362 |
| Females | 4 (5.1) | 75 (94.9) | |
| Age (years) | |||
| >18 | 6 (6.1) | 93 (93.9) | 0.062, 0.515 |
| <19 | 7 (6.9) | 94 (93.1) | |
| Education (years) | |||
| >12 | 7 (5.8) | 114 (94.2) | 0.258, 0.409 |
| <12 | 6 (7.6) | 73 (92.4) | |
The form of appendix associated with complains of the patients with appendicitis
The results showed that patients who gave vomiting, nausea and insomnia have the highest of complains in case of normal and inflamed appendix. So we can conclude that complains do not give us a firm judgment of the inflammation of the appendix Table 6.
Table 6.
The form of appendix associated with symptom of complains of the patients of appendicitis (n = 200)
| Variable | Normal no % | Inflamed no % | χ2, p value |
|---|---|---|---|
| Vomiting | |||
| Yes | 7 (53.8) | 91 (48.7) | 0.131, 0.470 |
| No | 6 (46.2) | 96 (51.3) | |
| Nausea | |||
| Yes | 22 (73.3) | 101 (59.4) | 0.002, 0.610 |
| No | 8 (26.7) | 69 (40.6) | |
| Diarrhea | |||
| Yes | 2 (15.4) | 28 (15.0) | 0.002, 0.610 |
| No | 11 (84.6) | 159 (85.0) | |
| Constipation | |||
| Yes | 2 (15.4) | 28 (15.0) | 0.002, 0.610 |
| No | 11 (84.6) | 159 (85.0) | |
| Frequent urination | |||
| Yes | 2 (15.4) | 65 (34.8) | 2.048, 0.128 |
| No | 11 (84.6) | 112 (65.2) | |
| Insomnia | |||
| Yes | 6 (46.2) | 118 (63.1) | 1.482, 0.178 |
| No | 7 (53.8) | 69 (36.9) | |
The histopathological examination of appendices
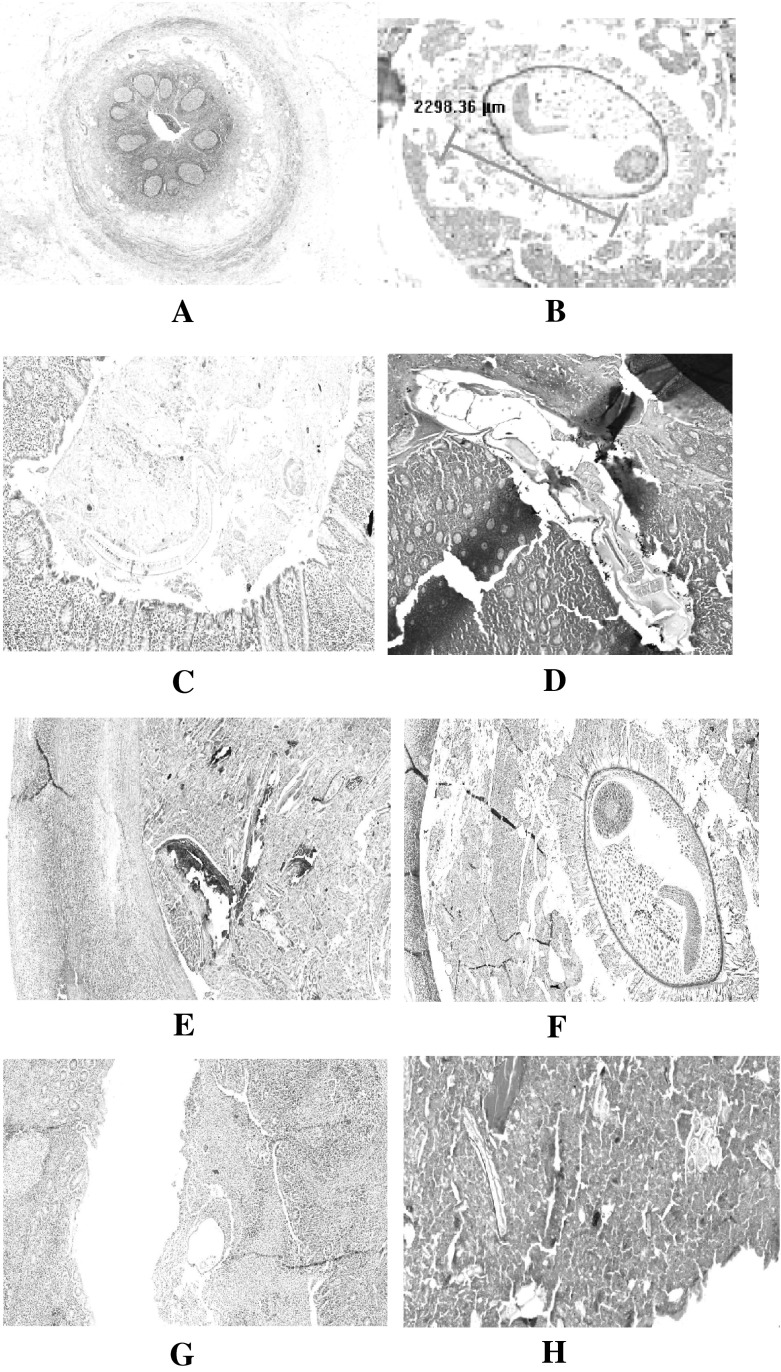
The present study showed a possible relationship between E. vermicularis and appendicitis. The histopathological processing of appendices showed the normal appendix (Fig. 2a.). Also most appendicitis showed the perforation in the tissue (Fig. 2b). The presence of E. vermicularis either as a whole (Fig. 2c), L.S. of Enterobius inside the appendix (Fig. 2d) or Calcified degenerated worm (Fig. 2e). Mucosa and E. vermicularis TS is illustrated in (Fig. 2f). Appendix and TS of E. vermicularis are shown in (Fig. 2g). Eggs and part of the adult worm are shown in (Fig. 2h) is more likely to cause the inflammation of appendix. In a few cases (23.1 % of studied cases), it was found that normal appendix have an E. vermicularis, but it did not cause any inflammation.
Fig. 2.
The histopahtological findings of E. vermicularis and appendix (From a to h). a normal appendix, b perforated appendix, c whole worm in appendix tissue, d L.S. of Enterobius inside the appendix, e calcified degenerated worm, f Mucosa and E. vermicularis TS, g. appendix and TS of E. vermicularis, h eggs and part of the adult worm
Discussion
Enterobius vermicularis (pinworm) is the most successful intestinal nematode to thrive among human populations with over 400 million infected people worldwide (Kucik et al. 2004).
In rare cases, enterobiasis has led to serious consequences such as appendicitis, eosinophilic colitis (Arca et al. 2004), intestinal obstruction, intestinal perforation, and ectopic infections (Quasem and Salam 2007).
A review of the published reports over the last 30 years does not settle this controversy. Some studies confirm the findings of acute or chronic inflammation in appendix specimens also found to have pinworms (Saxena et al. 2001). However, the majority of studies showed a lower incidence of inflammatory changes in patients with appendiceal pinworms (Batistatou et al. 2002).
In the present study by histological examination, we found that 30 appendices (15 %) out of 200 have an E. vermicularis. All of the patients have clinical symptoms of appendicitis. Most studies in the world regarding this topic may support our results.
In Thessaloniki; all 1085 surgical specimens removed at operation from patients with clinical appendicitis were evaluated. E. vermicularis was found in seven appendices, all of which were from patients with clinical symptoms of appendicitis. The prevalence of E. vermicularis was 0.65 % in cases of clinical appendicitis (Gialamas et al. 2012).
Another study supports these results, Columbus, Ohio; twenty-one of 1549 appendectomy patients (1.4 %) were noted to have intraluminal pinworms within the appendix specimen. The presence of E. vermicularis or eggs inside the tissue of appendix has changed the morphology of appendix tissue and resulted in the inflammation of some of the samples (Marjorie et al. 2004).
The present study found that the presence of E. vermicularis in acute appendicitis is 27, suspected appendicitis is 0, and normal appendices are 3, and these results are consistent with other studies conducted world wide.
Similarity to our results from Egyptian, 127 appendices specimens were examined, E. vermicularis worms were present also in 4 cases out of 76 cases diagnosed as acute appendicitis (5.3 %) and in 4 cases out of 28 cases diagnosed as chronic appendicitis (14.3 %) (Helmy et al. 2000). E. vermicularis worms were detected in 5.3 % of cases of acute appendicitis with some of them showed wall penetration by the worm suggesting the implication of these worms in the process of appendicitis as well as there role in inducing obstruction of the appendiceal lumen. E. vermicularis do invade the wall of the vermiform appendix, and related to these are inflammatory reactions. This invasion causes the symptoms that lead to appendectomy (Mogensen et al. 1985).
Although E. vermicularis may have a causal role in appendiceal pain and chronic inflammation due to obstructive phenomena, the overwhelming majority of cases are not associated with acute inflammation. Interestingly, the presence of pinworms in the appendix may cause a clinical “appendiceal syndrome” even without eliciting acute inflammation (Aydin 2007). This “syndrome”, also mentioned as appendiceal colic, consists of chronic right lower quadrant and pelvic pain, intermittent in nature, and can be explained by the hypothesis of appendiceal lumen obstruction. The situation in acute appendicitis is less clear (Gialamas et al. 2012).
In the present study the ages of patients with E. vermicularis in appendices with the highest incidence occurred in less than 18 years age group, this findings are contrast with a study in Thessaloniki, Greece, the ages of the patients with histologically proven E. vermicularis in appendices ranged from 15 to 33 years with a median age of 25 years (Gialamas et al. 2012). In another study that disagree with this results in Copenhagen, incidence 2267 appendices were examined, the highest age group from 6 to 15 year (Wiebe 1991).
The results of the present study showed that the prevalence of E. vermicularis in appendices is higher in females (16.5 %) than males (14.0 %). Our results were consistent with the results from Iran, thirty-eight of 5981 appendectomy patients, they were found in 38 cases there is pinworm that 67 % present of it relates to females and 33 % of it relates to males (Fallah and Dehgani 2011).
In one Brazilian study, 24 cases out of 1,600 appendectomies (1.5 %) with helminthes within the appendix were recorded during a 10-year period (Silva et al. 2007).
It is also well accepted that, one of the possible causes of “acute abdomen” in children may be parasitic infections. E. vermicularis is the most common parasite occurring in man infecting about 10 % of population in developed countries, the infection rate in children is even higher (Hwang et al. 2002). Where in the present study the individuals under 18 years had the highest infection with enterobiasis.
Conclusions and recommendations
Conclusions
The histopathology proved the presence of E. vermicularis in the appendices.
The study showed that 30 (15.0 %) of patients with appendix were infected with E. vermicularis.
The histopathology showed the tissue morphology of each appendix normal or inflamed.
The presence of E. vermicularis in appendix can cause inflammation of appendix.
And the normal appendix have an E. vermicularis, with no change of histological tissue, and may produce symptoms which resemble acute appendicitis.
The surgeon has the final opinion in the appendectomy.
Recommendations
Scotch tape preparation (STP) should be available in emergency department.
We recommend suspected patient with appendicitis to do E. vermicularis STP test before surgery, and this depend on the situation of the patients. So physician and surgeon could take the appropriate decision.
In case of children with appendicitis we recommend using STP test.
Acknowledgments
Many Thanks for all staff in Kamal Edwan, Al Shifa and European hospitals in Gaza Strip for their kindly cooperation during the study and thanks extended to all people who participated in the study. Thanks to Mr. Moein Redwan and Mr. Jamal Al-Shaiekh Deeb for samples processing in the lab.
Contributor Information
Abdel Monem Lubbad, Email: ahindi@iugaza.edu.
Adnan I. Al-Hindi, Email: ahindi@iugaza.edu.ps
References
- Akhigbe T, Smith F, Adeyemo A, Adeyanju T, Condon E, Waldron D.(2013) Pinworm And Appendicitis In Children. The Internet J Surgery 30(3)
- Al-Hindi AI. Prevalence of some intestinal parasites among school children in Deir-El-Balah town, Gaza strip, Palestine. Ann Saudi Med. 2002;22:3–4. doi: 10.5144/0256-4947.2002.273. [DOI] [PubMed] [Google Scholar]
- Al-Hindi AI. Diagnosis of gastrointestinal parasites among hospitalized patients attending Al-Nasser Paediatric Hospital, Gaza, Palestine. J Public Health. 2009;17:49–53. doi: 10.1007/s10389-008-0211-z. [DOI] [Google Scholar]
- Al-Hindi AI, AL-Louh M. Trends of Intestinal Parasites Prevalence in Gaza Strip, 1998-2007: The Use of Government Health Records. Turk J Med Sci. 2013;43:652–659. doi: 10.3906/sag-1208-86. [DOI] [Google Scholar]
- Al-Hindi A, Basil B, El-Kariri M. Enterobiasis among pre-school children in Gaza strip. J Al Azhar Univ-Gaza (Natural Sciences) 2013;15:53–70. [Google Scholar]
- Arca MJ, Gates RL, Groner JI, Hammond S, Caniano DA. Clinical manifestations of appendiceal pinworms in children: an institutional experience and a review of literature. Pediat Surg Int. 2004;20:372–375. doi: 10.1007/s00383-004-1151-5. [DOI] [PubMed] [Google Scholar]
- Aydin O. Incidental parasitic infestations in surgically removed appendices: a retrospective analysis. Diag Pathol. 2007;2:16. doi: 10.1186/1746-1596-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert AC. Appendicitis. In: Peterson Holger, Allison David., editors. The encyclopedia of medical imaging. The Nicer Institute: Oslo; 1999. [Google Scholar]
- Batistatou A, Solota V, Scopa CD. Images in pathology: oxyuris (Enterobius vermicularis) in human cecum and appendix. Int J Surg Pathol. 2002;10:58. doi: 10.1177/106689690201000109. [DOI] [PubMed] [Google Scholar]
- Budd JS, Armstrong C. Role of Enterobious vermicularis in the etiology of appendicitis. Br J Surgery. 1987;78:74–89. doi: 10.1002/bjs.1800740834. [DOI] [PubMed] [Google Scholar]
- Burkhart CN, Burkhart CG. Assessment of frequency, transmission, and genitourinary complications of enterobiasis (pinworms) Int J Dermatol. 2005;44:837–840. doi: 10.1111/j.1365-4632.2004.02332.x. [DOI] [PubMed] [Google Scholar]
- Cook GC. Enterobius vermicularis infection. Gut. 1994;35:1159–1162. doi: 10.1136/gut.35.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CG, Zumla IA. Manson’s tropical disease. 21. Philadelphia: W. B. Saunders Ltd; 2003. [Google Scholar]
- Fallah E, Dehgani A (2011) A study on Enterobius vermicularis infection in a appendices removed by surgery in Tabriz Hospitals. IntJ ParasiticDis ™ ISSN: 1559-4629 01/2011; 4
- Gialamas E, Papavramidis T, Michalopoulos N, Karayannopoulou G, Cheva A, Vasilaki O, Kesisoglou I, Papavramidis S. Enterobius vermicularis: a rare cause of appendicitis. Turkiye Parazitol Derg. 2012;36:37–40. doi: 10.5152/tpd.2012.09. [DOI] [PubMed] [Google Scholar]
- Gutiérrez Y (2000) Diagnostic pathology of parasitic infections with clinical correlations (second edition). Oxford University Press. pp. 354–366. ISBN 0-19-512143-0
- Helmy AH, Abou Shousha T, Magdi M, Sabri T. Appendicitis; appendectomy and the value of endemic parasitic infestation. Surgery and Pathology Departments, Theodore Bilharz Research Institute. Egyp J Surg. 2000;19:87–91. [Google Scholar]
- Hussein AS. Prevalence of intestinal parasites among school children in northern districts of West Bank-Palestine. Trop Med Int Health. 2011;16(2):240–244. doi: 10.1111/j.1365-3156.2010.02674.x. [DOI] [PubMed] [Google Scholar]
- Hwang KP, Tsai WS, Lincy LN. Detection of Enterobius vermicularis eggs in the submcosa of the transversecolon of a man presented with colon carcinoma. Am J Trop Med Hyg. 2002;67:546–548. doi: 10.4269/ajtmh.2002.67.546. [DOI] [PubMed] [Google Scholar]
- Kucik CJ, Martin GL, Sortor BV. Common intestinal parasites. Am Family Phys. 2004;69:1161–1168. [PubMed] [Google Scholar]
- Marjorie J, Arca R, Gates L, Groner JI, Hammond S, Caniano DA. Clinical manifestations of appendiceal pinworms in children. An institutional experience and a review of the literature. Pediat Surg Int. 2004;20:372–375. doi: 10.1007/s00383-004-1151-5. [DOI] [PubMed] [Google Scholar]
- Ministry of Health (2014) Surgery Department records, Gaza
- Mogensen K, Pahle E, Kowalski K. Enterobius vermicularis and acute appendicitis. Acta Chirurgica Scand. 1985;151:705–707. [PubMed] [Google Scholar]
- Quasem A, Salam A. Ectopic enterobiasis: a case report and review of literature. Pak J Med Sc. 2007;23:785–787. [Google Scholar]
- Ramezani MA, Dehghani MR. Relationship between Enterobius vermicularis and the incidence of acute appendicitis. Southeast Asian J Trop Med Public Health. 2007;38:20–23. [PubMed] [Google Scholar]
- Sah SP, Bhadani PP. Enterobius vermicularis causing symptoms of appendicitis in Nepal. Trop Doct. 2006;36:160–162. doi: 10.1258/004947506777978361. [DOI] [PubMed] [Google Scholar]
- Saxena AK, Springer A, Tsokas J, Willital GH. Laparoscopic appendectomy in children with Enterobius vermicularis. Surg Lap End Perc Tech. 2001;11:284–286. doi: 10.1097/00129689-200108000-00012. [DOI] [PubMed] [Google Scholar]
- Shtayeh A, Hamdan AH, Shaheen SF, Abu-Zeid I, Faidy YR. Prevalence and seasonal fluctuations of intestinal parasites infections in Nablus area, West Bank of Jordan. Ann Trop Med Parasitol. 1989;83:67–72. doi: 10.1080/00034983.1989.11812312. [DOI] [PubMed] [Google Scholar]
- Shubair ME, Yassin MM, Al-Hindi AI, Al-Wahaidi AA, Jadallah SY, Abu Shaaban ND. Intestinal parasites in relation to haemoglobin level and nutritional status of school children in Gaza. J Egypt Soc Parasitol. 2000;30:365–375. [PubMed] [Google Scholar]
- Silva DF, Silva RJ, Silva MG, Sartorelli AC, Rodrigues MA. Parasitic infection of the appendix as a cause of acute appendicitis. Parasitol Res. 2007;102:99–102. doi: 10.1007/s00436-007-0735-0. [DOI] [PubMed] [Google Scholar]
- Stephan S, Marks DS, Smith P, El Habbal MH, Lewis S (2006) The great Ormond street color handbook of pediatrics and child health
- Wiebe K. Appendicitis and Enterobius vermicularis. Scan J Gast. 1991;26:336–338. doi: 10.3109/00365529109025051. [DOI] [PubMed] [Google Scholar]
- Yassin MM, Shubair ME, Al-Hindi AI, Jadallah SY. Prevalence of intestinal parasites among school children in Gaza City, Gaza Strip. J Egypt Soc Parasitol. 1999;29:365–373. [PubMed] [Google Scholar]