Abstract
Cystic echinococcosis (CE) is a severe zoonotic disease which affects both human and animals. The disease has a considerable economic and social impact, because it has numerous complications leading to important disabilities and even death. CE is a widespread chronic endemic helminthic disease caused by infection with metacestodes of tapeworm Echinococcus granulosus. This study was conducted to diagnosis human CE by hydatid cyst antigens from camels and sheep. Hydatid fluid and protoscoleces crude antigens corresponding to camel and sheep were resolute by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and the protein bands of different antigens were exposed to infected patients serum CE through western blot (WB) assay. The camel hydatid fluid antigen revealed five polypeptide bands of 18–98.8 kDa by SDS-PAGE while sheep hydatid fluid antigen revealed four polypeptide bands of 20–100 kDa. Immune reactive bands were obtained through WB ranged from 25 to 125 kDa. The study showed prominent immune reactive bands of 92, 52.2 and 35.7 kDa which may helpful in diagnosis of human CE.
Keywords: Cystic echinococcosis (CE), Human, Camel, Sheep, SDS-PAGE, Western blot (WB)
Introduction
Human cystic echinococcosis (CE) is a serious life-threatening neglected zoonotic disease that occurs in both developing and developed countries and is recognized as major public health problem (Mandal and Mandal 2012). The geographical distribution of the members of genus Echinococcus is characterized by the presence of different strains existing in each country (Rausch 1995; Eckert and Deplazes 2004). The genetic characterization of the members of genus Echinococcus in different animals; sheep, pig, cattle and camel were also based on morphology, host specificity and molecular characteristics (Zhang et al. 2006; Tawfeek et al. 2009).
The diagnosis of human CE is usually established by a combination of imagistic methods; Ultrasound, CT scan, X-ray, MRI and serological immunodiagnosis (Constantea and Ciobanca 2007). Specific antigens of Echinococcus granulosus hydatidosis of camel and equines were characterized through SDS-PAGE, the antigens showed common shared protein bands at the level of 66, 55, 45 and 29 kDa. The WB assay recognized two polypeptides at 80 and 150 kDa which might be diagnostic bands in the two intermediate hosts (Mahmoud et al. 2008). IgG anti-echinococcus was determined by ELISA and WB and confirmed the positive results done by imagistic methods (Ciobanca and Junie 2011). The diagnostic efficacy of crude sheep hydatid cyst fluid (HCF), antigen B and its subunit (12 kDa) to detect IgG antibodies in CE patient serum was evaluated using ELISA assay, it was concluded that sheep was the most closely related isolate to human at antigen B level and recommended to use anti-HCF IgG ELISA for initial screening in large seroprevalence studies (Tawfeek et al. 2011).
In Egypt, several studies have been done on hydatidosis in both man and livestock for estimation of the prevalence of infection rates in camels, donkeys, horses, pigs and sheep which were 40, 7.69, 6.25, 0.92 and 0.77 % respectively (Derbala and Zayed 1997). The SDS-PAGE and WB analysis of human and camel hydatid fluid antigens revealed immune reactive 50 kDa band which was prominent in serum of patients (El Zayyat et al. 1999). Moreover, the electrophoretic make up of camel protoscoleces PCAg and camel hydatid fluid HFC Ag showed common bands of 205, 191 and 98 kDa and the WB analysis revealed three polypeptides of PC and HF Ag by rabbit anti PC and HFAg of molecular weight 191, 149 and 45 and 190, 149 and 33 kDa, respectively (Abdel-Rahman et al. 2003).
The performance of western blot assay (WB) using native antigen B which prepared from sheep hydatid cyst fluid (HFS) for diagnosis of human hydatidosis was evaluated, 8, 16 and 24 kDa subunits of antigen B was detected and the 8 kDa subunit, was highly recommended for the confirmatory diagnosis of hydatidosis (Sarkari et al. 2007). WB assay was stated as more confirmatory test than indirect haemagglutination assay (IHA) and the WB IgG technique indicated the presence of immune reactive bands against E. granulosus and Echinococcus multilocularis: 7, 12, 15, 24, 26 and 28 kDa in the patient serum samples and was considered specific for cystic echinococcosis (Logar et al. 2008).
However, it is still need of confirmation of clinical diagnosis and epidemiological surveys in detection of human Cystic Echinococcosis (Ito et al. 2003); So that the aim of the present work was to determine the antigenicity and sero-epidemiological value of some hydatid cyst antigens from camels and sheep in diagnosis of human Cystic Echinococcosis.
Materials and methods
Hydatid cysts collection
The hydatid cysts were collected from lung and liver of naturally infected camels and sheep slaughtered at Cairo abattoir. The cysts kept in ice boxes and immediately transferred to the laboratory. The fertile hydatid cysts that possess viable protoscoleces were chosen and marked according to the procedures of Himonas et al. (1994). The protoscoleces and hydatid fluids of each cyst were collected aseptically and preserved in normal saline at −20 °C for antigen preparation.
Antigens preparation
Protoscoleces crude antigen
The crude antigen of protoscoleces for both camels (PCAg) and sheep (PSAg) origin were prepared as the methods described by Gasser et al. (1988).
Hydatid fluid crude antigens
The hydatid cysts fluids were aseptically aspirated from fertile unilocular hydatid cysts of camel and sheep. The collected fluids of each animal type; HFC and sheep HFS were centrifuged separately at 12,000 rpm for 15 min at 4 °C. The supernatant fluids were dialyzed against 0.1 M phosphate buffer solution (PBS, pH 7.2) at 4 °C, transferred to sterile tube as HFC and HFS crude antigens and stored at −20 °C until use (Nasrieh and Abdel-Hafez 2004).
Detection of antigen protein concentration
The protein concentration of the both protoscoleces and hydatid fluid antigen was determined using the method of Lowry et al. (1951) with slight modification.
Serum of human cystic echinococcosis
Two serum samples were obtained from two clinic patients admitted to the Department of Tropical Medicine, Faculty of Medicine, Ain Shams University. The patients complained acute abdominal pain and showed soft abdominal masses (hydatids) of moderate size being confirmed by abdominal sonography and serologically using ELISA test. The present history of the two patients showed no other parasitic infection or chronic diseases. Stool examination and urine and blood analysis were done for confirmation.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
The assay was adopted for PC, PS, HFC and HFS antigens as the procedures of Laemmli (1970). The molecular weight marker was included into the gel. After separation, the gel was fixed in methanol and stained with silver stain according to the method of Wray et al. (1981).
Western immunoblot (WB)
Immunoblot assay was utilized to identify the immune reactive components. The assay was carried out as the method described by Towbin et al. (1979), briefly, the protein bands of different antigens were electrophoretically transferred from SDS-PAGE to nitrocellulose sheet and the human sera were diluted in 5 % BSA in 0.3 % PBS-T, and then a nitrocellulose sheet was exposed to the diluted patient sera for one hour for detection of specific diagnostic antigens of human CE.
The molecular weight of polypeptide bands obtained by SDS-PAGE and western-blot were calculated using image software; www.bio-rad.com/prd/en/US/LSR/PDP/Image-Lab-Software.
Results
Electrophoretic assay (SDS-PAGE)
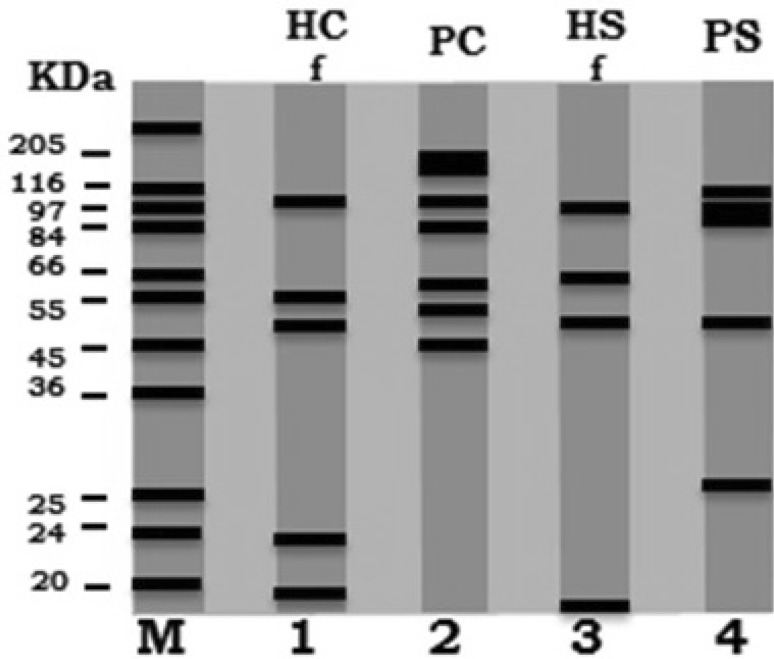
The electrophoretic profiles of camel and sheep hydatid fluids and protoscoleces crude antigens were assayed by SDS-PAGE; the electrophoretic pattern of camel hydatid fluid antigen (HFC) was separated into five bands with the molecular weights: 98.8, 61.2, 50.7, 23 and 18 kDa. While, camel protoscoleces antigen (PC) revealed seven bands with molecular weights of 141.7, 137.5, 99.7, 84, 60.5, 51.5 and 30.4 kDa. The electrophoretic profile of the sheep hydatid fluid antigen (HFS) showed four bands with molecular weights of 100, 60, 49.5 and 20 kDa, at the same time, the electrophoretic profile of the sheep protoscoleces antigen (PS) resulted in five bands with molecular weights of 103.1, 99.1, 96.5, 50.1 and 28.3 kDa (Table 1; Fig. 1).
Table 1.
Electrophoretic separation camel and sheep hydatid antigens (SDS-PAGE) followed by subsequent western blot assay (WB)
| Camel | Sheep | |||
|---|---|---|---|---|
| Hydatid fluid antigen (HFC) | Proto-scoleces antigen (PC) | Hydatid fluid antigen (HFS) | Proto-scoleces antigen (PS) | |
| SDS-PAGE | Five bands of MW: (98.8, 61.2, 50.7, 23 and 18) kDa | Seven bands of MW: (141.7, 137.5, 99.7, 84, 60.5, 51.5 and 30.4) kDa | Four bands of MW: (100, 60, 49.5 and 20) kDa | Five bands of MW: (103.1, 99.1, 96.5, 50.1 and 28.3) kDa |
| Western-blot | Four bands of MW:(125, 92, 52.2 and 33) KDa | Two bands of MW:(92 and 45.5) kDa | Three bands of MW: (100, 52.2 and 35.7) kDa | Three bands of MW: (35.7 and 25) kDa |
Fig. 1.
Comparative electrophoretic profile (SDS–PAGE): camel hydatid fluid (HC) lane 1, camel protoscoleces extract (PC) lane 2, sheep hydatid fluid (HS) lane 3, sheep protoscoleces extract (PS) lane 4 and molecular weight standards in kDa lane M
Western immunoblot analysis (WB)
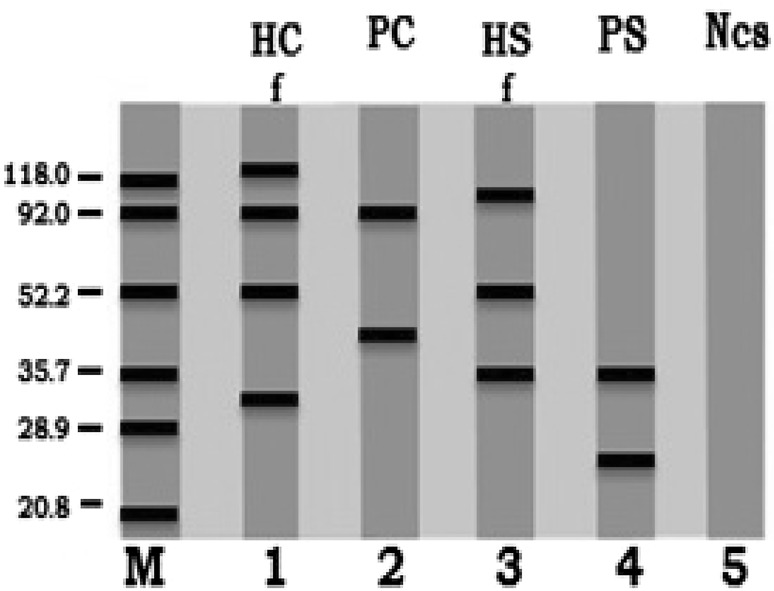
The immunoblot reactions were detected by WB assay where, the different separated protein bands of crude antigens (HFC, PC, HFS and PS) were passed against infected human patient’s antisera. The recognized immunoreactive bands were: 125, 92, 52.2, and 33 kDa for HFC antigen, 92 and 45.5 kDa for PC antigen, 100, 52.2 and 35.7 kDa for HFS antigen and 35.7 and 25 kDa for PS antigen (Table 1; Fig. 2).
Fig. 2.
Immunoreactive bands identified by infected human serum with hydatid cyst through western blot assay (WB): camel hydatid fluid (HC) lane 1, camel protoscoleces (PC) lane 2, sheep hydatid fluid (HS) lane 3, sheep protoscoleces (PS) lane 4 and negative control serum (Ncs) lane 5 and molecular weight standards in kDa lane M
Discussion
Evaluation of cystic fluid and protoscoleces as alternative source of antigen by using the immune diagnostic methods is important tool in sero-diagnosis of cystic echinococcosis infection (Mahmoud et al. 2008; Youssef et al. 2010). In the present study, 4 types of antigens (hydatid fluid and protoscoleces of both camel and sheep) were investigated with respect to the epidemiology of E. granulosus in human, camels and sheep. Also, the broad immunogenicity of some molecules detected in the current research raise the chance to be used as immunodiagnostic tools in human infections with hydatidosis.
Extraction of special protein fraction using SDS-PAGE electrophoretic analysis to use as antigens derived from cyst fluid (Heath et al. 1992) and protoscoleces (Hernandez and Nieto 1994) could be able to induce accurate diagnosis for hydatid infected animals and man. In our investigation SDS-PAGE of the 4 antigen types revealed separated similar or nearly identical protein molecular bands between hydatid fluid antigens 100 and 98.8; 61.2 and 60; 50.7 and 49.5 kDa from camel and sheep, respectively. Also, camel and sheep protoscoleces Ag revealed relatively similar molecular bands: 99.7 and 99.1; 51.5 and 50.1 kDa. The similarity between hydatid fluid antigens and between protoscoleces antigens obtained from different animal species was accepted as obtained by many other studies (Mahmoud et al. 2008; Derbala and Zayed 1997; El Zayyat et al. 1999).
The immune reactive bands detected by the western immunoblot against infected patient’s serum with hydatidosis revealed one similar band 92 kDa in both camel hydatid fluid and protoscoleces antigens and also one similar band of 35.7 kDa in both sheep hydatid fluid and protoscoleces antigenss. At the same time, one similar bands of molecular weights 52.2 kDa in camel and sheep hydatid fluid antigens; while, no sharing similar bands were found in PC and PS. The present results were in agreement with (El Zayyat et al. 1999), who detected a band of 50 kDa in running WB using camel hydatid antigens. On the other hand some studies showed high molecular weight band above 60 kDa in WB analysis (Mahmoud et al. 2008; Abdel-Rahman et al. 2003), while other ones showed low molecular weight ones, below 30 kDa (Ciobanca and Junie 2011; Tawfeek et al. 2011; Sarkari et al. 2007).
It is obvious from this study and many authors’ results which showed different data of human cystic Echinococcus infection that CE is still a public health problem and predominantly in many countries (Schantz 1991; Siracusano et al. 2008; Huang and Zheng 2012). Therefore, clinicians and health authorities should pay greater attention to CE and should make additional efforts to decrease or even eliminate this disease in the future.
Conclusion and recommendations
It could be concluded that immunological characterization of both camel and sheep hydatid fluid and protoscoleces antigens, beside the immune-reactive study may be helpful for diagnosis of hydatidosis in human. However, immunological procedures for the detection of antibodies to hydatid infection need epidemiological studies so effective measurements might become implemented for a better control of this zoonotic Cystic Echinococcosis which should be considered and need further examination and treatment.
References
- Abdel-Rahman EH, Abdel-Megeed KN, Abuel-Ezz NM. Cross-reaction: a common trait among helminthes. J Egypt Soc Parasitol. 2003;33:457–471. [PubMed] [Google Scholar]
- Ciobanca PT, Junie ML. Diagnosis confirmation of human cystic echinococcosis by imagistic methods and immunoserological determinations. Sci Parasitol. 2011;12(3):151–161. [Google Scholar]
- Constantea N, Ciobanca P. Studiul clinic pentru imunatatirea metodelor de diagnostic de laborator si profilaxia chistului hidatic. Rev Rom Parasitol. 2007;17:48–49. [Google Scholar]
- Derbala AA, Zayed AA. Prevalence, fertility and viability of cysticercosis and hydatidosis infection in some domestic animals. J. Union Arab Biol Cairo. 1997;7A:109–123. [Google Scholar]
- Eckert J, Deplazes P. Biological, epidemiological and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17(1):107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Zayyat EA, Ramzy RM, Abdel-Baki MH, Fahmi IA, Rifaat M, Helmy H, Abdel Hameed DM. Human cystic echinococcosis: diagnostic value of different antigenic fractions of hydatid cyst fluid with different specific immunoglobulin G subclasses by enzyme linked immuno-electrotransfer blot. J Egypt Soc Parasitol. 1999;29:817–830. [PubMed] [Google Scholar]
- Gasser RB, Lightowlers MW, Obendorf DL, Jenkins DJ, Rickard MD. Evaluation of a serological test system for the diagnosis of natural Echinococcus granulosus infection in dogs using E. granulosus protoscolex and oncosphere antigens. Aust Vet J. 1988;65:369–373. doi: 10.1111/j.1751-0813.1988.tb14274.x. [DOI] [PubMed] [Google Scholar]
- Heath DD, Lawrence SB, Yong WK. Echinococcus granulosus in sheep: transfer from ewe to lamb of Arc5 antibodies and onchospher-killing activity, but not protein. Int J Parasitol. 1992;22:1017–1021. doi: 10.1016/0020-7519(92)90063-Q. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Nieto A. Induction of protective immunity against murine secondary hydatidosis. Parasite Immunol. 1994;16:527–544. doi: 10.1111/j.1365-3024.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Himonas C, Antoniadou S, Papadopoulos E. Hydatidosis of food animals in Greece (prevalence of cysts containing viable protoscoleces) J Helminthol. 1994;68:311–313. doi: 10.1017/S0022149X00001541. [DOI] [PubMed] [Google Scholar]
- Huang M, Zheng H. Clinical and demographic characteristics of patients with urinary tract hydatid disease. PLoS One. 2012;7(11):1–6. doi: 10.1371/journal.pone.0047667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Urbani C, Jiamin Q, Vuitton DA, Dongchuan Q, Heath DD, Craig PS, Zheng F, Schantz PM. Control of echinococcosis and cysticercosis: a public health challenge to international cooperation in China. Acta Trop. 2003;86:3–17. doi: 10.1016/S0001-706X(02)00269-3. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logar J, Šoba B, Lejko-Zupanc T, Kotar T. Serological evidence for human cystic echinococcosis in Slovenia. BMC Infect Dis. 2008;8:1–4. doi: 10.1186/1471-2334-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:262–275. [PubMed] [Google Scholar]
- Mahmoud MS, Derbala AA, El-Massry AA, Maarouf OA. Sero-diagnostic potency of hydatid fluid and protoscoleces partially purified fractions of both camel and equine origin. Global Veterinaria. 2008;2(3):99–103. [Google Scholar]
- Mandal S, Mandal MD. Human cystic echinococcosis: epidemiologic, zoonotic, clinical, diagnostic and therapeutic aspects. Asian Pac J Trop Med. 2012;5(4):253–260. doi: 10.1016/S1995-7645(12)60035-2. [DOI] [PubMed] [Google Scholar]
- Nasrieh MA, Abdel-Hafez SK. Echinococcus granulosus in Jordan: assessment of various antigenic preparations for use in the serodiagnosis of surgically confirmed cases using enzyme immuno assays and the indirect haemagglutination test. Diagn Microbiol Infect Dis. 2004;48(2):117–123. doi: 10.1016/j.diagmicrobio.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Rausch RL. Life cycle patterns and geographic distribution of Echinococcus species. In: Thompson RCA, Lymbery AJ, editors. Echinococcus and hydatid disease. Wallingford: CAB International; 1995. pp. 89–134. [Google Scholar]
- Sarkari B, Sadjjadi SM, Abidi H, Izadpanah Kazemian S, Rafati A. Application of western blotting using native antigen B for serodiagnosis of human echinococcosis. Iranian J Parasitol. 2007;2(3):7–12. [Google Scholar]
- Schantz PM. Parasitic zoonoses in perspective. Int J Parasitol. 1991;21(2):161–170. doi: 10.1016/0020-7519(91)90006-S. [DOI] [PubMed] [Google Scholar]
- Siracusano A, Margutti P, Delunardo F, Profumo E, Rigano R. Molecular cross-talk in host-parasite relationships: the intriguing immunomodulatory role of Echinococcus antigen B in cystic echinococcosis. Int J Parasitol. 2008;38:1371–1376. doi: 10.1016/j.ijpara.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Tawfeek GM, Elwakil HS, Awad NS, El-Hoseiny L, Thabet HS, Sarhan RM, Darweesh SK, Anwar WA. Genetic variability of antigen B among Echinococcus granulosus Egyptian isolates. Korean J Parasitol. 2009;47(3):259–264. doi: 10.3347/kjp.2009.47.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfeek GM, Elwakil HS, El-Hoseiny L, Thabet HS, Sarhan RM, Awad NS, Anwar WA. Comparative analysis of the diagnostic performance of crude sheep hydatid cyst fluid, purified antigen B and its subunit (12 kDa), assessed by ELISA, in the diagnosis of human cystic echinococcosis. Parasitol Res. 2011;108(2):371–376. doi: 10.1007/s00436-010-2074-9. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;176:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W, Boulikas T, Wray VP, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Youssef MR, Hosseini SH, Hassan MT. Evaluation and comparison response in laboratory model to low antigen of fluid and protoscolex in hydatid cyst. Global Veterinaria. 2010;4(6):622–625. [Google Scholar]
- Zhang Y, Bart JM, Giraudoux P, Craig P, Vuitton D, Wen H. Morphological and molecular characteristics of Echinococcus multilocularis and Echinococcus granulosus mixed infection in a dog from Xinjiang. China Vet Parasitol. 2006;139(1–3):244–248. doi: 10.1016/j.vetpar.2006.03.003. [DOI] [PubMed] [Google Scholar]