Abstract
The study was carried out to assess the effect of condensed tannins (CT) supplementation through leaf meal mixture (LMM) on feed intake, humoral [Immunoglobulin G (IgG)], cell mediated immune response (CMI) and faecal egg counts in Haemonchus contortus infected sheep. Eighteen sheep were randomly divided into three groups (negative control—NC, infected control—C and Infected treatment—T) of six animals in each group in a completely randomized block design for a period of 90 days. Twelve H. contortus infected adult sheep were allocated into two equal groups C and T, supplemented with 0 and 1.5 % of CT, respectively. Six non-infected sheep of similar age and body weight of NC group were included in this study to compare their immune response with H. contortus C and CT supplemented T groups. Intake of dry matter and organic matter (g day−1 and % live weight) was statistically similar (P < 0.05) among the three groups. The anti-Haemonchus IgG and CMI response was higher in T group as compared to C group. The mean faecal egg counts was significantly (P < 0.001) higher in C group as compared to T group. It may be concluded that dietary supplementation of CT (1.5 %) through LMM improved humoral and CMI immune response and decreased worm load in H. contortus infected sheep.
Keywords: Condensed tannins, Immune response, Haemonchus contortus infection, Leaf meal mixture, Sheep
Introduction
Gastrointestinal nematode (GIN) infection is arguably the most serious constraint affecting small ruminant production worldwide. Economic losses are caused by reduced production, the costs of prophylaxis and the death of the infected animals (Pathak and Tiwari 2013). The control of GIN traditionally relies on anthelmintic treatment. The evolution of anthelmintic resistance in GIN threatens the success of anthelmintic treatment programs. Alternative strategies for control of GIN infections are being developed, and one approach is to take advantage of the host’s immune responses, which can be increased by protecting dietary protein degradation in the rumen. Dietary supplementation of condensed tannins (CT) decreases the degradation of dietary protein and ‘S’ amino acids to inorganic sulphide in the rumen and increase absorption of Methionine and Cystine in sheep (McNabb et al. 1993) and reduced GIN burden in animals. It has been suggested that the increased by-pass protein supply caused by the action of CT present in the forage helps counteract the protein losses caused by GIN infections. This high-quality protein bypass effect has the potential to enhance the immune response and increase resistance to GIN (Min et al. 2004). Supplementation of CT from potential tropical tanniferous tree leaves may be a possible alternative approach to reduce Haemonchus contortus load; however information regarding effect of CT from tropical tree leaves/leaf meal mixture (LMM) on H. contortus infection is scare. This raises the possibility that feeding locally available tree leaves/LMM containing CT may be an alternative method for controlling H. contortus infections, especially in areas such as the tropics and subtropics. Keeping this in view, it was proposed to investigate the effect of CT through LMM on immune response and H. contortus load in sheep.
Materials and methods
The experimental study was conducted at the Animal Nutrition Research Sheds of the IVRI, Izatnagar. A feeding trial of 90 days duration was undertaken in 18 non-descript adult sheep of either sex, similar age and mean body weight 25.03 ± 1.52 kg were selected from the well maintained herd and randomly allocated into three groups in a completely randomized design. Out of 18 sheep, 12 sheep were infected with infective third stage larvae of H. contortus @ 2000 larvae per sheep. All the sheep were divided into three different groups’ i.e. negative control (NC), control (C; H. contortus infected) and treatment (T; H. contortus infected + CT @ 1.5 % of the diet through LMM). A negative control group was taken to compare their performance with infected control and treatment groups. All experimental sheep were offered a basal diet of wheat straw ad libitum along with required amount of concentrate mixtures to meet their nutrient requirements for maintenance as per Kearl (1982) for a period of 90 days. One hundred gram oat hay was given to each sheep per day to meet their vitamin-A requirement. The LMM was prepared by mixing of Ficus infectoria and Psidium guajava in the ratio of 70:30 and incorporated in the concentrate mixture of T group by replacing concentrate so as to bring CT content to 1.5 % of diet.
Chemical analysis
Samples of concentrate mixture, LMM, oat hay and wheat straw were milled to pass through a 1 mm sieve and analyzed for their proximate principles (AOAC 1995). The fibre fractions, neutral detergent fibre (NDF) and acid detergent fibre (ADF) were estimated as per the methods of Van Soest et al. (1991). The estimation of CT content of LMM was done by Butanol-Hcl method (Makkar 2000). Blood samples were collected by jugular vein puncture at 30, 60 and 90 days feeding. About 3 ml of blood was collected from every sheep for serum collection. The collected sera samples were stored in deep freeze at −20 °C for estimation of IgG response.
For preparation of antigen, about 100 adult H. contortus worms (both male and female) were collected and washed properly in phosphate buffer saline (PBS; pH 7.2). The soluble proteins from adult worms were extracted by grinding in a pestle and mortar with autoclaved sand and PBS to form a paste at 4 °C. It was transferred to the homogenizer for few minutes in ice cold container to form a smooth homogeneous paste. The mixture was diluted with ice cold PBS to about 5 ml and centrifuged in a refrigerated centrifuge at 4 °C (10,000g) for 10 min. The supernatant was collected and filtered (0.22 µm syringe filter, Millipore, India) in previously autoclaved vial to avoid any bacterial contamination. The IgG response was determined by ELISA. The ELISA conditions were similar to those described by Cuquerella et al. (1991). The IgG response of all experimental sheep was estimated at 30 days interval after the administration H. contortus infection throughout the experimental feeding of 90 days duration.
Cell mediated immune (CMI) response was assessed through in vivo cutaneous delayed-type hypersensitivity (DTH) reaction against phytohaemagglutin-P (PHA-P). Towards the end of feeding, all experimental sheep were injected 125 µl of PHA-P intra-dermal on one side of neck region. Skin thickness was subsequently measured with the help of digital vernier calipers at the beginning (0 h) followed by 24 h intervals and up to 96 h.
Faecal samples from all experimental sheep were collected at fortnightly interval directly from the rectum for faecal egg counts (FECs). After collection, FECs were estimated using the modified McMaster technique (Anonymous 1984). One gm of faeces was weighed, mixed thoroughly with 14 ml saturated salt solution and charged in one chamber of McMaster slide having capacity of 0.3 ml. Total number of eggs counted in the chamber was multiplied by 50 to get the number of eggs present in one gm of faeces. At the beginning of the experiment the experimental sheep were free from any infection as indicated by FECs. However, after 19 days of H. contortus (L3) administration sheep in C and T groups showed passage of eggs in their faeces. Though, FECs were zero in sheep of negative control (NC) group throughout the experimental period, so they were not included in the statistical analysis.
Statistical analysis
The results obtained were subjected to analysis of variance using SPSS 11.0 software and treatment means were ranked using Duncan’s multiple range tests. The periodic alterations in FECs were analyzed using repeated measures design (General linear model, Multivariate). Significance of treatments with respect to different characters was declared at P < 0.05 unless otherwise stated. All the statistical procedure was done as per Snedecor and Cochran (1994).
Results and discussion
The chemical composition (% DM basis) of concentrate mixture, leaf meal mixture, oat hay and wheat straw offered to sheep for a period of 90 days and during metabolism trial is presented in the Table 1. The chemical composition of concentrate, LMM, oat hay and wheat straw used in the experiment was comparable with the values reported by many workers (Dutta and Sharma 2004; Patra et al. 2006; Dey et al. 2008). The NDF and ADF values were higher in LMM as compared to concentrate. This could be attributed to the high cell-wall constituents usually present in leaf meal (Anbarasu et al. 2004; Dey et al. 2008). The intake of dry matter and organic matter (g day−1 and % live weight) were statistically similar (P > 0.05) among the three groups. The roughage (oat hay (@ 100 g day−1) and wheat straw) intake (g) was also found to be statistically non significant (P > 0.05) under three dietary treatments. However, intake (g day−1) of concentrate mixture was reduced significantly (P < 0.01) in the T group as compared to NC and C group (Table 2). Present results are in agreement with the findings of Scharenberg et al. (2008), who reported non-significant difference in total intake of DM and OM in H. contortus infected lambs fed on diet with and without tanniferous sainfoin. The intake of DM by sheep was within the normal range (Kearl 1982) and this clearly indicates that all the experimental diets were palatable. Present results suggesting CT supplementation at low to moderate level without any adverse effect on nutrient intake are in consistency with the earlier reports (Terrill et al. 1992; Dey et al. 2008).
Table 1.
Chemical composition of feeds (on % DM bases)
| Attributes | Wheat straw | Oat hay | Concentrate mixture | Leaf meal mixture |
|---|---|---|---|---|
| OM | 92.72 | 90.95 | 91.79 | 90.82 |
| CP | 3.43 | 9.10 | 22.10 | 10.80 |
| EE | 1.55 | 1.63 | 2.98 | 3.21 |
| Total ash | 7.28 | 9.05 | 8.21 | 9.18 |
| NDF | 84.75 | 70.17 | 35.15 | 57.29 |
| ADF | 55.68 | 46.20 | 11.03 | 44.97 |
| Condensed tannins | – | – | – | 10.44 |
Table 2.
Effect of condensed tannins supplementation on nutrient intake by sheep
| Attributes | Group | SEM | P values | ||
|---|---|---|---|---|---|
| NC | C | T | |||
| Dry matter | |||||
| g day−1 | 624.27 ± 84.95 | 669.77 ± 96.22 | 683.37 ± 83.02 | 48.25 | 0.885 |
| % LW | 2.31 ± 0.24 | 2.59 ± 0.31 | 2.62 ± 0.33 | 0.16 | 0.720 |
| Organic matter | |||||
| g day−1 | 599.17 ± 78.11 | 642.39 ± 89.02 | 648.64 ± 77.98 | 44.72 | 0.898 |
| % LW | 2.22 ± 0.22 | 2.48 ± 0.29 | 2.49 ± 0.31 | 0.15 | 0.732 |
| Concentrate | |||||
| g day−1 | 188.18b ± 9.47 | 188.27b ± 13.43 | 143.73a ± 6.27 | 7.50 | 0.010 |
| % LW | 0.71b ± 0.01 | 0.74b ± 0.05 | 0.55a ± 0.03 | 0.03 | 0.003 |
| Leaf meal mixture | |||||
| g day−1 | 0.00 | 0.00 | 114.83 ± 16.85 | 14.15 | 0.000 |
| % LW | 0.00 | 0.00 | 0.44 ± 0.07 | 0.05 | 0.000 |
| Roughage | |||||
| g day−1 | 436.09 ± 78.49 | 481.50 ± 83.27 | 424.82 ± 60.86 | 41.01 | 0.853 |
| % LW | 1.60 ± 0.26 | 1.85 ± 0.27 | 1.63 ± 0.24 | 0.14 | 0.758 |
NC negative control, C control, T treatment
abMeans with different superscripts within a row differ significantly
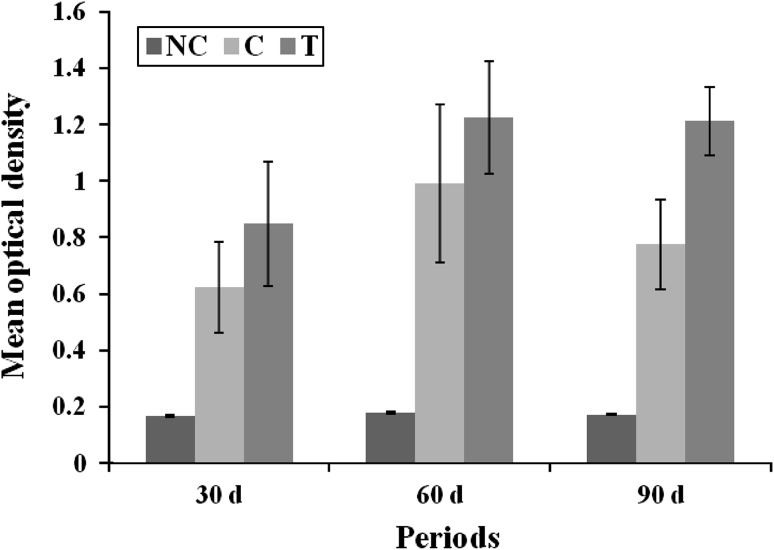
Effect of CT on IgG response in H. contortus infected sheep is depicted in the Fig. 1. The IgG response was significantly (P < 0.001) higher in both infected groups (C and T) than in negative control (NC). When IgG response was compared between infected groups, the higher (P < 0.001) response was evident in T group as compared to C group. The IgG response when compared among time intervals, the higher response was evident from 60 to 90 days of H. contortus infection. The serum of NC group did not alter any immunological response against H. contortus antigen throughout the experimental period. The specific IgG response is consistently associated with reduction of the helminth size and parasitic fecundity (Amarante et al. 2005), by metabolic enzyme neutralization, interfering with the feeding (Schallig 2000) and metabolism of H. contortus (Strain and Stear 2001). Alunda et al. (2003) observed a slight increase in the values of IgG after the primary H. contortus infection of 0.46 OD, other authors have observed increase in the serum levels of IgG in primary infection with OD values of 0.353 (Pena et al. 2004), 0.55 (Schallig et al. 2000) and 0.6 (Verelde et al. 2001).
Fig. 1.
IgG response of sheep against H. contortus as detected by ELISA due to CT supplementation
Present results are in accordance with the findings of Dutta et al. (2012), who reported perceptible encouraging impact on growth, antioxidant status and humoral immune response in kids given diet containing 1–2 % CT. Kumar et al. (2007) observed that the birds given tannins containing raw red sorghum exhibited higher humoral immune response assessed through HA titre. The role of tannins on immune response can be understood as flavonoids, tannins and micronutrients have been suggested to act as antioxidants and exert their antioxidant activity by scavenging the lipid peroxidation (Yuting et al. 1990).
The CMI response of experimental sheep under three different groups is presented in Table 3. Experimental sheep under C and T groups exhibited an increase in skin thickness following the intra dermal injection of PHA-P. The mean values for skin thickness differed significantly (P < 0.001) among different groups and the maximum (P < 0.001) skin thickness was observed in CT supplemented group (T), however, mean values of skin thickness were statistically similar (P > 0.05) between C and NC groups. Sheep fed diet supplemented with CT showed significantly higher CMI response as compared to C group. CMI response among treatment groups, periods and interaction was also significant (P < 0.001). The CMI response are in agreement with findings of Ross et al. (2001), who observed that the administration of Punica granatum fruit rind powder (PGFRP, containing tannins) in rabbits @ 100 mg kg−1 showed significant increased in the skin thickness compared to control group. Increase in delayed type hypersensitivity (DTH) reaction in response to thymus-dependent antigen revealed the stimulatory effect of PGFRP on T-cells and accessory cell types required for the expression of the reaction (Luster et al. 1982). The birds given red sorghum (containing tannins) exhibited higher immuno-responsiveness than their reconstituted counterparts assessed through cellular (footpad index) immune response (Kumar et al. 2007). Improved bio-availability of proteins has the potential to enhance the immune response and increase resistance to GIN (Min et al. 2004). By-passing amino acids like arginine, glutamine and cysteine can enhance immune responses as these amino acids regulate activation of T and B lymphocytes, natural killer cells and macrophages, gene expression and lymphocyte proliferation, and the production of antibodies, cytokines and other cytotoxic substances (Li et al. 2007).
Table 3.
Effect of condensed tannins supplementation on DTH response of sheep to PHA-P
| Group | Period (Hours post inoculation) | Mean ± SE | P values* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | 48 | 72 | 96 | G | P | G × P | ||
| NC | 2.92 ± 0.48 | 5.52 ± 0.57 | 4.83 ± 0.54 | 3.82 ± 0.33 | 3.33 ± 0.39 | 3.00 ± 0.46 | 3.90a ± 0.24 | 0.000 | 0.000 | 0.000 |
| C | 2.83 ± 0.20 | 5.70 ± 0.63 | 5.13 ± 0.43 | 4.13 ± 0.36 | 3.45 ± 0.19 | 3.03 ± 0.27 | 4.05a ± 0.23 | |||
| T | 2.45 ± 0.20 | 10.35 ± 1.21 | 7.75 ± 0.45 | 6.22 ± 0.42 | 4.90 ± 0.28 | 3.77 ± 0.37 | 5.91b ± 0.49 | |||
| Mean ± SE | 2.73a ± 0.31 | 7.19e ± 1.24 | 5.91d ± 0.71 | 4.72c ± 0.57 | 3.89b ± 0.41 | 3.27ab ± 0.38 | ||||
NC negative control, C control, T treatment
* G × P: Interaction between group and period
abcdeMeans with different superscripts within a row and column differ significantly
Effect of CT on FECs in H. contortus infected sheep is shown in the Table 4. Mean FECs were significantly (P < 0.001) higher in C group as compared to T group. The present findings are in agreement with the previous report (Sokerya and Preston 2003), who reported that dietary supplementation of CT may be used as an alternative parasite management strategy. Moore et al. (2008) reported that feeding SL hay can reduce FEC and increase performance of goats compared with Bermuda grass (BG) hay. The effect of reduced GIN infection levels in kids fed SL hay confirms the reports with sheep and goats fed this forage in different dried forms (Shaik et al. 2006; Lange et al. 2006).
Table 4.
Effect of condensed tannins supplementation on faecal egg count per gram by sheep
| Groups | Period | Mean ± SE | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| C | 337.50Aa | 954.17Ab | 2,004.17ABa | 3,712.50BCb | 5,412.50Cb | 7,979.17Db | 3,400.00b ± 523.07 |
| T | 116.67Aa | 266.67Aa | 658.33Aa | 1,100.00ABa | 1,758.33ABa | 2,741.67Ba | 1,106.94 ± 256.89 |
| Mean ± SE | 227.08 ± 67.66 | 610.42 ± 128.86 | 1,331.25 ± 282.90 | 2,406.25 ± 530.49 | 3,585.42 ± 801.92 | 5,360.42 ± 1,093.57 | |
C control, T treatment
ABCDMean values with different superscripts within a row differ significantly
abMean values with different superscripts within a column differ significantly
Reduced FEC have been attributed to both direct (reduced fecundity, killing of adult worms; Shaik et al. 2006) and indirect, the dietary supplementation of CT improved immune function against GIN through enhanced tissue protein supply (higher absorption of amino acid; AA) that is required for repair and immune response (Niezen et al. 2002). Pathak et al. (2013) observed that CT extracts from various tree leaves can disrupt the life cycle of H. contortus by preventing their eggs from hatching and larval development to the infective stage and by direct killing of adult H. contortus. Shaik et al. (2006) reported a predominantly direct effect of SL hay on GIN in goats with approximately 70 % less adult H. contortus compared with animals fed BG hay. Lange et al. (2006) suggested that the effect of SL hay feeding on GIN in sheep was a combination of reduced fecundity and direct killing of the worms. Both direct and indirect effects against GIN infection were apparently evident in the present study.
Conclusion
On the basis of present results, it may be concluded that dietary supplementation of condensed tannins @1.5 % of diet through a mixture of F. infectoria and P. guajava leaves significantly improved the humoral and cell mediated immune response and decreased FECs in H. contortus infected sheep without any adverse effect on voluntary feed intake.
Acknowledgments
This study was financially supported by funds provided by the Indian Council of Agriculture Research, New Delhi, India.
References
- Alunda JM, Angulo-Cubillan F, Cuquerella M. Immunization against ovine haemonchosis with three low molecular weight somatic antigens of adult Haemonchus contortus. J Vet Med Ser B. 2003;50(2):70–74. doi: 10.1046/j.1439-0450.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- Amarante AF, Bricarello PA, Huntley JF, Mazzolin LP, Gomes JC. Relationship of abomasal histology and parasite-specific immunoglobulin A with the resistance to H. contortus infection in three breeds of sheep. Vet Parasitol. 2005;128:99–107. doi: 10.1016/j.vetpar.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Anbarasu C, Dutta N, Sharma K, Rawat M. Response of goats to partial replacement of dietary protein by a leafmeal mixture containing Leucaena leucocephala, Morus alba and Tectona grandis. Small Rumin Res. 2004;51:47–56. doi: 10.1016/S0921-4488(03)00203-7. [DOI] [Google Scholar]
- Anonymous (1984) Manual of veterinary investigation. Vol. 2 reference book 390. Ministry of agriculture, fisheries and food. Her Majestry-Stationary Office, London, pp 161–187
- AOAC (1995) Animal feeds. Official methods of analysis of AOAC international, vol 1, 16th edn. AOAC, International Virginia, USA, pp 2201–3301
- Cuquerella M, Gomez-Munoz MT, Alunda JM. Serum IgG response of Manchego lambs to infections with Haemonchus contortus and preliminary characterization of adult antigens. Vet Parasitol. 1991;38:131–143. doi: 10.1016/0304-4017(91)90123-D. [DOI] [PubMed] [Google Scholar]
- Dey A, Dutta N, Sharma K, Pattanaik AK. Effect of dietary inclusion of Ficus infectoria leaves as a protectant of proteins on the performance of lambs. Small Rumin Res. 2008;75:105–114. doi: 10.1016/j.smallrumres.2007.06.013. [DOI] [Google Scholar]
- Dutta N, Sharma K. Replacement of wheat bran by the rice polishing as an economic supplement to wheat straw diet for lactating buffaloes in northern plains of India. Anim Nutr Feed Technol. 2004;4:113–120. [Google Scholar]
- Dutta N, Dubey M, Banerjee PS, Pattanaik AK, Sharma K, Kumar P, Narang A (2012) Effect of supplementing tanniferous tree leaves mixture on immune response and GI nematodes in kids. Live Res Rural Dev. 24(2). http://www.lrrd.org/lrrd24/2/dutt24035.htm
- Kearl LC (1982) Nutrient requirements of ruminants in developing countries. International Feed Stuffs Institute, Utah Agricultural Experiment Station, Utah State University, Logan, Utah, 84322, USA, pp 45–81
- Kumar V, Elangovan AV, Mandal AB, Tyagi PK, Bhanja SK, Dash BB. Effects of feeding raw or reconstituted high tannin red sorghum on nutrient utilization and certain welfare parameters of broiler chickens. Br Poult Sci. 2007;48(2):198–204. doi: 10.1080/00071660701251089. [DOI] [PubMed] [Google Scholar]
- Lange KC, Olcott DD, Miller JE, Mosjidis JA, Terrill TH, Burke JM, Kearney MT. Effect of sericea lespedeza (Lespedeza cuneata) fed as hay, on natural and experimental H. contortus infections in lambs. Vet Parasitol. 2006;141:273–278. doi: 10.1016/j.vetpar.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Li P, Yu-long Y, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- Luster ML, Dean JF, Boorman GA. Cell mediated immunity and its application in toxicology. Environ Health Perspect. 1982;43:31–36. doi: 10.1289/ehp.824331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar HPS (2000) Quantification of tannins in tree foliage—a laboratory manual. FAO/IAEA Working Document, Vienna, Austria, pp 1–26
- McNabb WC, Waghorn GC, Barry TN, Shelton ID. The effects of condensed tannin in Lotus pedunculatus upon the digestion and metabolism of methionine, cystine and inorganic sulphur in sheep. Br J Nutr. 1993;70:647–661. doi: 10.1079/BJN19930155. [DOI] [PubMed] [Google Scholar]
- Min BR, Pomroy WE, Hart SP, Sahlu T. The effect of short-term consumption of a forage containing condensed tannins on gastrointestinal nematode parasite infections in grazing wether goats. Small Rumin Res. 2004;51:279–283. doi: 10.1016/S0921-4488(03)00204-9. [DOI] [Google Scholar]
- Moore DA, Terrill TH, Kouakou B, Shaik SA, Mosjidis JA, Miller JE, Vanguru M, Kannan G, Burke JM (2008) The effects of feeding sericea lespedeza hay on growth rate of goats naturally infected with gastrointestinal nematodes. J Anim Sci, online May 9 [DOI] [PubMed]
- Niezen JH, Charleston WAG, Robertson HA, Shelton D, Waghorn GC, Green R. The effect of feeding sulla (Hedysarum coronarium) or lucerne (Medicago sativa) on lambs parasite burdens and immunity to gastrointestinal nematodes. Vet Parasitol. 2002;105:229–245. doi: 10.1016/S0304-4017(02)00014-6. [DOI] [PubMed] [Google Scholar]
- Pathak AK, Tiwari SP (2013) Effect of high plane of nutrition on the performance of Haemonchus contortus infected kids. Vet World. doi:10.5455/vetworld.2013.22-26
- Pathak AK, Dutta N, Banerjee PS, Sharma K. Effect of tannin extracts from tropical tree leaves on larvae and adult Haemonchus contortus. Indian Vet J. 2013;90(1):32–34. [Google Scholar]
- Patra AK, Sharma K, Dutta N, Pattanaik AK. Effect of partial replacement of dietary protein by a leaf meal mixture on nutrient utilization by goats in pre and late gestation. Small Rumin Res. 2006;63:66–74. doi: 10.1016/j.smallrumres.2005.02.008. [DOI] [Google Scholar]
- Pena MT, Miller JE, Horohov DW. Effect of dexamethasone treatment on the immune response of gulf coast native lambs to Haemonchus contortus infection. Vet Parasitol. 2004;119(2–3):223–235. doi: 10.1016/j.vetpar.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ross GR, Selvasubramanian S, Jayasundar S. Immunomodulatory activity of Punica granatum in rabbits-a preliminary study. J Ethnopharmacol. 2001;78:85–87. doi: 10.1016/S0378-8741(01)00287-2. [DOI] [PubMed] [Google Scholar]
- Schallig HDFH. Immunological responses of sheep to Haemonchus contortus. Parasitology. 2000;120:S63–S72. doi: 10.1017/S003118209900579X. [DOI] [PubMed] [Google Scholar]
- Schallig HDFH, Van Der Aar WM, Boersema JH, Cornelissen AWCA. The effect of oxfendazole terminated infections with Haemonchus contortus on the development of immunity in sheep. Vet Parasitol. 2000;88:61–72. doi: 10.1016/S0304-4017(99)00193-4. [DOI] [PubMed] [Google Scholar]
- Scharenberg A, Heckendorn F, Arrigo Y, Hertzberg H, Gutzwiller A, Hess HD, Kreuzer M, Dohme F. Nitrogen and mineral balance of lambs artificially infected with Haemonchus contortus and fed tanniferous sainfoin (Onobrychis vicifolia) J Anim Sci. 2008;86:1879–1890. doi: 10.2527/jas.2007-0448. [DOI] [PubMed] [Google Scholar]
- Shaik SA, Terrill TH, Miller JE, Kouakou B, Kannan G, Kaplan RM, Burke JM, Mosjidis JA. Sericea lespedeza hay as natural deworming agent against gastrointestinal nematode infection in goats. Vet Parasitol. 2006;139:150–157. doi: 10.1016/j.vetpar.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 8. New Delhi: East West Press Pvt. Ltd.; 1994. [Google Scholar]
- Sokerya S, Preston TR (2003) Effect of grass or cassava foliage on growth and nematode parasite infestation in goats fed low or high protein diets in confinement. Live Res Rural Develop 15(8). http://www.cipav.org.co/lrrd/lrrd15/8/kery158.htm
- Strain SA, Stear MJ. The influence of protein supplementation on the immune response to Haemonchus contortus. Parasitol Immunol. 2001;23:527–531. doi: 10.1046/j.1365-3024.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- Terrill TH, Douglas GB, Foote AG, Purchas RW, Wilson GF, Barry TN. Effect of condensed tannins upon body growth, wool growth and rumen metabolism in sheep grazing sulla (Hedysarum coronarium) and perennial pasture. J Agric Sci Camb. 1992;119:265–273. doi: 10.1017/S0021859600014192. [DOI] [Google Scholar]
- Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Verelde L, Kooyman FNJ, Van Leeuwen MAW, Schallig HDFH, McKellar A, Huntley JF, Cornelissen AWCA. Age related protective immunity after vaccination with Haemonchus contortus excretory/secretory proteins. Parasitol Immunol. 2001;23:419–426. doi: 10.1046/j.1365-3024.2001.00391.x. [DOI] [PubMed] [Google Scholar]
- Yuting C, Rongliang Z, Zhongjian J, Yong J. Flavonoids as superoxide scavengers and antioxidants. Free Radical Biol Med. 1990;9:19–23. doi: 10.1016/0891-5849(90)90045-K. [DOI] [PubMed] [Google Scholar]