Abstract
Giardiasis is a parasitic cosmopolitan disease that the rate of infection in developing countries is considerable. This infection directly is associated with poor hygienic conditions, poor water quality control, and overcrowding. Reinfection and drug resistance are two major problems in endemic areas. Recently, researchers are concentrating on herbal drugs as a proper solution. Therefore, the objective of the present study was to survey on efficacy of chloroformic extract of Artemisia annua against Giardia lamblia trophozoite and cyst in vitro. G. lamblia cysts were prepared from faces of giardiasis patients from different hospitals of Mazandaran Medical University. Four concentrations (1, 10, 50 and 100 mg/ml) of chloroformic extract of A. annua were utilized for 1, 5, 30, 60 and 180 min. Viability of G. lamblia cysts was confirmed by 0.1 % Eosin staining. Cyst and trophozoite contact (intermix) of G. lamblia with extract of A. annua with variant concentrations (1, 10, 50 and 100 mg/ml) after 1 and 180 min caused following cyst and trophozoite elimination rates: (67, 69, 71 and 73 %), (65, 67, 67 and 72 %), (94, 96, 97 and 99 %) and (100, 100, 100 and 100 %), respectively. Authors from the current investigation draw a conclusion that chloroformic extract of A. annua has the ability to eliminate G. lamblia cysts and trophozoites in vitro.
Keywords: Plant extract, Parasite, Antiprotozoal activity, Intestinal parasites, Giardia lamblia, In vitro
Introduction
Giardiasis or beaver fever is a parasitic intestinal disease and its causative agent is Giardia intestinalis. This flagellate protozoon has a wide range of mammalian hosts besides humans, thus making it very difficult to eradicate (Larry and Janovy 2006; Choubisa et al. 2012). The prevalence rate of giardiasis in developing countries is 20–30 % (Sayyari et al. 2005) and G. intestinalis in the United State and Jamaica is the most common intestinal parasite (Kappus 1994; Lindo et al. 1998). A range of clinical syndromes may occur, with gastrointestinal syndromes being the most prevalent such as mal absorption syndrome (diarrhea, abdominal pain, asthenia and weight loss) and extra intestinal symptoms, such as fever, maculopapular rash, pulmonary infiltrates, lymphadenopathy, polyarthritis and urticarial (Larry and Janovy 2006). Failures of remedy have been reported with whole of the commonly used antigiardial drugs and drug resistance to all available drugs, has been proven in the laboratory (Wright et al. 2003).
Within the last decade and so, isolated bioactive compounds from plants were utilized against a wide range of microorganisms particularly parasites. Among these plants, Artemisia annua has an undeniable allotment and a plenty of researches were carried out in order to study on impact of this immensely precious plant (Bone and Morgan 1992). The species A. annua also is known as Sweet Wormwood, Sweet Annie or Annual Wormwood. This highly aromatic herbaceous plant belongs to the family Asteraceae and is a common type of wormwood that in temperate Asia is considered native, but naturalized all over the world (Bailey and Bailey 1976; Simon et al. 1984). It was used since A.D. 341 by Chinese herbalists who exploited it for the treatment of malaria fever which is considered as dreadful disease (Hilen and White 1993: Mahdavi et al. 2013).
Ridley and Hudson (1998) described that artemisinin ruins the cells of parasitic organisms by either the production of highly reactive oxygen-based free radicals or electrophilic intermediates. It has been proven that the impact of artemisinin is equally mediated through disruption of membrane potential by interacting with the electron transport chain in the mitochondrial membrane, as a result of free radical damage and dysfunctional mitochondria (Li et al. 2005). Jorge et al. (2010) stated that the flavonoids present in A. annua leaves have been associated to repression of CYP450 enzymes which is responsible for altering the absorption and metabolism of artemisinin in body, but also have been connected to a beneficial immunomodulatory activity in subjects afflicted with parasitic and chronic diseases. Consumption of extracts and the essential oil of A. annua as an anti-parasitic drug in herbal therapies received particular attention and the following studies were performed and they indicates that this plant is effective and active against trypanosomiasis or “sleeping sickness” (Mishina et al. 2007), schistosomiasis (Utzinger et al. 2001), toxoplasmosis (Oliverira et al. 2009), leishmaniasis (Chawla and Madhubala 2010), cryptosporidiosis (Arab et al. 2006), coccidiosis (Brisibe et al. 2008). Furthermore, Basic compound of artemisinin which is an antimalarial drug was extracted from A. annua (Oliverira et al. 2009).
Although A. annua is widely distributed in Mazandaran province there is not adequate and documented information of influence on this herb against G. lamblia cysts and trophozoites. The present survey was conducted to survey on efficacy of chloroformic extract of A. annua against G. lamblia trophozoite and cyst in vitro.
Materials and methods
Giardia lamblia cysts were collected from faces of giardiasis patients from different hospitals of Mazandaran Medical University. All specimens were processed immediately after arrival, ordinarily within 48 h after excretion. By the sucrose flotation method a highly purified cyst and trophozoite suspension was achieved. Purified cysts were resuspended in distilled water and stored at 4 °C for a maximum of 3 days prior to use. The method of Bingham et al. (1979) was used for excystations. Initial experiments demonstrated that survival of most of the primary cultures could be favorably affected by the addition of bile and by increasing the serum component to 20 % (Laarman et al. 1986). Therefore the excystation medium used consisted of filter-sterilized TYI-S-33 culture medium, with bovine bile added (Keister 1983) supplemented with 20 % heat inactivated fetal calf serum. Screw capped borosilicate glass culture tubes containing 8 ml of the medium, supplemented with penicillin (500 mg/ml) and streptomycin (500 mg/ml), and was incubated at 37 °C in a slant.
Artemisia annua were dried under shade, and powdered mechanically. To obtain the chloroformic extract, 70 g of dry powder was added to 350 ml of pure chloroform and mixed gradually for 60 min using a magnetic stirrer. The obtained solution was left at room temperature for 24 h. The solution was stirred again and filtered and then the solvent was removed by evaporation in a rotating evaporator. The remaining semisolid material was freeze-dried. The obtained filtrate (5.5 g) was placed into a sterile glass container and stored at 4 °C for further use. In the current research, four concentrations (1, 10, 50 and 100 mg/ml) of the A. annua extract were applied for 5, 10, 30, 60 and 180 min. 2 ml of each solution was placed in test tubes, to which 10,000 washed cysts was added. The contents of the tubes were gently mixed. The tubes were incubated at 37 °C for 5, 10, 30, 60 and 180 min. At the end of each incubation time the upper phase was carefully removed so as not to interrupt the cysts. 2 ml of 0.1 % eosin stain was then added to the remaining settled cysts and mixed gently. The upper portion of the solution was discarded after 15 min of incubation. The remaining pellet of cysts was then smeared on a glass slide, covered with a cover glass and examined under a light microscope. The viability of were determined by counting 500 cysts. Non treated cysts were considered a control group in each experiment. The experiments were performed in triplicate. In the present study eosin stain with the concentration of 0.1 % (1 g of eosin powder in 1,000 ml distilled water) was used to check the viability of the cysts. The cysts with no absorbed dye were considered potentially viable otherwise they were recorded as dead (Fig. 1).
Fig. 1.
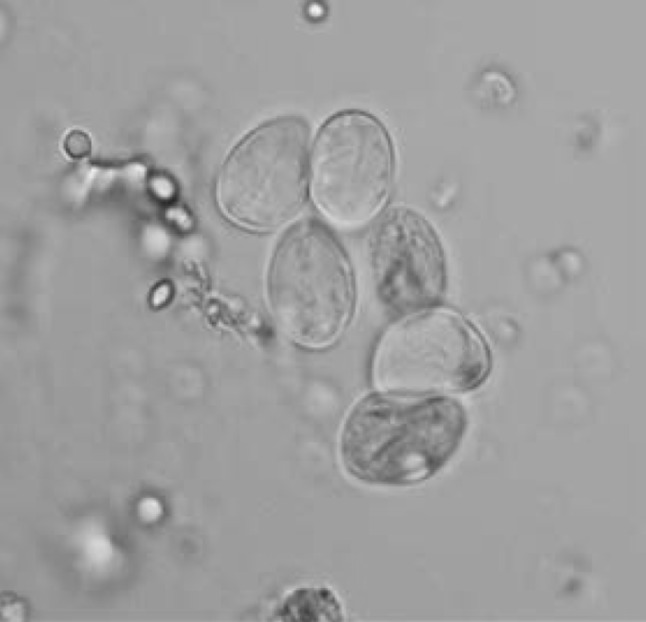
Viable cysts with no absorbed dye and dead cysts with absorbed dye
Statistical analysis was performed by means of one-way ANOVA (analysis of variance) considering a level of significance of 95 % (P < 0.05), with SPSS software.
Results
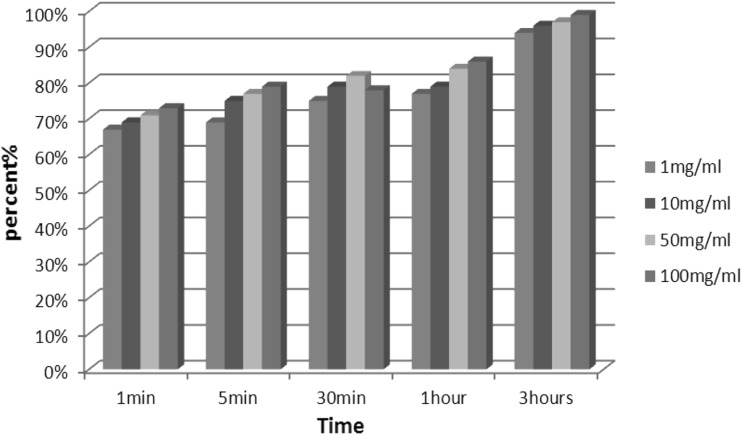
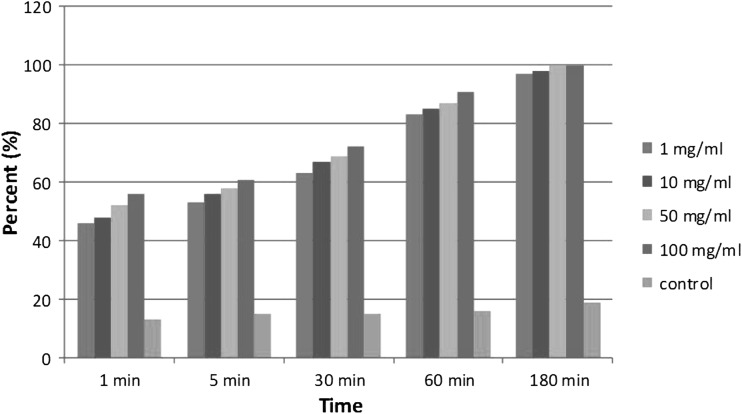
Table 1 illustrates recovered results of using the following different concentrations and time exposure of chloroformic extract of A. annua against G. lamblia cyst and trophozoite respectively: (1, 10, 50 and 100 mg/ml) (5, 10, 30, 60 and 180 min). Intermix of cysts with the extract of A. annua with dilution of 1, 10, 50 and 100 mg/ml after 180 min caused the following cyst and trophozoite demolishing rates: (94, 96, 97 and 99 %) and (100, 100, 100 and 100 %), respectively; whereas mentioned concentrations after 1 min just led to (67, 69, 71 and 73 %) and (65, 67, 67 and 72 %) of cyst and trophozoite elimination, respectively. On the whole of examination cyst elimination by increase of dilution without change of time raised except of 30 min by consumption of 100 mg/ml that was just 78 % compared to 82 % in the similar time but different concentration (50 mg/ml). In addition, Charts 1 and 2 depicts recovered results of using different concentrations and time exposure of chloroformic extract of A. annua against G. lamblia cyst and trophozoite.
Table 1.
Effect of different concentrations and time exposure of chloroformic extract of A. annua on G. lamblia cyst and trophozoite in vitro
| Time exposure (min) | 1 mg/ml (%) | 10 mg/ml (%) | 50 mg/ml (%) | 100 mg/ml (%) | Control (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cyst | Trophozoite | Cyst | Trophozoite | Cyst | Trophozoite | Cyst | Trophozoite | Cyst | Trophozoite | |
| 1 | 67 | 65 | 69 | 67 | 71 | 67 | 73 | 72 | 3 | 9 |
| 5 | 69 | 78 | 75 | 79 | 77 | 84 | 79 | 86 | 3 | 9 |
| 30 | 75 | 83 | 79 | 87 | 82 | 88 | 87 | 92 | 5 | 9 |
| 60 | 77 | 89 | 79 | 92 | 84 | 96 | 86 | 100 | 7 | 11 |
| 180 | 94 | 100 | 96 | 100 | 97 | 100 | 99 | 100 | 9 | 11 |
Chart 1.
The effect of different concentrations and time exposure of chloroformic extract of A. annua on G. lamblia cyst in vitro
Chart 2.
Effect of different concentrations and time exposure of chloroformic extract of A. annua on G. lamblia trophozoite in vitro
Discussion
Recently there is a high tendency among researchers to solve drug resistance which is a major problem in endemic areas against parasitic diseases particularly giardiasis by using herbal therapies (Rahimi-Esboei et al. 2012). In a review of the literatures, following reports were found concerning effect of herbal remedies on giardiasis:
On the screen of some methanolic extracts of Mexican plants which are used traditionally in treatment of gastrointestinal disorders it was concluded that Sennavillosa, Dorsteniacontrajeva and Rutachalepensis were effective against G. lamblia with IC50 <38 mg/ml (Calzada et al. 2006). In another study, testing of two antimalarial drugs (Mefloquine and Artesunate) on G. lamblia in experimentally infected hamsters shown that the number of G. lamblia cysts was significantly reduced (William and Ramzy 2008). In addition to, antiprotozoal activity of A. absinthium has been proven versus Naegleria fowleri (Mendiola et al. 1991) and G. lamblia (Gurra et al. 2001). In the earlier study on laboratory rats, aqueous extract of Artemisia spp., (4 g/kg) was active against intestinal flagellate G. lamblia (Shanawa 1995). The residents of North East of Mexico Previously utilized an infusion of leaves from A. ludoviciana as a therapeutic drug (antidiarrhoeal). The authors presumed that the aqueous acetone, hexane and methanol leaf extracts of mature plants were found to have an active antiprotozoal role in vitro against Entamoeba histolytica and G. lamblia (Salvador et al. 2005).
According to our results not only concentration of chloroformic extract of A. annua for destroying of G. lamblia cyst and trophozoite plays prominent role but also time is another undeniable factor. Because increasing of time has an effective impact regarding rate of cyst and trophozoite elimination under laboratory conditions. By considering that in the present study a variant dilution (from 1 to 100 mg/ml) and time (from 1 to 180 min) were applied and cyst and trophozoite elimination rate at least and at most were (67 and 99 %) and (65 and 100 %), respectively. It seems reasonable to assume that the trophozoite is more susceptible to chloroformic extract of A. annua in comparison with cyst and the results of the current investigation correspond closely to this fact; as we observed the elimination of whole of trophozoites (100 %) in time exposure of 180 min in mentioned different concentrations.
Owing to successful outcomes of the current study, chloroformic extract of A. annua can be considered as an alternative choice for patients who are engaging in either acute phase that excretes trophozoites or chronic phase that excretes cysts of Giardia as well.
Though there is not any reports regarding significant adverse or side effects of toxicity of A. annua in patients who were treated with therapeutic dosages, it is noteworthy that some cautions are advisable for consumption of A. annua: it can initially cause a worsening of symptoms, allergic reactions and some intestinal irritation. Moreover, A. annua should not be used during pregnancy (Vries and Dient 1996). Therefore, from this study it could be concluded that chloroformic extract of A. annua has the capability to destroy G. lamblia cysts and trophozoites. Hence, this is a value in endemic areas where people are prone to develop drug resistance and prevalently use antigiardiasis drugs. Further trials are demanded in order to assess the influence of A. annua in vivo both prescribing monotherapy and taking as a complementary treatment particularly in endemic areas.
Acknowledgments
The authors are grateful to express their deep gratitude to Mazandaran University of Medical Science and Young Researchers Club of Islamic Azad University, Babol Branch.
References
- Arab HA, Rahbari S, Rassouli A, Moslemi MH, Khosravirad F. Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extract in broiler chickens. Trop Anim Health Prod. 2006;38:497–503. doi: 10.1007/s11250-006-4390-8. [DOI] [PubMed] [Google Scholar]
- Bailey LH, Bailey EZ. Hortus third. New York: MacMillan Publ Co; 1976. pp. 145–146. [Google Scholar]
- Bingham AK, Jarroll EL, Jr, Meyer EA, Radulescu S. Giardia sp.: physical factors of excystation in vitro, and excystation versus eosin exclusion as determinants of viability. Exp Parasitol. 1979;47:284–291. doi: 10.1016/0014-4894(79)90080-8. [DOI] [PubMed] [Google Scholar]
- Bone K, Morgan M. Clinical applications of ayurvedic and Chinese herbs: monographs for the Western herbal practitioner. Warwick: Phytotherapy Press; 1992. pp. 7–12. [Google Scholar]
- Brisibe EA, Umoren UE, Owai PU, Brisibe F. Dietary inclusion of dried Artemisia annua leaves for management of coccidiosis and growth enhancement in chickens. Afr J Biol. 2008;7:4083–4092. [Google Scholar]
- Calzada F, Mulia LY, Aguilarb A. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharm. 2006;108:367–370. doi: 10.1016/j.jep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Chawla B, Madhubala R. Drug targets in Leishmania. J Parasit Dis. 2010;34:1–13. doi: 10.1007/s12639-010-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubisa SL, Jaroli VJ, Choubisa P, Mogra N. Intestinal parasitic infection in Bhil tribe of Rajasthan. India J Parasit Dis. 2012;36:143–148. doi: 10.1007/s12639-012-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurra M, Torres D, Martinez L. Validación del uso tradicional de plantas medicinales cultivadas en Cuba. Rev Cubana Plant Med. 2001;2:48–51. [Google Scholar]
- Hilen TT, White NJ. Qinghaosu. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-K. [DOI] [PubMed] [Google Scholar]
- Jorge FS, Ferreira L, Devanand LL, Saki T, Heyerick A. Flavonoids from Artemisia annua as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules. 2010;15:3135–3170. doi: 10.3390/molecules15053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappus KD. Intestinal parasitism in the United States: update on a continuing problem. Am J Hyg Trop med. 1994;50:705–713. doi: 10.4269/ajtmh.1994.50.705. [DOI] [PubMed] [Google Scholar]
- Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Laarman JJ, Hautus M, Abdillahi H. Isolation of stocks of Giardia lamblia from stools and their use in the determination of specific immunoglobulins in patient sera. Trop Geo Med. 1986;38:320. [Google Scholar]
- Larry SR, Janovy J. Foundations of parasitology. 7. New York: McmGraw, Hill companies; 2006. [Google Scholar]
- Li W, Mo W, Shen D, Sun L, Wang J, Lu S, Gitschier JM, Zhou B. Yeast model uncovers dual roles of mitochondria in the action of artemisinin. PLoS Gen. 2005;1:0329–0334. doi: 10.1371/journal.pgen.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindo JF, Levy VA, Baum MK, Palmer CJ. Epidemiology of giardiasis and cryptosporidiosis in Jamaica. Am J Hyg Trop med. 1998;59:717–721. doi: 10.4269/ajtmh.1998.59.717. [DOI] [PubMed] [Google Scholar]
- Mahdavi SA, Raeesi A, Faraji L, Youssefi MR, Rahimi MT. A case of misdiagnose of malaria infection. Asian Pac J Trop Biomed. 2013;3:748–750. doi: 10.1016/S2221-1691(13)60150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Bousa M, Perez N, Hernandez H, Torres D. Extracts of Artemisia abrotanum and Artemisia absinthium inhibit growth of Naegleria fowleri in vitro. Trans R Soc Trop Med Hyg. 1991;85:78–79. doi: 10.1016/0035-9203(91)90165-U. [DOI] [PubMed] [Google Scholar]
- Mishina YV, Krishina S, Haynes RK, Meade JC. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. Antimicrob Agents Chemother. 2007;51:1852–1854. doi: 10.1128/AAC.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliverira TC, Silva D, Rostkoaska C, Bela SR, Ferro E, Magalhaes PM. Toxoplasma gondii: effects of Artemisia annua L. on susceptibility to infection in experimental models in vitro and in vivo. Exp Parasitol. 2009;122:233–241. doi: 10.1016/j.exppara.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Rahimi-Esboei B, Gholami SH, Azadbakht M, Ziaei H. Effect of Hydroalcholic extract of Artemisia annua on cysts of Giardia lamblia in Invitro. J Mazand Univ Med Sci. 2012;22:72–80. [Google Scholar]
- Ridley RG, Hudson N. Oxidative stress and antimalarial drugs. Curr Biol. 1998;8:346–349. doi: 10.1016/S0960-9822(98)70218-0. [DOI] [Google Scholar]
- Salvador SF, Guerra MCR, Cardenas BDM, Villarreal JV, Trevino LV. In vitro antiprotozoal activity of the leaves of Artemisia ludoviciana. Fitoterapia. 2005;76:466–468. doi: 10.1016/j.fitote.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Sayyari AA, Imanzadeh F, Bagheri Yazdi SA, Karami H, Yaghoobi M. Prevalence of intestinal parasitic infections in the Islamic Republic of Iran. East Med Health J. 2005;11:377. [PubMed] [Google Scholar]
- Shanawa BH. Biological and immunological studies on Giardia lamblia (Stiles, 1915) Coll Sci. 1995;102:34–37. [Google Scholar]
- Simon JE, Chadwick AF, Craker LE. Herbs: an indexed bibliography, 1971–1980. The scientific literature on selected herbs, and aromatic and medicinal plants of the temperate zone. Hamden: Archon Books; 1984. [Google Scholar]
- Utzinger T, Shuhua X, Keiser J, Minggan C, Jiang Z, Tanner M. Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr Med Chem. 2001;8:1841–1859. doi: 10.2174/0929867013371581. [DOI] [PubMed] [Google Scholar]
- Vries PJ, Dient TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. doi: 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- William S, Ramzy F. Testing two antimalarial drugs on Giardia lamblia in experimentally infected Hamsters. Re J Med Sci. 2008;41:1–6. [Google Scholar]
- Wright JM, Dunnla P, Upcroft JA. Efficacy of antigiardial drugs. Expert Opin Drug Safe. 2003;2:529–541. doi: 10.1517/14740338.2.6.529. [DOI] [PubMed] [Google Scholar]