Abstract
Schistosomosis has been recognised as one of the major parasitic diseases of livestock and human beings. Schistosoma spindale is the major cause of visceral schistosomosis among bovines of Kerala State. Besides pathology in animals, it has been long known that cercariae of S. spindale are a common cause of dermatitis in human beings in Asia. However, detection of this disease based on coprology has underestimated the prevalence of this economically important disease among cattle of the State. An efficient diagnostic tool providing unequivocal evidence of infection in living animals is perhaps, the key to formulate and deliver control measures to the target population. It is also crucial for an enhanced understanding of parasite epidemiology. The utility of excretory–secretory proteins as diagnostic and vaccine candidates for schistosomosis has been a focus of medical research since long. There exists a paucity of information with regard to analysis of ES proteins of S. spindale and their incorporation to develop sensitive and specific serodiagnostic tool. Hence a study was designed to evaluate the efficacy of Dot-ELISA incorporating different antigens of S. spindale and to validate the test under field conditions.
Keywords: Schistosoma spindale, Excretory–secretory antigens, Dot-ELISA
Introduction
Schistosomes are a group of digenean blood flukes which evoke a strong immunological response in the host which is potentially protective as well as incite an immunopathological reaction. The Indian species, Schistosoma spindale, located in portal and mesenteric veins of buffalo, ox, sheep, goat, rarely horse and donkey and rodents causes pseudotubercle formation in the liver and intestine along with thrombosis of periportal veins (Kalapesi and Purohit 1954). The disease is widely distributed in India, Sri Lanka, Thailand, Malaysia, Indonesia, Vietnam and Laos (Kumar 1999). It is noteworthy that abattoir surveys reveal a significant prevalence of S.spindale among slaughtered cattle of Kerala (Ravindran et al. 2007; Lakshmanan et al. 2011). As per the Animal Disease Surveillance Report (AHD 2008), which was based on routine coprological diagnosis, schistosomosis accounted for only 0.38 % of the total cases of parasitism in cattle of Kerala. This represents a true picture of underestimation of bovine schistosomosis in the State, which could be attributed to the fact that natural S. spindale infections characterised with diarrhoea and loss of production are seldom diagnosed by routine faecal sample examination (Agarwal 1999).
Serological assays have always played a crucial role as an adjunct to routine parasitological methods in diagnosis of parasitic diseases. Immunodiagnostic methods based on the detection of antibodies continued to be the most effective and practical method for diagnosis of imported schistosomosis (Tsang and Wilkins 1997). Serodiagnosis of S. haematobium (Khalil et al. 1993), S. japonicum (Hwu et al. 1978) and S.mansoni (Boctor et al. 1987) have been attempted using various immunodiagnostic assays with antigens from adult worms and eggs. Enzyme linked immunosorbent assay (ELISA) is a simple, sensitive and rapid serodiagnostic technique which has been widely used for detection of the circulating antibodies and antigens related to many parasitic diseases (Voller et al. 1976). Different methods have been adopted of which enzyme linked immunosorbent assay is most widely developed for human schistosomosis (Hamilton et al. 1998). Dot-ELISA, a modification of standard ELISA, is a widely employed practical tool for field studies. Only limited work has been attempted in diagnosis of S. spindale using whole worm antigens (Sumanth et al. 2003; Singh et al. 2004; Divya et al. 2013; Murthy et al. 2013).There has been no attempts to evaluate the diagnostic efficacy of excretory–secretory and egg antigens of S. spindale. The objective of the present study was to develop a simple field level Dot-ELISA for serodiagnosis of S. spindale, to compare the efficacy of different antigens for its suitability as diagnostic candidates as well as to compare the efficacy of Dot-ELISA with routine faecal sample examination in diagnosis of the disease among bovines, in field conditions, in Kerala, South India.
Materials and methods
Excretory–secretory antigens
Adult S. spindale worms were separated from the mesentery of cattle as per the method of Lakshmanan et al. (2011). Excretory–secretory antigens (ESA) of S. spindale were prepared based on the method of Liu et al. (2009) with some modifications. Eight hundred adult worms were soaked in 1X phosphate buffered saline (PBS, pH 7.4) for 2 h at room temperature followed by overnight incubation at 4 °C. The viability and tegumental integrity of the worms were checked under a stereo zoom microscope to ensure that all worms were intact and live. The worms were carefully removed under sterile conditions, the remaining fluid was centrifuged at 10,000×g for 30 min at 4 °C and the supernatant was used as ESA. The protein concentration of ESA was found to be 2.56 mg/ml using protein estimation kit by Lowry’s method (Merck GeNei™, Bangalore) in a spectrophotometer (Lambda 750, Perkin Elmer) at 660 nm and was further aliquoted and stored at −20 °C.
Whole worm antigens
The recovered flukes were washed in several changes of chilled PBS (pH 7.2) and used for whole worm antigen (WWA) preparation as described by Sumanth et al. (2003) with some modifications. Around 200 adult male S. spindale flukes were homogenized at 4 °C in PBS, containing cocktail protease inhibitors (Sigma-Aldrich, Bangalore). The homogenate was subjected to sonication for 15 s each, for 2–3 min, interrupted by a five second pause at 80 kHz, on an ice bath. It was later centrifuged at 12,060×g for 30 min at 4 °C in a cooling centrifuge. The supernatant had a protein content of 12.6 mg/ml estimated as mentioned above and was stored at −20 °C.
Egg antigens
Eggs of S. spindale were concentrated by density gradient centrifugation with 20 % percoll in 0.25 M sucrose as per Baltz et al. (1982) and further processed for antigen as per Ashton et al. (2001). Egg antigen had a protein concentration of 264 µg/ml.
Serum
A total of 30 serum samples were collected from slaughtered animals in which the mesenteries harboured adult schistosomes. These formed the known positive sera. The serum collected from worm negative animals with a history of being reared in confined farms of non endemic areas served as known negative control (n = 30). Besides 100 serum samples were collected from cattle in field conditions reared in semi intensive system with access to water logged areas in Thrissur and Palakkad districts of Kerala State.
Raising hyper immune serum in rabbits
Hyper immune serum was raised against E/S, WWA and egg antigens of S. spindale in adult New Zealand White rabbits as per Murthy et al. (2013). Hyper immune serum raised against mixed amphistomes species WWA, available in the Department of Veterinary Parasitology, were also used as specificity controls.
Faecal samples
Rectal faecal samples collected from cattle in field conditions were processed using 10 % formalin and examined for the presence of typical napoleon hat shaped S. spindale ova.
Dot-ELISA
Three different dilutions of WWA and ESA each were prepared in carbonate bicarbonate buffer (pH 9.6) viz., 500, 250 and 100 ng/µl. The egg antigen was diluted to 250 and 100 ng/µl in the same buffer. One microlitre each of the diluted antigens was coated on the centre of nitrocellulose membrane (NCM) strips of approximately 1 cm2 size. The air dried strips were incubated in blocking buffer at 37 °C for 1 h. Later the strips were incubated in serum samples (1:50 dilution of bovine serum/hyperimmune serum in blocking buffer) at room temperature for 1 h and then, washed six times with 0.05 % Tween-20 in 1X PBS (PSBT) for 10 min each. Further, the NCM strips were incubated with the conjugate (1:2,000 dilution of antispecies IgG-HRP conjugate was used as per the serum selected) at room temperature for 1 h. The strips were washed four times in PBST as described earlier and were immersed in chromogenic visualisation solution, containing diaminobenzidine, with mild rocking at room temperature, in dark, for 2 to 3 min. The reaction was terminated by washing the strips with distilled water. The NCM strips were air dried and photographed. Development of brown coloured spot indicated a positive reaction.
The three antigens were tested at different protein concentrations with serum from animals that were positive for schistosomes in the mesentery and the hyperimmune serum raised against schistosome antigens as the known positive controls. The different antigen combinations were also tested with serum of mesentery negative animals and foetal bovine serum which served as known schistosome negative controls. Sera from animals in which faecal samples revealed ova of amphistome, as well as the hyperimmune serum raised in rabbits against mixed amphistome species antigens, available in the department, were used to check the specificity of all the antigens in different dilutions. Field samples were also subjected to Dot-ELISA for testing seroprevalence of S. spindale in dairy cattle of endemic areas.
Results
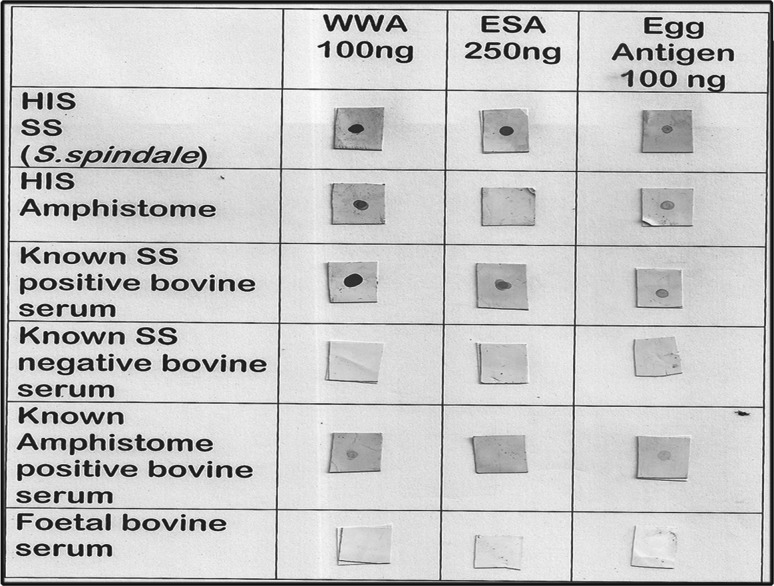
The minimum antigen concentrations that yielded a positive Dot-ELISA signal were 100 ng/µl each for WWA and egg antigen and 250 ng/µl for ESA and hence were determined as optimum for further specificity studies. Serum dilution was fixed at 1:50 to rule out the possibility of cross reaction with heterologous serum. The test was performed with known schistosome positive and negative bovine serum, hyper immune serum raised against WWA of mixed amphistome species and against S. spindale, as well as with serum of cattle harbouring amphistome infection. The test was repeated six times. The results are represented in the Fig. 1.
Fig. 1.
Dot-ELISA with different antigens of S. spindale
The ESA yielded positive Dot-ELISA signals with known positive bovine control serum and with hyperimmune serum raised against S. spindale in all replicates. It failed to react non specifically with either known negative bovine controls or amphistome positive controls. The WWA gave positive results with known schistosome positive bovine and hyperimmune sera but not with known schistosome negative bovine sera or foetal bovine sera, in all six replicates. However, WWA also gave a less intense positive signal with hyperimmune serum raised against amphistomes as well as with serum from amphistome infected cattle, in all replicates. Though egg antigen yielded positive signals with schistosome positive sera, weak positive signals were obtained using amphistome positive sera, as well in all the replicates. However, negative reaction was observed with known schistosome negative bovine serum and foetal bovine serum. Owing to the enhanced specificity, the ESA was selected as diagnostic antigen candidate for further validation of Dot-ELISA with field serum samples.
Anti-schistosome antibodies could be detected in 32 out of the 100 bovine sera collected from field, by Dot-ELISA using S. spindale ESA at a concentration of 250 ng/µl. Schistosomosis could be diagnosed only in 12 animals (12 %) by routine coprology. There was statistically significant difference between the tests [Cal χ2 > Table χ2 (5 %)] and coprology was found to have a low detection level. The results of Dot-ELISA with known negative and positive sera, was also compared with mesentery sample examination. It revealed that Dot-ELISA could detect anti-schistosome antibodies with 100 % sensitivity and specificity when mesentery sample examination was fixed as the test standard (Table 1).
Table 1.
Comparative evaluation of Dot-ELISA using ESA to detect anti-schistosome antibodies
| Abattoir samples | Field samples | |||
|---|---|---|---|---|
| Mesentery examination | Dot-ELISA | Coprology | Dot-ELISA | |
| No. positive | 30 | 30 | 12 | 32a |
| No. negative | 30 | 30 | 88 | 68 |
| Total | 60 | 60 | 100 | 100 |
aSignificant [Cal χ2 > Table χ2 (5 %)]
Discussion
Excretory–secretory antigens were found to be more specific for detection of anti-schistosome antibodies in cattle. The alternate methodology adopted for ESA preparation in the present study was also relatively simple when compared to the traditional in vitro culture technique in supplemented RPMI-1640 medium described for schistosome ESA preparation. Moreover according to Morphew et al. (2007) the excretion and secretion behaviour of the parasites could be altered due to removal from natural host tissues and in vitro maintenance in chemical mixture.
Though crude antigens were reported to possess a high sensitivity and specificity equivalent to those of defined or characterised antigens, cross reactions of S. japonicum WWA and egg antigens with Ascaris sp. and Paragonimus sp. infected human sera have been documented (Mott et al. 1987). But Boctor et al. (1987) could not observe any cross reaction between S. mansoni WWA and Fasciola sp. infected human serum in Dot-ELISA. Soluble WWA preparations of S. mansoni at a concentration of 250 ng/dot were also reported to yield false positive results in malaria and filaria positive human subjects (Montenegro et al. 1999). Noya et al. (2002) had also observed false positive reactions in western blot and ELISA with crude WWA and egg antigens of S. mansoni particularly in individuals infected with nematodes, though these antigens possessed high sensitivity for the diagnosis of infection. In the present study S. spindale ESA had not exhibited cross reaction with amphistome positive sera in Dot-ELISA. The high specificity of ESA in serodiagnosis has been observed by several workers for detecting other trematode infections as well viz, Clonorchis sinensis (Choi et al. 2003), Paragonimus sp. (Narain et al. 2005), F. hepatica (Awad et al. 2009), Opisthorchis felineus (Gomez-Morales et al. 2013) and Paramphistomum cervi (Anuracpreeda et al. 2013). On perusal of available literature, it is understood that ESA of S. spindale have not yet been utilised for Dot-ELISA. The absence of cross reaction with amphistomes, one of the most prevalent flukes infecting cattle of Kerala, highlights the utility of ESA as a potential diagnostic candidate for detecting intestinal schistosomosis serologically. However future studies are to be conducted towards purifying the antigens and to rule out the possibility of cross reaction with other helminths.
Detection of anti-schistosome antibodies does not preclude the possibility of existence of past infection or persistence of antigens after specific treatment. However such conclusions do not seem to influence the positive results of Dot-ELISA in the present study. This is because the only available anti-schistosome drug, praziquantel, is not being used to treat bovine schistosomosis in Kerala. The current practice of using antimony compounds leads to energy depletion, reduced fecundity and “hepatic shift” (Kumar 1999) instead of destroying the adult worms. Hence ELISA positive animals in the study could be considered as those harbouring schistosomes.
In the present study, schistosomosis could be detected among 32 % of bovines in Kerala especially in waterlogged panchayats of the State, using Dot-ELISA. The wide disparity between immunodiagnosis and egg detection, revealed in this study points out to the possibility that S. spindale infection among bovines of the State was under diagnosed. To strengthen our view, abattoir surveys have revealed a significant prevalence of S. spindale in various parts of Kerala. The significant prevalence of infection among live animals demands urgent attention as the disease is characterised with morbidity, mortality, reduced productivity and poor subsequent reproductive performance (Mc Cauley et al. 1984) and increased susceptibility to other diseases (Dargie 1980). Dot-ELISA is simple, cheap and easy to perform under field conditions and offer best possibility for timely and effective diagnosis of the disease, identification of endemic areas and thus in planning prevention and control strategies.
Acknowledgments
The authors acknowledge the facilities provided by Kerala Veterinary and Animal Sciences University, Wayanad and Dean, College of Veterinary and Animal Sciences, Mannuthy, Kerala, to carry out the study.
Contributor Information
Bindu Lakshmanan, Phone: 0487-2372070, Email: bindul@kvasu.ac.in.
K. Devada, Email: devada@kvasu.ac.in
Siju Joseph, Email: siju@kvasu.ac.in.
References
- Agarwal MC. Schistosomosis: an underestimated problem in animals in South Asia. World Anim Rev. 1999;92:55–57. [Google Scholar]
- AHD [Department of Animal Husbandry] (2008) Animal disease surveillance annual report. Department of animal husbandry, Government of Kerala, pp 156
- Anuracpreeda P, Poljaroen J, Chotwiwatthananku C, Tinikul Y, Sobhon P. Antigenic components, isolation and partial charaterization of excretion-secretion fraction of Paramphistomum cervi. Exp Parasitol. 2013;133:327–333. doi: 10.1016/j.exppara.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Ashton PD, Harrop R, Shah B, Wilson RA. The schistosome egg: development and secretions. Parasitology. 2001;122:329–338. doi: 10.1017/S0031182001007351. [DOI] [PubMed] [Google Scholar]
- Awad WS, Ibrahim AK, Sahb FA. Using indirect ELISA to assess different antigens for the serodiagnosis of Fasciola gigantica infection in cattle, sheep and donkeys. Res Vet Sci. 2009;86:466–471. doi: 10.1016/j.rvsc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Baltz T, Lacassie I, Duret JT, Tribouley J. Density gradient separation of Schistosoma mansoni eggs. J Parasitol. 1982;68:963–965. doi: 10.2307/3281019. [DOI] [PubMed] [Google Scholar]
- Boctor FN, Stec MJ, Petert JB, Kamal R. Simplification and standardisation of Dot-ELISA for human schistosomiasis mansoni. J Parasitol. 1987;73:589–592. doi: 10.2307/3282141. [DOI] [PubMed] [Google Scholar]
- Choi MH, Park C, Li S, Hong ST. Excretory–secretory antigen is better than crude antigen for the serodiagnosis of clonorchiasis by ELISA. Korean J Parasitol. 2003;41:35–39. doi: 10.3347/kjp.2003.41.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargie JD. The impact on production and mechanisms of pathogenesis of trematode infections in cattle and sheep. Int J Parasitol. 1980;17:453–463. doi: 10.1016/0020-7519(87)90121-4. [DOI] [PubMed] [Google Scholar]
- Divya SP, Lakshmanan B, Joseph Siju. Efficacy of counterimmunoelectrophoresis in diagnosis of Schistosoma spindale infection in cattle. Indian Vet J. 2013;90:20–22. [Google Scholar]
- Gomez-Morales MA, Ludovisi A, Amati M, Pozio E (2013) Validation of an excretory/secretory antigen based ELISA for the diagnosis of Opisthorchis felineus infection in human from low trematode endemic areas. PLoS One [Online] 8:2267. http://www.plosone.org. Accessed 01 Jan 2014 [DOI] [PMC free article] [PubMed]
- Hamilton JV, Klinkert M, Doenhoff MJ. Diagnosis of schistosomiasis: antibody detection with notes on parasitological and antigen detection methods. Parasitology. 1998;117:S41–S45. doi: 10.1017/S0031182099004205. [DOI] [PubMed] [Google Scholar]
- Hwu HR, Banzon TC, Cross JH. Counter current immunoelectrophoresis in detection of antibodies to S. japonicum. Am J Trop Med Hyg. 1978;27:276–280. doi: 10.4269/ajtmh.1978.27.276. [DOI] [PubMed] [Google Scholar]
- Kalapesi RM, Purohit BL. Observation on histopathology of morbid tissue from a case of natural infection with Schistosoma spindale in a bovine. Indian Vet J. 1954;30:336–340. [Google Scholar]
- Khalil HM, Abdelbaki MH, Adallah HM, el Zayat EA, Aziz A. Diagnosis of active urinary schistosomiasis using homologous adult Schistosoma haematobium antigen. J Egypt Soc Parasitol. 1993;23:323–330. [PubMed] [Google Scholar]
- Kumar V. Trematode infections and diseases of man and animals. Netherlands: Kluwer Academic Publishers; 1999. p. 362. [Google Scholar]
- Lakshmanan B, Rauoof A, Fawaz M, Subramanian H. Abattoir survey of Schistosoma spindale infection in Thrissur. J Vet Anim Sci. 2011;42:53–54. [Google Scholar]
- Liu F, Cui SJ, Hu W, Feng Z, Wang ZG, Han ZG. Excretory–secretory proteome of the adult developmental stage of human blood fluke, Schistosoma japonicum. Mol Cell Proteomics. 2009;8:1236–1251. doi: 10.1074/mcp.M800538-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Cauley EH, Majid AA, Tayeb A. Economic evaluation of the production impact of bovine schistosomiasis and vaccination in Sudan. Prev Vet Med. 1984;6:735–754. doi: 10.1016/0167-5877(84)90030-8. [DOI] [Google Scholar]
- Montenegro SML, Silva JDB, Brito MEF, Caravalho LB. Dot-ELISA for schistosomosis using Dacron as solid base. Rev Soc Bras Med Trop. 1999;32:139–143. doi: 10.1590/S0037-86821999000200004. [DOI] [PubMed] [Google Scholar]
- Morphew RM, Wright HA, LaCourse EJ, Woods DJ, Brophy PM. Comparative proteomics of excretory–secretory proteins released by the liver fluke Fasciola hepatica in sheep host bile and during in vitro culture ex host. Mol Cell Proteomics. 2007;6:963–972. doi: 10.1074/mcp.M600375-MCP200. [DOI] [PubMed] [Google Scholar]
- Mott KE, Dixon H, Carter CE, Garcia E, Ishi A, Matsuda H, Mitchell G, Owhashi M, Tanaka H, Tsang VC. Collaborative study on antigens for immunodiagnosis of Schistosoma japonicum infection. Bull World Health Organ. 1987;65:233–244. [PMC free article] [PubMed] [Google Scholar]
- Murthy GSS, D’Souza PE, Isloor KS. Evaluation of a polyclonal antibody based sandwich ELISA for the detection of faecal antigens in Schistosoma spindale infections in bovines. J Parasite Dis. 2013;37:47–51. doi: 10.1007/s12639-012-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narain K, Devi KR, Mahanta J. Development of enzyme-linked immunosorbent assay for serodiagnosis of human paragonimiasis. Indian J Med Res. 2005;121:739–746. [PubMed] [Google Scholar]
- Noya O, Alarcon de Noya B, Losada S, Colmenares C, Guzman C, Lorenzo MA, Bermudez H. Laboratory diagnosis of Schistosomiasis in areas of low transmission.A review of a line of research. Mem Inst Oswaldo Cruz. 2002;97:167–169. doi: 10.1590/S0074-02762002000900032. [DOI] [PubMed] [Google Scholar]
- Ravindran R, Bindu L, Ravishankar C, Subramanian H. Visceral schistosomosis among domestic ruminants slaughtered in Wayanad, South India. Southeast Asian J Trop Med Public Health. 2007;38:6. [PubMed] [Google Scholar]
- Singh A, Singh A, Chaudhri SS. Visceral schistosomiasis of domestic animals in India: humoral immune status of infected sheep and goats against major polypeptide antigens of Schistosoma indicum and S. spindale. Parasite Immunol. 2004;26:167–175. doi: 10.1111/j.0141-9838.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- Sumanth S, D’Souza PE, Jagannath MS. Immunodiagnosis of nasal and visceral schistosomosis in cattle by Dot-ELISA. Indian Vet J. 2003;80:495–498. [Google Scholar]
- Tsang VCW, Wilkins PP. Immunodiagnosis of schistosomiasis. Immunol Investig. 1997;26:175–188. doi: 10.3109/08820139709048925. [DOI] [PubMed] [Google Scholar]
- Voller A, Bartlett A, Bidwell DE. Enzyme immunoassays for parasitic diseases. Trans R Soc Trop Med. 1976;70:98–106. doi: 10.1016/0035-9203(76)90163-2. [DOI] [PubMed] [Google Scholar]