Abstract
Background:
Pathologically low-risk endometrial cancer patients do not receive postoperative treatment; however, 10–15% of these patients show recurrence with poor prognosis. We evaluated the clinical importance of cyclin-dependent kinase 4/6 (CDK4/6) activity, and its significance as a novel biomarker for the prognosis and chemo-sensitivity of endometrioid endometrial carcinoma (EEC).
Methods:
Cyclin-dependent kinase 4/6 expression and enzyme activity in 109 tumour samples from patients with EEC were examined with a cell-cycle profiling (C2P) assay. CDK4/6-specific activity (CDK4/6SA) was determined, and its relationship with clinicopathological factors and expression of Ki-67 was analysed.
Results:
CDK4/6-specific activity was significantly correlated with Ki-67 (P=0.035), but not with any other clinicopathological characteristics. CDK4/6SA was significantly higher (P=0.002) in pathologically low-risk patients (not receiving adjuvant chemotherapy, n=74) than in intermediate- or high-risk patients (receiving adjuvant chemotherapy, n=35). In addition, patients with high CDK4/6SA (>3.0) showed significantly (P=0.024) shorter progression-free survival (PFS) than those with low CDK4/6SA (<3.0). Although Ki-67 expression itself was not a marker for prognosis, the combination of high CDK4/6SA and high Ki-67 expression (>15%) was robustly associated with shorter PFS (P=0.015), and this combination was an independent poor prognostic factor in the low-risk group. Inversely, in the intermediate-/high-risk group, patients with high CDK4/6SA had a tendency of a more favourable prognosis compared with patients with low CDK4/6SA (P=0.063).
Conclusions:
CDK4/6-specific activity can be used as a biomarker to predict prognosis and, possibly, chemo-sensitivity. The combination of Ki-67 expression might strengthen the clinical usefulness of CDK4/6SA as a biomarker.
Keywords: cyclin-dependent kinase, Ki-67 expression, endometrial cancer, low risk, chemo-sensitivity
Endometrial cancer is the fourth most common malignancy among women in the United States (Siegel et al, 2015). The main therapeutic options are surgery, chemotherapy, and radiation (Baekelandt and Castiglione, 2008). Despite recent notable developments in molecular biology, the only current indicators for the use of adjuvant chemotherapy in endometrial cancer are clinicopathological risk factors. Risk in surgically treated endometrial cancer patients is generally determined based on the patient's pathological diagnosis, including the stage, histological subtypes, tumour grade, lymph-node status, and depth of myometrial invasion, which serve to classify patients into three main risk groups: low-risk, intermediate-risk, and high-risk (Baekelandt and Castiglione, 2008; Wright et al, 2012). Adjuvant chemotherapy is mainly used for high-risk patients, while indicators for intermediate-risk patients remain controversial (Baekelandt and Castiglione, 2008; Wright et al, 2012). However, 10–15% of early-stage patients who do not receive adjuvant chemotherapy show recurrence, and their prognoses are exclusively poor with median survival of <10 months and a 5-year survival rate close to 15% (Morrow et al, 1991; Creutzberg et al, 2000). These statistics demonstrate the necessity of identifying novel biomarkers to select poor prognostic patients with indications of adjuvant treatments.
Histologically, >80% of all endometrial cancer cases are diagnosed as endometrioid endometrial carcinoma (EEC) (Baekelandt and Castiglione, 2008). Endometrioid endometrial carcinoma is frequently associated with somatic mutations in genes involved in the RAS/PI3K pathway, such as KRAS, PIK3CA, PTEN, AKT1, CTNNB1, and CCND1 (Fukuchi et al, 1998; Moreno-Bueno et al, 2003; Oda et al, 2005, 2008; Shoji et al, 2009; Musgrove et al, 2011; Ikeda et al, 2012, 2013). Cyclin D1 is upregulated by the RAS/PI3K/beta-catenin pathway and cooperates with cyclin-dependent kinase 4 and 6 (CDK4/6) to promote the cell cycle from the G1 to the S phase (Musgrove et al, 2011). Elevated CDK4 expression is observed in 34–77% of EEC cases and is considered to be an early event of neoplastic transformation in EEC (Tsuda et al, 2000; Shih et al, 2003). Although CDK6 interacts with CDK4, little is known about the role of CDK6 activity in EEC (Shiozawa et al, 1997; Nakashima et al, 1999; Wright et al, 2012). Moreover, the enzymatic activities of CDK4 and CDK6 in EEC have not been analysed in detail. On basis of the high frequency of RAS/PI3K-Cyclin D1 pathway-related mutations in EEC, we hypothesised that the aggressiveness of this disease might be associated with abnormally high CDK4/6 activity.
We developed a cell-cycle profiling (C2P) assay as a novel diagnostic technique to evaluate the activities and expression levels of CDKs (Ishihara et al, 2005). The C2P assay can be used to determine the specific activity (SA; activity/expression) of CDKs, as kinase activity can be compensated by protein expression. Previous studies using this assay focussed on analyses of CDK1/2SA; for example, in breast cancer, high CDK1/2SA was associated with significantly poor prognosis (Kim et al, 2008). Furthermore, Ki-67 expression is commonly used as a marker of cell proliferation, and the combination of CDK1/2SA and Ki-67 expression was found to strongly predict the sensitivity to paclitaxel in breast cancer (Nakayama et al, 2009; Kim et al, 2012).
In the present study, we established a novel C2P method to accurately measure CDK4/6SA, and to clarify the clinical importance of CDK4/6 to EEC.
Materials and methods
Tumour samples
Surgical samples were obtained from 119 patients who had primary EEC and underwent tumour resection at the University of Tokyo Hospital. All tumour specimens were freshly frozen and stored at −80 °C until use. All the patients provided informed consent for the collection and research use of their samples, and the use of tissues in this study was approved by the appropriate institutional ethics committees. All patients underwent primary surgery, including hysterectomy, bilateral salpingo-oophorectomy, and systematic lymphadenectomy, and were diagnosed using the International Federation of Gynecology and Obstetrics (FIGO) 2009 staging criteria. The intermediate-/high-risk group included stage I–III patients with at least one of the following factors: deep myometrial invasion (>1/2), positive lymph nodes, adnexal metastases, and pelvic peritoneal metastases. Adjuvant therapy was administered to these patients as previously described (Onda et al, 1997, Murayama-Hosokawa et al, 2010). Stage IB patients received adjuvant radiation therapy (whole pelvis with 50.4 Gy in 28 fractions). Adjuvant chemotherapy with 3–6 cycles of platinum-based chemotherapy was indicated for the other intermediate-/high-risk patients. Stage IV patients (in the high-risk group) were treated post-operatively with 6–8 cycles of platinum-based chemotherapy. All other patients (low-risk group) were closely monitored, but did not receive any additional therapy.
C2P assay
The C2P assay (Sysmex Corporation, Kobe, Japan) was performed as described previously (Ishihara et al, 2005; Kim et al, 2012). Cyclin-dependent kinase 4/6 activities were determined via the C2P assay from frozen surgical tissue samples. CDK4/6-specific activity was calculated using the following formula:
The lower detection limit of the expression assay of CDK4 and CDK6 was defined with 4 eU μl−1 of the lysate. A ‘not informative case' was defined when the expression levels of a sample were less than the lower limits for both the CDK4 and CDK6 expression assays.
CDK4 and Ki-67 expression
For CDK4 and Ki-67 immunohistochemical analysis, 4-μm sections were cut from the tissue microarray. Immunohistochemical staining was performed according to standard techniques using a Ventana Benchmark XT autostainer (Ventana Medical Systems Inc., Tucson, AZ, USA).
Anti-CDK4 (Invitrogen, Waltham, MA, USA) and anti-Ki-67 (Dako, Carpinteria, CA, USA) monoclonal antibodies were applied at a dilution of 1 : 200 and 1 : 100, respectively. The extent of CDK4 expression was scored as follows: 0 (0%), 1+ (0–5%), 2+ (5–50%), and 3+ (>50%) along with the previous study (Shiozawa et al, 1997). The extent of Ki-67 staining was scored as follows: 0 (0–4%), 1+ (5–14%), 2+ (15–49%), and 3+ (>50%). The results were scored using two slides, and the average was recorded as the final expression score.
Statistical analysis
The association of variables with clinical characteristics was evaluated using the Fisher's exact test and the Student's t-test. In all tests, differences were considered to be significant at P<0.05. Survival curves were constructed using the Kaplan–Meier method and were compared with the log-rank test. Multivariate analyses were evaluated using the Cox's proportional hazard model.
Results
Characteristics of patients
Among the 119 patients enrolled in the study, 9 samples were excluded due to the lack of informative results based on the C2P assay; 7 tumours showed low CDK4/6 expression, and assay failure was noted for 2 tumours. Another sample was excluded because of a history of neo-adjuvant chemotherapy. As shown in Table 1, the remaining 109 patients were eligible for assessment in this study, and the median follow-up time was 64 months. According to their clinicopathological characteristics, 67 patients (61%) were at stage I and 35 patients (32%) considered to be pathologically intermediate/high risk were treated with adjuvant chemotherapy. After primary treatment, 18 patients (17%) experienced recurrence. Overall, nine patients (8%) were alive with disease and six patients (6%) died from the disease.
Table 1. Clinicopathological background in 109 patients with endometrioid endometrial cancer.
| Parameter | Criteria | N (%) |
|---|---|---|
| Median follow-up | 64 months | |
| Age | <60 | 68 (62) |
| ⩾60 | 41 (38) | |
| Stage | I | 67 (61) |
| II | 9 (8) | |
| III | 28 (26) | |
| IV | 5 (5) | |
| Histological grade | 1 | 77 (71) |
| 2 | 22 (20) | |
| 3 | 10 (9) | |
| Tumour size | ⩽5 cm | 64 (59) |
| >5 cm | 45 (41) | |
| Myometrial invasion | No invasion | 17 (16) |
| ⩽1/2 | 70 (64) | |
| >1/2 | 22 (20) | |
| Lymph-node metastasis | Positive | 23 (21) |
| Negative | 86 (79) | |
| Vascular invasion | Positive | 22 (20) |
| Negative | 87 (80) | |
| Lymphatic Invasion | Positive | 23 (21) |
| Negative | 86 (79) | |
| Adjuvant chemotherapy | Yes | 35 (32) |
| No | 74 (68) | |
| Regimen | Paclitaxel and carboplatin | 28 (80) |
| Adriamycin and cisplatin (with or without cyclophosphamide) | 7 (20) | |
| Radiotherapy (whole pelvis) | Yes | 17 (16) |
| No | 92 (84) | |
| Recurrence | Yes | 18 (17) |
| No | 91 (83) | |
| Prognosis | No evidence of disease | 94 (86) |
| Alive with disease | 9 (8) | |
| Death of disease | 6 (6) |
CDK4/6SA is significantly higher in pathologically low-risk patients, and is correlated with Ki-67 expression
The median CDK4/6SA obtained via the C2P assay was 4.8 (range: 0–350.3). Analysis of CDK4 and Ki-67 expression was performed in 103 cases with samples that were suitable for immunohistochemistry. The average CDK4 expression scores determined by immunohistochemistry (CDK4-IHC) were 1.0 or higher in 53 cases (51%). The average Ki-67 expression scores were 1.5 or higher in 69 cases (67%). CDK4/6-specific activity was significantly associated with Ki-67 expression (P=0.035), but not with any other clinicopathological factor such as age, stage, lymph-node metastasis, histological grade, tumour size, myometrial invasion, lymph vascular invasion, and CDK4-IHC expression (Table 2).
Table 2. Association between CDK4/6SA and various clinicopathological backgrounds, including Ki-67 expression.
| CDK4/6SA | ||||
|---|---|---|---|---|
| Parameter | Criteria | ⩽4.8 | >4.8 | P-value |
| Age | <60 | 35 | 33 | 0.845 |
| ⩾60 | 20 | 21 | ||
| Stage | ⩽II | 36 | 41 | 0.293 |
| ⩾III | 19 | 13 | ||
| Lymph-node metastasis | No | 44 | 44 | 0.760 |
| Yes | 7 | 5 | ||
| Histological grade | I | 34 | 41 | 0.140 |
| II&III | 20 | 12 | ||
| Tumour size | <5 cm | 30 | 30 | 1.000 |
| ⩾5 cm | 22 | 23 | ||
| Myometrial invasion | ⩽1/2 | 44 | 43 | 0.798 |
| >1/2 | 10 | 8 | ||
| Vascular invasion | No | 41 | 44 | 0.474 |
| Yes | 13 | 9 | ||
| Lymphatic invasion | No | 40 | 44 | 0.347 |
| Yes | 14 | 9 | ||
| CDK4 IHC average score | <1.0 | 25 | 25 | 0.928 |
| ⩾1.0 | 28 | 25 | ||
| Ki-67 IHC average score | <1.5 | 12 | 22 | 0.035 |
| ⩾1.5 | 41 | 28 | ||
Abbreviations: CDK4=cyclin-dependent kinase 4; CDK4/6SA=CDK4/6-specific activity; IHC=immunohistochemistry.Bold entries denote P<0.05 (significant).
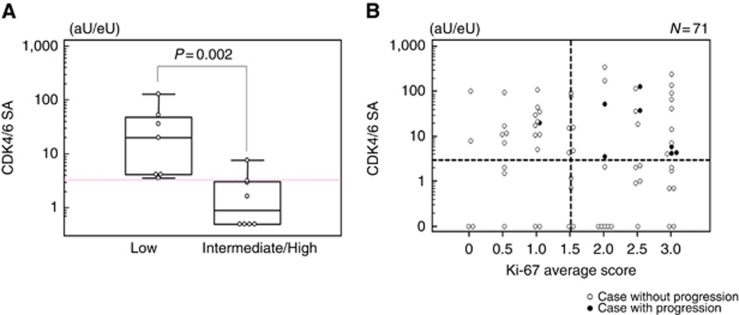
We further focussed on variation in CDK4/6SA among patients classified based on the pathological risk, which is the main determinant for the indication of adjuvant chemotherapy. In particular, CDK4/6SA was significantly higher in low-risk patients than in intermediate-/high-risk patients (P=0.002; Figure 1A). Receiver operating characteristic (ROC) curve analysis in the low-risk patients revealed that the Youden Index was maximised at 3.2 for CDK4/6SA (area under the curve=0.759, P=0.003; Supplementary Figure 1). Therefore, we defined the CDK4/6SA cutoff value as 3, and this value was employed for further analyses. The number of recurrent cases in each group according to pathological risk and/or CDK4/6SA is summarised in Supplementary Table 1.
Figure 1.
CDK4/6SA in low-risk and intermediate-/high-risk patients, and its association with Ki-67 expression. (A) Discordance of CDK4/6SA between low-risk and intermediate-/high-risk groups. Statistical analysis was performed using Mann–Whitney's U-test. (B) Scatter diagram of CDK4/6SA and the average Ki-67 expression score in low-risk patients.
As both CDK4/6 and Ki-67 are associated with cell-cycle progression, we also assessed the correlation between CDK4/6SA and Ki-67 expression using a scatter diagram with CDK4/6SA expressed on a logarithmic scale. As a result, in the low-risk group, 7 of 8 (88%) cases with progressive disease were plotted in the zone representing high CDK4/6SA (>3) and high Ki-67 expression (>1.5), whereas only 17 of 66 (26%) cases without progression were classified in the same zone, representing a statistically significant difference (P=0.002; Figure 1B). On the other hand, in the intermediate-/high-risk group only 2 of 10 (20%) cases with progression and 9 of 22 (41%) cases without progression were plotted in the zone of high CDK4/6SA and high Ki-67 expression, which did not reflect a significant difference (P=0.43; Supplementary Figure 2).
CDK4/6SA is a useful biomarker for predicting recurrence in pathologically low-risk EEC patients
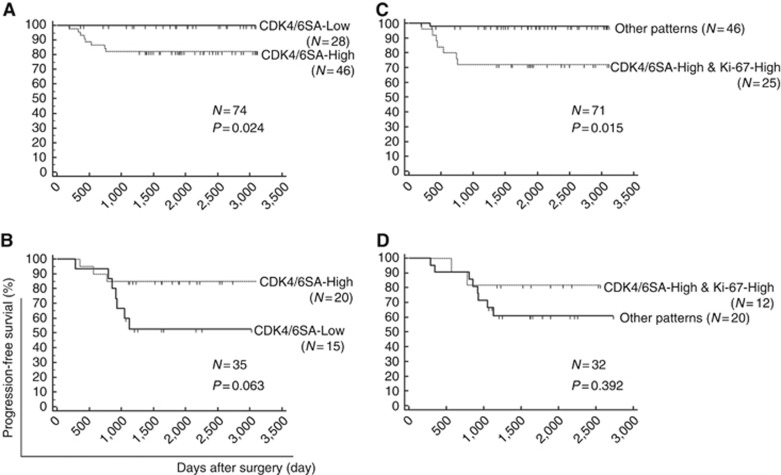
Next, we explored the prognostic importance of Ki-67 expression using Kaplan–Meier survival analysis. Ki-67-High (Ki-67 average score >1.5) was not significantly associated with prognosis in both the low-risk (P=0.191, N=71) and intermediate-/high-risk patients (P=0.392, N=31) (Supplementary Figure 3). We next examined the prognostic impact of CDK4/6SA. The CDK4/6SA value was divided into CDK4/6SA-High (CDK4/6SA >3) and CDK4/6SA-Low (CDK4/6SA <3), and progression-free survival (PFS) was compared between the groups. In low-risk patients, CDK4/6SA-High was associated with a significantly worse prognosis than CDK4/6SA-Low (P=0.024, N=74; Figure 2A). However, the result tended to be inversed in intermediate-/high-risk patients, showing a more favourable prognosis in patients with CDK4/6SA-High (P=0.063, N=35; Figure 2B).
Figure 2.
Prognostic impact of CDK4/6SA alone and in combination with Ki-67 expression. The prognostic value of CDK4/6SA was explored using Kaplan–Meier methods and the log-rank test. (A, B) Comparison between CDK4/6SA-High (CDK4/6SA >3) and CDK4/6SA-Low (CDK4/6SA >3) in (A) low-risk (those not receiving adjuvant chemotherapy) and (B) intermediate-/high-risk (those receiving adjuvant chemotherapy) patients. (C, D) Analysis of the combination of CDK4/6SA and Ki-67 expression. Cases with both CDK4/6SA-High and Ki-67-High (Ki-67 average score >1.5) were compared with other combinations in (C) low-risk and (D) intermediate-/high-risk patients.
The combination of CDK4/6SA-High and Ki-67-High was significantly associated with poor prognosis, compared with other combinations of these factors (CDK4/6SA-Low and/or Ki-67-Low) in the low-risk patients (P=0.015, N=71; Figure 2C). However, this combination did not significantly influence prognosis in the intermediate-/high-risk patients (P=0.392, N=32; Figure 2D).
CDK4/6SA is an independent prognostic factor in low-risk EEC patients, and its predictive value is reinforced by Ki-67 expression
We conducted univariate and multivariate analyses in the low-risk group to more comprehensively analyse the relationship between the potential biomarkers and clinicopathological features. CDK4/6-specific activity alone was only associated with PFS in the univariate analyses (hazards ratio [HR]=10.79, 95% confidence interval [CI]: 1.34–86.87, P=0.026; Supplementary Table 2). Multivariate analysis among CDK4/6SA, age, and tumour size indicated that CDK4/6SA is an independent prognostic factor in the low-risk group (HR=10.79, 95% CI: 1.34–86.87, P=0.036; Supplementary Table2). Next, we combined Ki-67 expression with CDK4/6SA in the low-risk group. As shown in Table 3, univariate analysis showed that the combination of CDK4/6SA-High and Ki-67-High was significantly associated with poor prognosis (HR=11.80, 95% CI: 1.44–97.3, P=0.022). The CDK4-IHC score was not significantly associated with prognosis (HR=1.6, 95% CI: 0.62–4.12, P=0.33). Multivariate analysis using the CDK4/6SA-High and Ki-67-High combination, vascular invasion, and tumour size showed that only the combination of CDK4/6SA-High and Ki-67-High was an independent poor prognostic marker (HR=11.39, 95% CI: 1.23–105, P=0.033). These data suggest that CDK4/6SA could be used to effectively predict ‘high-risk' patients with EEC in the pathologically low-risk group, and the combination of Ki-67 expression could enhance the clinical significance of CDK4/6SA.
Table 3. Uni/Multivariate analysis of CDK4/6SA in combination with Ki-67 in low-risk patients.
|
Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Criteria | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age | >60 | 2.00 | 0.150–8.85 | 0.336 | |||
| ⩽60 | |||||||
| Stage | ⩽II | 3.40 | 0.414–28.1 | 0.256 | |||
| ⩾III | |||||||
| Vascular invasion | No | 3.00 | 0.583–15.2 | 0.192 | 1.59 | 0.248–10.2 | 0.628 |
| Yes | |||||||
| Histological grade | I | 2.30 | 0.209–10.0 | 0.287 | |||
| II and III | |||||||
| Tumour size | <5 cm | 2.70 | 0.618–12.2 | 0.187 | 2.86 | 0.508–16.1 | 0.236 |
| ⩾5 cm | |||||||
| Myometrial invasion | ⩽1/2 | 0.90 | 0.167–4.36 | 0.849 | |||
| >1/2 | |||||||
| Lymphatic invasion | No | 1.50 | 0.180–12.2 | 0.716 | |||
| Yes | |||||||
| CDK4-IHC | <1.5 | 1.60 | 0.624–4.12 | 0.328 | |||
| ⩾1.5 | |||||||
| CDK4/6SA and Ki-67 | High and high | 11.80 | 1.44–97.3 | 0.022 | 11.39 | 1.23–105 | 0.033 |
| Others | |||||||
Abbreviations: CDK4=cyclin-dependent kinase 4; CDK4/6SA=CDK4/6-specific activity; IHC=immunohistochemistry. Bold entries denote HR>10 or P<0.2 (significant). Italic entries indicate P<0.05 (significant).
Discussion
In this analysis of 109 clinical EEC samples, we demonstrated (i) a novel analysis method for examining the activity and expression of CDK4/6 using a C2P assay, (ii) an association between CDK4/6SA and Ki-67 expression, (iii) CDK4/6SA as a significant predictor of recurrence in the pathologically low-risk group, and (iv) the combination of CDK4/6SA and Ki-67 expression as a more robust predictor of recurrence in the low-risk group. In addition, our data indicated that CDK4/6SA might enhance the chemo-sensitivity in patients with adjuvant chemotherapy in the intermediate-/high-risk group, and that CDK4/6SA is an independent prognostic factor in the low-risk group.
Various useful clinicopathological prognostic factors for EEC have been previously reported, including stage, tumour grade, depth of myometrial invasion, lymphovascular invasion, and tumour size and age (Salvesen et al, 2012). In addition, several biomarkers, including chromosomal instability, microsatellite instability, and TP53 mutations, have been analysed with respect to EEC (Lax et al, 2000; Murayama-Hosokawa et al, 2010). However, the status of adjuvant treatment has not yet been considered in detail, and several biomarkers might not be unveiled according to different treatment protocols. Moreover, no appropriate biomarkers have been identified to predict chemo-sensitivity in EEC. Because of the high rate of recurrence in patients with non-adjuvant chemotherapy, novel biomarkers are warranted to better identify high-risk patients who are not categorised as ‘high-risk' based on the conventional pathological criteria. In our analysis, CDK4/6SA in pathologically low-risk patients (those not receiving adjuvant chemotherapy) was more broadly distributed than that in intermediate-/high-risk patients (those receiving adjuvant chemotherapy). Because CDK4/6SA indicates the activity of CDK4/6 per unit, and CDK4/6 is strongly associated with the cell cycle (Bates et al, 1994), we hypothesised that high CDK4/6SA might be strongly associated with poor prognosis, especially in cases diagnosed as ‘low-risk' based on the pathological criteria. This hypothesis was verified, as CDK4/6SA was significantly associated with prognosis in the low-risk patients, suggesting that high CDK4/6SA promotes cell proliferation and tumour growth.
Interestingly, CDK4/6SA-High showed better prognosis in the intermediate-/high-risk patients. These data indicate that tumours with high CDK4/6SA might be more sensitive to platinum-based chemotherapy. These data are in agreement with a report demonstrating that CDK4/6 activity is required for DNA-damaging anticancer drugs to effectively suppress cell proliferation (Roberts et al, 2012). We recently reported that mutant cyclin D1 (T286I) promoted the cell cycle by regulating Rb function (Ikeda et al, 2013). As CDK4/6 cooperates with cyclin D1 to accelerate the cell cycle, CDK4/6SA-high tumours might be associated with rapid cell proliferation and promote the cell cycle from the G1 to the S phase. As platinum-DNA adducts cause various cellular responses, including cell-cycle arrest and apoptosis (Wang and Lippard, 2005; He et al, 2011), it is reasonable to suggest that the cell-cycle augmentation induced by high CDK4/6SA might be associated with sensitivity to platinum-based chemotherapy (He et al, 2011; Roberts et al, 2012).
Although both CDK4/6 and Ki-67 are associated with cell proliferation (Gerdes et al, 1984), in our study, Ki-67 expression did not show prognostic significance on its own. This might be related to the fact that CDK4/6 activity can be directly augmented via activation of the RAS/PI3K-cyclin D1 pathway in endometrial cancer (Fukuchi et al, 1998; Moreno-Bueno et al, 2003; Oda et al, 2005, 2008; Shoji et al, 2009; Musgrove et al, 2011; Ikeda et al, 2012, 2013), which could more robustly reflect tumour aggressiveness than the expression level of Ki-67. These data support the significance of CDK4/6SA as determined through the C2P assay. Taken together with previous reports showing that PI3K pathway-related activity is associated with chemo-sensitivity (Guinea Viniegra et al, 2002; Bender et al, 2011), CDK4/6SA might be a suitable marker for assessing the necessity of adjuvant chemotherapy, and information on Ki-67 expression could strengthen the significance of CDK4/6SA. This additional post-operative treatment that would not be received otherwise based on the pathological criteria alone might improve the prognosis of clinicopathologically low-risk patients with high CDK4/6SA.
This study has some limitations that should be mentioned. First, the number of samples was limited, especially with respect to the number of recurrent cases, and the overall prognosis of patients was more favourable compared with the previous reports (Todo et al, 2010). Further validation with a larger number of samples is warranted to strengthen our results. Second, the relationship between chemo-sensitivity and CDK4/6SA should be further clarified directly. Third, C2P analysis is not available for formalin-fixed specimens, and the requirement of freshly frozen samples might hinder its clinical application.
In conclusion, we determined the significance of CDK4/6 activity in EEC, using a new modification of the C2P assay. CDK4/6-specific activity can be used as a biomarker to predict prognosis and, possibly, chemo-sensitivity. In addition, combination with Ki-67 expression could enhance the predictive value of CDK4/6SA for pathologically diagnosed low-risk EEC patients. The selection of clinicopathologically low-risk and biologically high-risk patients based on the C2P analysis should provide useful information for establishing personalised medicine in the treatment of endometrial cancer.
Acknowledgments
We thank Toshiyuki Sato and Hirokazu Kurata from Sysmex Co. Ltd., and Keiko Shoji, Satoko Kojima, Yuichiro Miyamoto, Kensuke Tomio, Michihiro Tanikawa, Kazunori Nagasaka, and Reiko Kurikawa from the University of Tokyo for their support and assistance. This work was supported by Sysmex Co. Ltd. and by a Grant-in-Aid for Scientific Research (C) (grant number 26462515 to KO), Grant-in-Aid for Young Scientists (B) (grant numbers 15K20128 to KS and 25861471 to TA), and Grant-in-Aid for Research Activity Start-up (grant number 25893229 to YI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This study was also supported by a research program from the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct), Ministry of Education, Culture, Sports, Science, and Technology of Japan (to TY).
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The funding for this study was partly provided by Sysmex Co. Ltd. (to KO and DA). HI is an employee of Sysmex Co. Ltd. C2P assay was performed at Sysmex Co. Ltd., but nobody in the company was involved in data analysis, or decision to submit.
Supplementary Material
References
- Baekelandt MM, Castiglione M (2008) Endometrial carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 19(Suppl 2): ii19–ii20. [DOI] [PubMed] [Google Scholar]
- Bates S, Bonetta L, MacAllan D, Parry D, Holder A, Dickson C, Peters G (1994) CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene 9(1): 71–79. [PubMed] [Google Scholar]
- Bender A, Opel D, Naumann I, Kappler R, Friedman L, von Schweinitz D, Debatin KM, Fulda S (2011) PI3K inhibitors prime neuroblastoma cells for chemotherapy by shifting the balance towards pro-apoptotic Bcl-2 proteins and enhanced mitochondrial apoptosis. Oncogene 30(4): 494–503. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, De Winter KA, Lutgens LC, van den Bergh AC, van de Steen-Banasik E, Beerman H, van Lent M (2000) Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 355(9213): 1404–1411. [DOI] [PubMed] [Google Scholar]
- Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S (1998) Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res 58(16): 3526–3528. [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133(4): 1710–1715. [PubMed] [Google Scholar]
- Guinea Viniegra J, Hernandez Losa J, Sanchez-Arevalo VJ, Parada Cobo C, Fernandez Soria VM, Ramon y Cajal S, Sanchez-Prieto R (2002) Modulation of PI3K/Akt pathway by E1a mediates sensitivity to cisplatin. Oncogene 21(46): 7131–7136. [DOI] [PubMed] [Google Scholar]
- He G, Kuang J, Khokhar AR, Siddik ZH (2011) The impact of S- and G2-checkpoint response on the fidelity of G1-arrest by cisplatin and its comparison to a non-cross-resistant platinum(IV) analog. Gynecol Oncol 122(2): 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Oda K, Hiraike-Wada O, Koso T, Miyasaka A, Kashiyama T, Tanikawa M, Sone K, Nagasaka K, Maeda D, Kawana K, Nakagawa S, Fukayama M, Tetsu O, Fujii T, Yano T, Kozuma S (2013) Cyclin D1 harboring the T286I mutation promotes oncogenic activation in endometrial cancer. Oncol Rep 30(2): 584–588. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Oda K, Nakagawa S, Murayama-Hosokawa S, Yamamoto S, Ishikawa S, Wang L, Takazawa Y, Maeda D, Wada-Hiraike O, Kawana K, Fukayama M, Aburatani H, Yano T, Kozuma S, Taketani Y (2012) Genome-wide single nucleotide polymorphism arrays as a diagnostic tool in patients with synchronous endometrial and ovarian cancer. Int J Gynecol Cancer 22(5): 725–731. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Yoshida T, Kawasaki Y, Kobayashi H, Yamasaki M, Nakayama S, Miki E, Shohmi K, Matsushima T, Tada S, Torikoshi Y, Morita M, Tamura S, Hino Y, Kamiyama J, Sowa Y, Tsuchihashi Y, Yamagishi H, Sakai T (2005) A new cancer diagnostic system based on a CDK profiling technology. Biochim Biophys Acta 1741(3): 226–233. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Nakayama S, Miyoshi Y, Taguchi T, Tamaki Y, Matsushima T, Torikoshi Y, Tanaka S, Yoshida T, Ishihara H, Noguchi S (2008) Determination of the specific activity of CDK1 and CDK2 as a novel prognostic indicator for early breast cancer. Ann Oncol 19(1): 68–72. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Nakayama S, Shimazu K, Tamaki Y, Akazawa K, Tsukamoto F, Torikoshi Y, Matsushima T, Shibayama M, Ishihara H, Noguchi S (2012) Recurrence risk score based on the specific activity of CDK1 and CDK2 predicts response to neoadjuvant paclitaxel followed by 5-fluorouracil, epirubicin and cyclophosphamide in breast cancers. Ann Oncol 23(4): 891–897. [DOI] [PubMed] [Google Scholar]
- Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L (2000) The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 88(4): 814–824. [PubMed] [Google Scholar]
- Moreno-Bueno G, Rodriguez-Perales S, Sanchez-Estevez C, Hardisson D, Sarrio D, Prat J, Cigudosa JC, Matias-Guiu X, Palacios J (2003) Cyclin D1 gene (CCND1) mutations in endometrial cancer. Oncogene 22(38): 6115–6118. [DOI] [PubMed] [Google Scholar]
- Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, Graham JE (1991) Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 40(1): 55–65. [DOI] [PubMed] [Google Scholar]
- Murayama-Hosokawa S, Oda K, Nakagawa S, Ishikawa S, Yamamoto S, Shoji K, Ikeda Y, Uehara Y, Fukayama M, McCormick F, Yano T, Taketani Y, Aburatani H (2010) Genome-wide single-nucleotide polymorphism arrays in endometrial carcinomas associate extensive chromosomal instability with poor prognosis and unveil frequent chromosomal imbalances involved in the PI3-kinase pathway. Oncogene 29(13): 1897–1908. [DOI] [PubMed] [Google Scholar]
- Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL (2011) Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 11(8): 558–572. [DOI] [PubMed] [Google Scholar]
- Nakashima R, Fujita M, Enomoto T, Haba T, Yoshino K, Wada H, Kurachi H, Sasaki M, Wakasa K, Inoue M, Buzard G, Murata Y (1999) Alteration of p16 and p15 genes in human uterine tumours. Br J Cancer 80(3–4): 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Torikoshi Y, Takahashi T, Yoshida T, Sudo T, Matsushima T, Kawasaki Y, Katayama A, Gohda K, Hortobagyi GN, Noguchi S, Sakai T, Ishihara H, Ueno NT (2009) Prediction of paclitaxel sensitivity by CDK1 and CDK2 activity in human breast cancer cells. Breast Cancer Res 11(1): R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K, Okada J, Timmerman L, Rodriguez-Viciana P, Stokoe D, Shoji K, Taketani Y, Kuramoto H, Knight ZA, Shokat KM, McCormick F (2008) PIK3CA cooperates with other phosphatidylinositol 3'-kinase pathway mutations to effect oncogenic transformation. Cancer Res 68(19): 8127–8136. [DOI] [PubMed] [Google Scholar]
- Oda K, Stokoe D, Taketani Y, McCormick F (2005) High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res 65(23): 10669–10673. [DOI] [PubMed] [Google Scholar]
- Onda T, Yoshikawa H, Mizutani K, Mishima M, Yokota H, Nagano H, Ozaki Y, Murakami A, Ueda K, Taketani Y (1997) Treatment of node-positive endometrial cancer with complete node dissection, chemotherapy and radiation therapy. Br J Cancer 75(12): 1836–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, Zamboni WC, Wong KK, Perou CM, Sharpless NE (2012) Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst 104(6): 476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen HB, Haldorsen IS, Trovik J (2012) Markers for individualised therapy in endometrial carcinoma. Lancet Oncol 13(8): e353–e361. [DOI] [PubMed] [Google Scholar]
- Shih HC, Shiozawa T, Kato K, Imai T, Miyamoto T, Uchikawa J, Nikaido T, Konishi I (2003) Immunohistochemical expression of cyclins, cyclin-dependent kinases, tumor-suppressor gene products, Ki-67, and sex steroid receptors in endometrial carcinoma: positive staining for cyclin A as a poor prognostic indicator. Hum Pathol 34(5): 471–478. [DOI] [PubMed] [Google Scholar]
- Shiozawa T, Nikaido T, Shimizu M, Zhai Y, Fujii S (1997) Immunohistochemical analysis of the expression of cdk4 and p16INK4 in human endometrioid-type endometrial carcinoma. Cancer 80(12): 2250–2256. [DOI] [PubMed] [Google Scholar]
- Shoji K, Oda K, Nakagawa S, Hosokawa S, Nagae G, Uehara Y, Sone K, Miyamoto Y, Hiraike H, Hiraike-Wada O, Nei T, Kawana K, Kuramoto H, Aburatani H, Yano T, Taketani Y (2009) The oncogenic mutation in the pleckstrin homology domain of AKT1 in endometrial carcinomas. Br J Cancer 101(1): 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1): 5–29. [DOI] [PubMed] [Google Scholar]
- Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N (2010) Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet 375(9721): 1165–1172. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Yamamoto K, Inoue T, Uchiyama I, Umesaki N (2000) The role of p16-cyclin d/CDK-pRb pathway in the tumorigenesis of endometrioid-type endometrial carcinoma. Br J Cancer 82(3): 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4(4): 307–320. [DOI] [PubMed] [Google Scholar]
- Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ (2012) Contemporary management of endometrial cancer. Lancet 379(9823): 1352–1360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.