Abstract
Purpose
The purpose of this nested case-control study was to identify baseline, incident, and progressive MRI findings visible on standard MRI clinical sequences that were associated with development of incident knee pain in subjects at risk for OA over a period of 48 months.
Methods
We analyzed 60 case knees developing incident pain (WOMACpain = 0 at baseline and WOMACpain ≥ 5 at 48 months) and 60 control knees (WOMACpain = 0 at baseline and WOMACpain = 0 at 48 months) from the Osteoarthritis Initiative. 3 T knee MRIs were analyzed using a modified WORMS score (cartilage, meniscus, bone marrow) at baseline and after 48 months. Baseline and longitudinal findings were grouped into logistic regression models and compared using likelihood-ratio tests. For each model that was significant, a stepwise elimination was used to isolate significant MRI findings.
Results
One baseline MRI finding and three findings that changed from baseline to 48 months were associated with the development of pain: at baseline, the severity of a cartilage lesion in the medial tibia was associated with incident pain—(odds ratio (OR) for incident pain = 3.05; P = 0.030). Longitudinally, an incident effusion (OR = 9.78; P = 0.005), a progressive cartilage lesion of the patella (OR = 4.59; P = 0.009), and an incident medial meniscus tear (OR = 4.91; P = 0.028) were associated with the development of pain.
Conclusions
Our results demonstrate that baseline abnormalities of the medial tibia cartilage as well as an incident joint effusion, progressive patella cartilage defects, and an incident medial meniscus tear over 48 months may be associated with incident knee pain. Clinically, this study helps identify MRI findings that are associated with the development of knee pain.
Keywords: Osteoarthritis, Pain, MRI, Knee, Osteoarthritis initiative
Introduction
Knee pain from osteoarthritis (OA) is one of the primary causes of disability-adjusted life years in the United States [1, 2]. While knee structural changes in OA such as cartilage loss, meniscal tears, and bone marrow edema patterns have been well described [3, 4], it is unclear which particular morphologic findings are associated with prevalent and incident pain. Identifying such morphologic abnormalities could lead to treatments targeting specific pain-related structural changes, thus improving quality of life for OA patients.
The discrepancies between pain and morphologic evidence of OA have been well documented [5, 6]: there is evidence that subjects with radiographic OA may not have pain and vise-versa. For example, studies have shown that 50% of patients with radiographic OA did not have symptoms [7], and a large variation in pain scores was evident among subjects with the same radiographic score [8]. In addition, multiple studies have evaluated the relationship between MRI findings and Western Ontario and McMaster Universities (WOMAC) [9] pain scores in knee OA; however, a distinct temporal relationship has not been reliably demonstrated [10, 11], potentially due to intra- and inter-subject pain tolerance variability. These studies highlight the complexity and multifactorial nature of OA, and emphasize the need for longitudinal evaluation of predictors for symptomatic disease.
The purpose of this nested case-control study was to identify baseline, incident, and progressive MRI findings visible on standard MRI clinical sequences that were associated with development of incident knee pain in subjects at risk for OA over a period of 48 months. The current study design is unique from previous research, as it utilizes the Osteoarthritis Initiative database (OAI) [12] to assess longitudinal changes in knee morphology (including the cartilage, meniscus, bone marrow) in subjects that develop pain over 48 months. Evaluating the same subjects over time mitigates errors due to inter-subject variations in pain tolerance. In addition, assessing subjects from the OAI, a longitudinal database with MR images of subjects scanned annually over 8 years, facilitates identifying subjects that develop pain and evaluating their concurrent changes in knee morphology.
Methods
Subjects
Subjects were selected from the Osteoarthritis Initiative (OAI) [12], a large multicenter, longitudinal cohort study of patients either with or at risk for developing knee OA. The OAI dataset includes both MRI and radiographic images of subjects scanned annually over 8 years. This database can be used to longitudinally evaluate MRI biomarkers for the development and progression of OA. The study protocol, amendments, and informed consent documentation were reviewed and approved by the local institutional review boards.
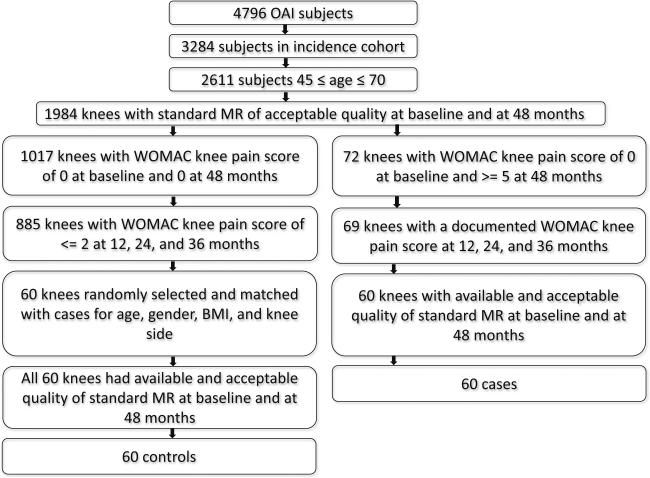
Figure 1 shows a flowchart of subject selection. Of the 4, 796 subjects enrolled in the OAI study, knees with a pain increase and controls were selected based on the following 6 inclusion criteria: (1) age 45–70 years old at baseline; (2) subjects did not have symptoms at baseline, but were considered high risk for developing symptomatic knee OA due to being overweight, prior knee injury or surgery, family history of knee replacement, Heberden's nodes or frequent knee bending activity; (3) WOMAC knee pain score of 0 at baseline in the index knee, (4) complete WOMAC knee pain data at 12, 24, 36 and 48 month follow-up, (5) availability of Kellgren-Lawrence (KL) scores at baseline [13], and (6) availability and acceptable quality of baseline and 48-month follow-up MRI exams. Case knees were those that had a WOMAC pain score of ≥5 at 48 months. There were only 60 knees that met these criteria. Eligible control knees had a WOMAC knee pain score ≤2 at all follow-ups between 0 and 48 months. A WOMAC pain score threshold of ≤2 was chosen based on a previous study [14], which showed that using the Visual Analogue Scale (VAS), a minimal perceptible change in pain required a 10% difference, which equates to a change greater than 2 using the Likert scale.
Fig. 1.
Patient selection from the OAI database
From the total of 885 knees eligible from the control cohort, 60 subjects were randomly selected frequency-matched for age, gender, body mass index (BMI), and knee side (left or right). The frequency matching (in addition to adjustment in the statistical analysis) was used to account for any potential confounding effects in the analysis [15, 16]. The case knees and the knees eligible for the control cohort showed an equal distribution of Kellgren-Lawrence grades; therefore, we did not frequency-match controls by this criterion. We excluded subjects with interval acute events such as trauma or infection.
WOMAC questionnaires
To assess pain severity and monitor pain progression, we used the WOMAC Osteoarthritis Index [9] which quantifies pain, stiffness, and physical function [9]. This well-established and validated questionnaire was administered in yearly intervals to all 4,796 OAI subjects in a standardized manner. As subjects with a WOMAC pain score ≥5 have at least one activity with moderate amounts of pain [15], we used a threshold of ≥5 to define significant knee pain.
Magnetic resonance imaging
Knee MRIs were obtained at 3 T at baseline and after 48 months, using identical MR scanners (Trio, Siemens, Erlangen) and standard knee coils (Siemens, Erlangen, Germany) at four different sites. Four MRI sequences from the OAI protocol [12] were analyzed, and the scanning parameters are listed in Table 1.
Table 1.
OAI knee MRI protocol acquisition parameters, from Peterfy et al. [12]
| Scan | COR IW 2D TSE | SAG 3D DESS WE | COR T1W 3D FLASH WE | SAG IW 2D TSE FS |
|---|---|---|---|---|
| Plane | Coronal | Sagittal | Coronal | Sagittal |
| Fat saturation | No | WE | WE | FS |
| Number of slices | 35 | 160 | 80 | 37 |
| FOV (mm) | 140 | 140 | 160 | 160 |
| Slice thickness/gap (mm/mm) | 3/0 | 0.7/0 | 1.5/0 | 3/0 |
| Flip angle (°) | 180 | 25 | 12 | 180 |
| TE/TR (ms/ms) | 29/3700 | 4.7/16.3 | 7.57/20 | 30/3200 |
| Bandwidth (Hz/pixel) | 352 | 185 | 130 | 248 |
| X-resolution (mm) | 0.365 | 0.365 | 0.313 | 0.357 |
| Y-resolution (mm) | 0.456 | 0.456 | 0.313 | 0.511 |
MR image analysis
Following standard clinical radiology practice, paired readings of baseline and 48-month MRI exams were performed on a PACS workstation (Agfa, Ridgefield Park, NJ) using the modified Whole-Organ MR Imaging Scoring (WORMS) method, which has been established for evaluating early degenerative joint disease [3, 16]. Cartilage and subchondral bone marrow edema abnormalities were assessed in six regions: patella, trochlea, medial/lateral tibia, and medial/lateral femur. Cartilage lesions were graded using the modified WORMS scale [3]. Subchondral bone marrow edema pattern was defined as poorly marginated areas of increased T2 signal intensity and graded using a modified 4-point WORMS scale (0, none; 1, diameter 0–5 mm; 2, 5–20 mm; 3, >20 mm) [19], as well as a volumetric quantification described previously [17].
Meniscal lesions were graded separately in 6 regions (medial/lateral and anterior/body/posterior) using the following 4-point scale: 0, normal; 1, intrasubstance signal; 2, non-displaced tear; 3, displaced or complex tear; 4, complete destruction/maceration. As established in previous studies [16, 18], intrasubstance degeneration was added to the WORMS classification to permit the inclusion and quantification of early degenerative disease [19]. Each MRI was evaluated for the presence or absence of an effusion. An “incident effusion” was defined as absent at baseline, but present at 48 months.
Sequences were analyzed blinded to subject data. A “training period” was performed in which three radiologists (S.W.H., 3rd year radiology resident; S.L., board-certified radiologist with 1 year of MSK fellowship training; L.N., 4th year radiology resident) and a senior MSK radiologist (T.M.L., more than 20 years of experience in MSK radiology) together analyzed 30 MRI exams in order to arrive at a consensus reading and to calibrate thresholds for grading abnormalities. The remaining studies were then reviewed independently by S.W.H., S.L., and L.N with two radiologists reading each study. In those instances where scores were not identical, consensus readings were performed with the senior MSK radiologist (T.M.L.). Regarding changes in findings between baseline and 48 months, a finding was considered incident if it was absent at baseline but present at 48 months, and was considered progressive if it was present at baseline but worse at 48 months.
Statistical analysis
Independent t-tests (for numeric variables) and chi-square tests (for categorical variables) were used to evaluate differences in subject characteristics between cases and controls. Baseline and longitudinal predictors were assessed in logical groups using logistic regression and likelihood-ratio tests. In total, ten different statistical models were employed. All models were adjusted for age, sex, and BMI. Incident pain was predicted using three different models for baseline findings and seven different models for changes in findings between baseline and 48 months. The MRI findings were grouped into statistical models according to the type of tissue the lesion affected. Three models for baseline findings included meniscal pathology, cartilage lesions, and bone marrow edema pattern. Six models for longitudinal changes included the menisci, cartilage, and bone marrow, with each type of tissue having one model for incident lesions and one model for progressive lesions. The final model evaluated an incident effusion.
The baseline models included: model 1 investigated baseline pathology of the anterior horn, body, and posterior horn of both the medial and lateral menisci; model 2 evaluated baseline pathology of the patella, trochlea, medial femoral condyle, lateral femoral condyle, medial tibia, and lateral tibia cartilage; model 3 tested baseline pathology of bone marrow edema pattern, (same compartments as model 2). All baseline models utilized the raw WORMS scores and not the binary data.
The longitudinal models included: models 4 and 5 investigated incident and progressive pathology, respectively, of the medial and lateral menisci at 48 months; models 6 and 7 evaluated incident and progressive pathology, respectively, of cartilage, with the same compartments as model 2; models 8 and 9 tested incident and progressive pathology, respectively, of bone marrow edema pattern, again with the same compartments as model 2; model 10 investigated an incident effusion. All longitudinal models utilized binary (yes/no) predictor variables representing incidence or progression.
The likelihood-ratio test was used to compare each model to a base model consisting of only age, sex, and BMI. For those models that were significantly different from the base model, a backward-stepwise method using the likelihood ratio test was implemented to reduce the number of variables to a significant subset. This two-stage approach (first requiring a statistically significant improvement over the base model) protects against multiple testing of the individual components for each model. Thus, omnibus tests were used to control for the overall error rate before proceeding to the stepwise regressions. A final logistic regression model, which included the significant variables that remained, was used to determine the odds for developing incident pain. This model was adjusted for age, gender and BMI, and Wald tests were used for calculating P-values. Statistical analysis was performed using Stata/IC Version 13 (StataCorp, College Station, TX).
Results
Subject characteristics
Table 2 shows the baseline demographics and clinical characteristics of 120 knees (in 120 subjects) included in our study. The cases and controls had similar distributions of age, gender, BMI, and KL grade.
Table 2.
Baseline demographics are displayed for control and case subjects.
| Case (n = 60) | Control (n = 60) | P-value | |
|---|---|---|---|
| Gender (male) | 25 (41.7%) | 25 (41.7% | * |
| Knee side (right) | 34 (56.7%) | 34 (56.7%) | * |
| WOMAC pain score at baseline | 0±0 | 0±0 | * |
| WOMAC pain score at 48 months | 6.4 ± 1.4 | 0±0 | * |
| Age (mean ± SD) (years) | 59.0 ± 6.8 | 59.2 ± 7.1 | 0.91 |
| BM (kg/m2) | 29.4 ± 4.8 | 28.6 ± 4.1 | 0.31 |
| History of knee injury | 29 (48.3%) | 24 (44.4%) | 0.68 |
| History of knee surgery | 11 (18.3%) | 9 (16.7%) | 0.82 |
| Family history of knee replacement surgery | 8 (13.3%) | 5 (9.3%) | 0.49 |
| PASEa score at baseline | 177.34 ± 87.76 | 175.34 ± 79.82 | 0.89 |
| Change in PASE score (48 months – baseline) | −18.52 ± 81.98 | −8.98 ± 94.11 | 0.56 |
| Kellgren-Lawrence grade ≥2 at baseline | 20 (33.3%) | 15 (25.0%) | 0.32 |
| Kellgren-Lawrence grade ≥2 at 48 months | 27 (45.0%) | 26 (43.3%) | 0.85 |
PASE physical activity scale for the elderly
Data are presented as mean ± SD or number of subjects (n), (proportion to total in percentage).
P-values of intergroup differences were assessed using independent t-tests and Chi-square tests, for numerical and categorical variables, respectively.
signifies that these variables were included as part of the study design
Baseline MRI findings predictive of development of incident pain
Baseline knee morphologic characteristics were considered to be associated with the development of knee pain if case knees had more frequent MRI findings than control knees, as assessed using a likelihood ratio test. The statistical models evaluating baseline MRI findings (models 1–3) are shown in Table 3.
Table 3.
Ten statistical regression models were evaluated. Models 1-3 evaluated baseline MR findings. Models 4-10 evaluated changes in MR findings frombaseline to 48 months. All 10 models were adjusted for age, sex, and BMI. P-values were calculated using a likelihood ratio test comparing each model to a base model. The base model consisted of only age, sex, and BMI
| Model number | Model | Variables | Number of variables added to base model | P value |
|---|---|---|---|---|
| 1 | Baseline meniscal findings | Anterior horn, posterior horn, and body of medial and lateral meniscus | 6 | 0.110 |
| 2 | Baseline cartilage findings | Cartilage of patella, trochlea, medial and lateral femoral condyles, medial and lateral tibia | 6 | 0.028 |
| 3 | Baseline bone marrow findings | Bone marrow of patella, trochlea, medial and lateral femoral condyles, medial and lateral tibia | 6 | 0.226 |
| 4 | Incident meniscal tear | Medial and lateral meniscus | 2 | <0.0001 |
| 5 | Progressive meniscal tear | Medial and lateral meniscus | 2 | 0.121 |
| 6 | Incident cartilage lesion | Cartilage of patella, trochlea, medial and lateral femoral condyles, medial and lateral tibia | 6 | 0.438 |
| 7 | Progressive cartilage lesion | Cartilage of patella, trochlea, medial and lateral femoral condyles, medial and lateral tibia | 6 | <0.0001 |
| 8 | Incident bone marrow lesion | Bone marrow of patella, trochlea, medial and lateral femoral condyles, medial and lateral tibia | 6 | 0.034 |
| 9 | Progressive bone marrow lesion | Bone marrow of patella, trochlea, medial and lateral femoral condyles, medial and lateral tibia | 6 | 0.055 |
| 10 | Incident effusion | Incident effusion | 1 | 0.0003 |
Bold signifies p < 0.05
Model 2, which evaluated cartilage pathology, was significantly different from the base model. A backward stepwise method using the likelihood ratio test for model 2 identified a cartilage lesion of the medial tibia to be significant. At baseline, a cartilage lesion of the medial tibia was present in 18.3% of knees in the case cohort, compared to 3.3 % of knees in the control cohort (Table 4). This finding was associated with the development of incident pain with an odds ratio (OR) of 3.05 using the likelihood ratio test (P = 0.030, CI= 1.11–8.36).
Table 4.
A cartilage lesion in the medial tibia at baseline, the presence of an incident effusion, the presence of a progressive cartilage lesion in the patella, and the presence of an incident medial meniscus tear were associated with new knee pain at 48 months. A knee was defined as having an incident effusion if it had no effusion at baseline, but developed an effusion at 48 months. A progressive cartilage lesion was defined as a cartilage lesion at baseline, which evolved into a higher grade and/or larger size at 48 months. A knee was defined as having an incident medial meniscus tear if it did not have a medial meniscus tear at baseline, but developed one at 48months. Data are presented as number of subjects (proportion to total in percentage). The odds ratios were calculated using logistic regression models, adjusted for age, gender, and BMI (withWald tests for the calculation of P-values)
| Case | Control | |
|---|---|---|
| No medial tibia cartilage lesion at baseline | 49 (81.7%) | 58 (96.7%) |
| Medial tibia cartilage lesion at baseline | 11 (18.3%) | 2 (3.3%) |
| Odds ratio = 3.05, P-value = 0.030, CI = 1.11-8.36 | ||
| No incident effusion | 45 (75.0%) | 58 (96.7%) |
| Incident effusion | 15 (25.0%) | 2 (3.3%) |
| Odds ratio = 9.78, P-value = 0.005 CI: 1.99-18.08 | ||
| No progressive cartilage lesion in the patella | 44 (73.3%) | 55 (91.6%) |
| Progressive cartilage lesion in the patella | 16 (26.7%) | 5 (8.3%) |
| Odds ratio = 4.59, P-value = 0.009, CI = 1.46-14.43 | ||
| No incident medial meniscus tear | 48 (80.0%) | 57 (95.0%) |
| Incident medial meniscus tear | 12 (20.0%) | 3 (5.0%) |
| Odds ratio = 4.91, P-value = 0.028, CI = 1.18-20.33 |
Bold signifies p<0.05
Changes in MRI findings associated with development of incident pain
A change in an MRI finding at 48-months needed to be more frequent in case knees than in control knees to be considered associated with development of incident pain. The statistical models evaluating baseline MRI findings (models 4–10) are shown in Table 3.
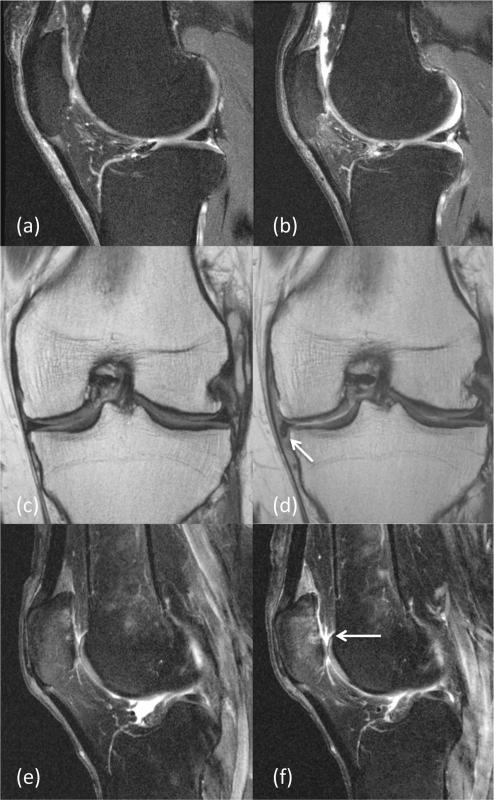
Models 4, 7, 8, and 10, which evaluated incident meniscal tears, progressive cartilage lesions, incident bone marrow lesions, and an incident effusion, respectively, differed significantly from the base model. A backward-stepwise method identified an incident medial meniscus tear (≥ grade 2), a progressive cartilage lesion of the patella (increase ≥1 grade), and an incident effusion to be significant (models 4, 7 and 10). Incident bone marrow lesions were not significant following backward-stepwise regression. Of all degenerative knee changes over 48 months, an incident effusion had the highest odds for the development of pain. An incident effusion was present in 25.0% of knees in the case cohort, compared with 3.3% of knees in the control cohort (Table 4). This finding was associated with development of incident pain with an OR of 9.78 using the likelihood ratio test (P = 0.005, CI = 1.99–48.08). Figure 2 shows an example of an incident effusion, which was associated with the development of incident pain. In addition, a progressive cartilage lesion of the patella was present in 26.7% of knees in the case cohort, compared with 8.3% of knees in the control cohort (Table 4; Fig. 2). This finding also was associated with development of incident pain, with an OR of 4.59 using the likelihood ratio test (P = 0.009, CI= 1.46–14.43). Finally, an incident medial meniscus tear was present in 20.0% of knees in the case cohort, compared with 5.0% of knees in the control cohort (Table 4; Fig. 2). This finding was associated with development of incident pain, with an OR of 4.91 using the likelihood ratio test (P = 0.028, CI = 1.18–20.33).
Fig. 2.
Sagittal intermediate-weighted fast spin echo images (TR 3200 ms, TE 30 ms) at baseline (a) and at 48 months (b) show an incident effusion. A finding was considered incident if it was absent at baseline, but present at 48 months. Coronal intermediate-weighted fast spin echo images (TR 3,700 ms, TE 29 ms) at baseline (c) and at 48 months (d) show an incident medial meniscal flap tear (arrow). The medial meniscus body WORMS score was 0 at baseline and 4 at 48 months. Sagittal intermediate-weighted fast spin echo images (TR 3200 ms, TE 30 ms) at baseline (e) and at 48 months (f) show a progressive patellar cartilage lesion. The patellar cartilage WORMS score was 3 (partial thickness cartilage loss) at baseline and was 5 at 48 months with incident full thickness defects (arrow)
In addition, 54.17% of cases vs. 17.5 % of controls had at least one of the significant abnormalities (baseline medial tibial cartilage lesion, incident medial meniscal tear, incident effusion, or progressive patellar cartilage lesion), which yielded an odds ratio of 7.31 (P < 0.0001, CI = 3.05–17.22).
Discussion
In this analysis of the OAI longitudinal data set, a medial tibial cartilage lesion at baseline, an incident effusion, a progressive cartilage lesion in the patella, and an incident medial meniscus tear were associated with incident pain in subjects enrolled in the OAI. Collectively, these results may implicate the origins of pain in knee OA, and also facilitate radiologists to identify clinically relevant knee MRI findings associated with pain and attributable to morphologic joint degeneration in OA in a clinical population.
The only baseline morphologic finding associated with the development of pain was the severity of cartilage lesions in the medial tibia. These results corroborate prior studies, which have reported an association between cartilage lesions and pain [20–22]. In addition, the results are consistent with prior knowledge that the medial compartment of the knee is the most load-bearing [23]. Furthermore, cartilage lesions in the medial tibia are signs of more substantial cartilage damage compared to other cartilage compartments, especially in early stages of disease. The cartilage of the medial tibia is relatively thin compared to other compartments such that one of these lesions may be more likely osteochondral involving the underlying subchondral bone. Osteochondral lesions are more indicative of joint disruption and potentially able to initiate symptomatic OA (regardless of whether the lesion remains unchanged or progresses).
The MRI finding most strongly associated with development of incident knee pain was an incident effusion. This result is consistent with prior literature, as multiple studies have correlated an effusion with pain [24–26], possibly due to capsular distention [26]. While joint effusion is not generally regarded as a cause of OA, it often develops during the course of disease. Of note, one study showed that change in synovitis, assessed by synovial thickness rather than change in an effusion was associated with pain [27]. However, the same study also demonstrated poor to moderate range of inter-reader reliability on the synovial assessment of the non-contrast study. It is possible that the incident effusions are more closely associated with acute inflammatory component and synovitis than progressive or unchanged effusions at 48 months.
Another central finding in this study was that progression of cartilage lesions in the patella was associated with the development of pain. An association between cartilage degeneration and pain has been demonstrated in previous studies [28, 29]; however, a direct link between cartilage degeneration and pain may be ambiguous to identify, since cartilage is aneural and avascular. Degenerative changes in the patellar cartilage in particular may be a by-product of its unique tissue properties such as shear load bearing as well as greater thickness than the other cartilage compartments. In addition, the progression of lesions in the patella may alter the surface geometry and trochlear tracking patterns, which may lead to degenerative changes such as patellar instability, chondromalacia, and consequently anterior knee pain [30], thus corroborating the results of the current study.
The results showed that an incident medial meniscus tear was associated with the development of incident knee pain, corroborating previous studies demonstrating that meniscus abnormalities are associated with pain [26, 31]. Since one of the primary functions of the meniscus is shock absorption and load bearing in the joint, the occurrence of a meniscal tear may compromise these functions, thus increasing the contract stresses in the knee [32]. Meniscal tears not only shift the mechanical stress distributions in the surrounding tissues including the cartilage, but also alter whole joint kinematics and suggest that it is possible that the disruption to knee joint mechanics may be associated with the development of symptoms.
An incident bone marrow edema pattern in the medial tibia demonstrated a trend toward being predictive of development of pain, but was not statistically significant (dropped out of the final stepwise regression model). Given that a cartilage lesion of the medial tibia was predictive of pain in our study, it would be reasonable for bone marrow edema pattern of the medial tibia also to be predictive. Our finding of a statistically insignificant trend is expected, as multiple studies have demonstrated a strong association [10, 11], while others have shown no association [33]. Further research is required, possibly including analysis of bone attrition, given that one prior study suggested that bone attrition is required for bone marrow edema pattern to contribute to pain severity [26].
One limitation of our study is a focus on development of incident pain without stratification of severity of pain. While analysis of pain severity is compelling, we chose to focus our study on incident knee pain in order to target early stages of symptomatic OA. Nonetheless, further investigation into which morphologic MR findings are associated with increased severity of pain is warranted. The small sample size was a limiting factor: with additional subjects, other possible knee joint parameters may have been significant. In addition, the low prevalence of abnormalities highlights that OA is a heterogeneous disease, and that there may be other possible contributing factors related to pain that imaging may not explain.
In conclusion, our study identified MRI findings, including baseline abnormalities of medial tibia cartilage, an incident effusion, progressive patella cartilage pathology, and incident medial meniscus tears, which were associated with development of incident knee pain in subjects with OA risk factors that volunteered in the OAI. The results of this study may help a radiologist and a referring clinician better understand the clinical significance of MR findings responsible for the development of pain, and may also aid to understand the evolution of symptomatic OA in a clinical population.
Footnotes
Compliance with ethical standards All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest The authors declare that they have no conflict of interest.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Lawrence R, Felson D, Helmick C, Arnold L, Choi H, Deyo R, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthrit Rheuma. 2007;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKenna MT, Michaud CM, Murray CJ, Marks JS. Assessing the burden of disease in the United States using disability-adjusted life years. Am J Prev Med. 2005;28:415–23. doi: 10.1016/j.amepre.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthrit Cartilage / OARS Osteoarthrit Res Soc. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Alizai H, Virayavanich W, Nardo L, Joseph GB, Nevitt MC, Lynch Ja, et al. Comparison of a novel quantitative assessment score with established semi-quantitative scoring systems for cartilage lesions in early osteoarthritis: data from the osteoarthritis initiative. Osteoarthrit Cartil. 2011;19:S164. [Google Scholar]
- 5.Lachance L, Sowers M, Jamadar D, Jannausch M, Hochberg M, Crutchfield M. The experience of pain and emergent osteoarthritis of the knee. Osteoarthritis Cartilage. 2001;9(6):527–32. doi: 10.1053/joca.2000.0429. [DOI] [PubMed] [Google Scholar]
- 6.Sowers MF, Hayes C, Jamadar D, Capul D, Lachance L, Jannausch M, et al. Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and X-ray-defined knee osteoarthritis. Osteoarthrit Cartilage / OARS Osteoarthrit Res Soc. 2003;11:387–93. doi: 10.1016/s1063-4584(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 7.Javaid MK, Kiran A, Guermazi A, Kwoh CK, Zaim S, Carbone L, et al. Individual magnetic resonance imaging and radiographic features of knee osteoarthritis in subjects with unilateral knee pain: the health, aging, and body composition study. Arthritis Rheum. 2012;64(10):3246–55. doi: 10.1002/art.34594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker K, Lamb SE, Toye F, Jackson S, Barrington S. Association between radiographic joint space narrowing, function, pain and muscle power in severe osteoarthritis of the knee. Clin Rehabil. 2004;18:793–800. doi: 10.1191/0269215504cr754oa. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 10.Felson D, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthrit Rheuma. 2007;56:2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf E, Kortekaas M, Watt I, Huizinga T, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis. 2011;70:60–7. doi: 10.1136/ard.2010.131904. [DOI] [PubMed] [Google Scholar]
- 12.Peterfy C, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellgren J, Lawrence J. Radiologic assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27(11):2635–41. [PubMed] [Google Scholar]
- 15.Baum T, Joseph G, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the osteoarthritis initiative. Arthritis Care Res. 2012;64:248–55. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stehling C, Lane N, Nevitt M, Lynch J, Mcculloch C, Link T. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18:776–86. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl R, Jain S, Lutz J, Wyman B, Graverand-Gastineau M, Vignon E, et al. Osteoarthritis of the knee at 3.0 T: comparison of a quantitative and a semi-quantitative score for the assessment of the extent of cartilage lesion and bone marrow edema pattern in a 24-month longitudinal study. Skeletal Radiol. 2011;40:1315–27. doi: 10.1007/s00256-011-1156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stehling C, Liebl H, Krug R, Lane NE, Nevitt M, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254:509–20. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laberge M, Baum T, Virayavanich W, Nardo L, Nevitt M, Lynch J, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects: data from the osteoarthritis initiative. Skeletal Radiol. 2012;41:633–41. doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckstein F, Cotofana S, Wirth W, Nevitt M, John M, Dreher D, et al. Greater rates of cartilage loss in painful knees than in pain-free knees after adjustment for radiographic disease stage: data from the osteoarthritis initiative. Arthrit Rheuma. 2011;63:2257–67. doi: 10.1002/art.30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Link T, Steinbach L, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226:373–81. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 22.Sharma L, Chmiel JS, Almagor O, Dunlop D, Guermazi A, Bathon JM, et al. Significance of preradiographic magnetic resonance imaging lesions in persons at increased risk of knee osteoarthritis. Arthrit Rheumatol. 2014;66(7):1811–9. doi: 10.1002/art.38611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson F. The distribution of load across the knee: a comparison of static and dynamic measurements. J Bone Joint Surg. 1980;62-B:346–9. doi: 10.1302/0301-620X.62B3.7410467. [DOI] [PubMed] [Google Scholar]
- 24.Kornaat P, Bloem J, Ceulemans RY, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239:811–7. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 25.Hayes CW, Jamadar DA, Welch GW, Jannausch ML, Lachance LL, Capul DC, et al. Osteoarthritis of the knee: comparison of MR imaging findings with radiographic severity measurements and pain in middle-aged women. Radiology. 2005;237:998–1007. doi: 10.1148/radiol.2373041989. [DOI] [PubMed] [Google Scholar]
- 26.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthrit Cartilage / OARS Osteoarthrit Res Soc. 2006;14:1033–40. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Hill C, Hunter D, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter DJ, March L, Sambrook PN. The association of cartilage volume with knee pain. Osteoarthritis Cartilage. 2003;11(10):725–9. doi: 10.1016/s1063-4584(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 29.Wluka AE, Wolfe R, Stuckey S, Cicuttini FM. How does tibial cartilage volume relate to symptoms in subjects with knee osteoarthritis? Ann Rheum Dis. 2004;63(3):264–8. doi: 10.1136/ard/2003.007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali SA, Helmer R, Terk MR. Analysis of the patellofemoral region on MRI: association of abnormal trochlear morphology with severe cartilage defects. AJR Am J Roentgenol. 2010;194(3):721–7. doi: 10.2214/AJR.09.3008. [DOI] [PubMed] [Google Scholar]
- 31.Sowers M, Karvonen-Gutierrez C, Jacobson J, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg. 2011;93:241–51. doi: 10.2106/JBJS.I.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee: a preliminary report. Am J Sports Med. 1986;14(4):270–5. doi: 10.1177/036354658601400405. [DOI] [PubMed] [Google Scholar]
- 33.Kornaat P, Kloppenburg M, Sharma R, Botha-Scheepers S, Graverand M, Coene L, et al. Bone marrow edema-like lesions change in volume in the majority of patients with osteoarthritis: associations with clinical features. Eur Radiol. 2007;17:3073–8. doi: 10.1007/s00330-007-0711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]