Abstract
The marine natural product (−)-8,15-diisocyano-11(20)-amphilectene (1), isolated from the Caribbean sponge Svenzea flava, was used as scaffold to synthetize five new products, all of which were tested against laboratory strains of Plasmodium falciparum and Mycobacterium tuberculosis H37Rv. The scaffold contains two isocyanide units that are amenable to chemical manipulation, enabling them to be elaborated into a small library of sulfur and selenium compounds. Although most of the analogs prepared were less potent than the parent compound, 5 was nearly equipotent showing IC50 values of 0.0066 µM and 0.0025 µM, respectively, against two strains (Dd2 and 3D7) of the malaria parasite. On the other hand, when assayed against the tuberculosis bacterium, analogs 5 and 6 were found to be more potent than 1.
Keywords: Malaria, Tuberculosis, Isothiocyanate, Isoselenocyanate, Isocyanide, Synthesis of natural product derivatives
Tuberculosis and Malaria are two of the world’s deadliest diseases, with more than two million deaths worldwide in 2013, most of them in sub-Saharan Africa, South-East Asia and Western Pacific regions.1 Plasmodium falciparum has for some time been developing resistance against known antimalarial drugs, and therefore new drugs are urgently needed.2 Chloroquine was the first drug produced on a large scale for treatment and prevention of malaria infection. Chloroquine has activity against the blood stages of Plasmodium ovale, P. malariae, and susceptible strains of P. vivax and P. falciparum.3 Widespread resistance in most malariaendemic countries has led to a continual decline in its use for the treatment of P. falciparum, although it remains effective for treatment of P. ovale, P. malariae, and, in most regions, P. vivax.4
Tuberculosis (TB) is second only to HIV/AIDS as the greatest killer worldwide due to a single infectious agent, Mycobacterium tuberculosis (Mtb).5 Standard antimycobacterial drugs (isoniazid, rifampicin, pyrazidamide, ethambutol, streptomycin) have been used for decades, and resistance to the medicines is also widespread. If a patient is unable to tolerate isoniazid, or if isoniazid-resistant TB is present, rifampicin, ethambutol, and pyrazidamide are usually used for 18 months. If rifampicin-resistant TB is present, the regimen usually consists of isonizaid, ethambutol, and pyrazidamide for 18 months. If there is resistance to both isoniazid and rifampicin, the disease is very difficult to treat.6 Disease strains that are resistant to a single anti-TB drug have been documented in every country surveyed. In some cases more severe drug resistance can develop. Extensively drug-resistant TB, XDR-TB, is a form of multi-drug resistant tuberculosis (MDR-TB) that responds to even fewer available medicines, including the most effective second-line anti-TB drugs. About 480,000 people developed MDR-TB in the world in 2013. More than half of these cases were in India, China and the Russian Federation. It is estimated that about 9.6% of MDR-TB cases had XDR-TB.5,7 Hence, the search for new antitubercular drugs is a priority so as to overcome the problem of drug resistance and to finally eradicate TB.
The marine sponge metabolite (−)-8,15-diisocyano-11(20)-amphilectene (1) was first reported by Faulkner et al. from Hymeniacidon amphilecta in 1978, and has been shown subsequently to exhibit potent in vitro anti-infective activity.8,9 Several structurally related natural products as well as a small number of synthetic analogs prepared from diisocyanide 1 also exhibit antimalarial and antimycobacterial potential.10 Whilst comparison among their activities reveals that the biological activity is generally dependent on the presence of the isocyanide functionality, the structural features of the carbon backbone and the location of the isocyanide groups also seem to play a pivotal role.11 Notwithstanding, the observation that a plethora of sponge-derived isocyanide-, isothiocyanate-, isocyanate-, and formamide-containing diterpenoids based on amphilectane, cycloamphilectane, isocycloamphilectane, and isoneoamphilectane skeletons are often active (usually in the low nanomolar range), suggests that the biological activity does not depend strictly on the presence of the isocyanide functionality.12 This observation implies that the metabolite’s carbon skeleton can also modulate biological activity.
As part of our continued drug discovery programme in search of new agents for the treatment of Malaria and Tuberculosis, we became interested in the synthesis of a limited number of amphilectane-based isothiocyanate and isoselenocyanate diterpenes for biological evaluation. Of the two classes of congeneric compounds, organic isoselenocyanates are of particular interest to us since so far they have received much less attention compared to their sulfur and oxygen analogs.13 We targeted diisocyanide 1 as a suitable starting material, a well-known antimalarial and antimycobacterial pharmacophore accessible to us which contains both a rigid amphilectane skeleton and two isocyanide “handles” with potential for further synthetic elaboration.8 We anticipated that comparison among the biological activities exhibited by the strickly related amphilectane analogs with those of 1 would reveal definite structure-activity relationships. While the isothiocyanate moiety is found in many natural products only two isothiocyanate-containing amphilectane diterpenoids with antiplasmodial activity have been documented.12a Remarkably, no studies assessing the potential antiplasmodial or antimycobacterial properties of isoselenocyanate-containing compounds (synthetic or natural) have been reported so far.14 In the present work, the syntheses of analogs 2–6 were swiftly accomplished through the isothio- and isoselenocyanation of metabolite 1, previously isolated by us from the marine sponge Svenzea flava.9 All compounds were characterized by detailed inspection of 1H NMR, 13C NMR, DEPT-NMR, 2D NMR (COSY, HSQC, HMBC, and NOESY), mass spectrometry, and UV and IR spectra. The purity of these compounds was ascertained by TLC, HPLC and spectroscopic analysis. All of the semi-synthetic derivatives exhibited strong to potent in vitro inhibition of Plasmodium falciparum Dd2 and 3D7 strains with some exhibiting greater antiplasmodial activity than the standard drug chloroquine. Likewise, the new compounds have shown sub-micromolar to low micromolar in vitro antimycobacterial activity. In order to assess their microbe-specific selectivity (i.e. whether the observed antimicrobial activity was a specific or general toxic effect) the cytotoxic effects of compounds 1–6 using a mammalian Vero cell line were also investigated. The results obtained are further evidence of the anti-infective potential of these novel amphilectane-based chemotypes.
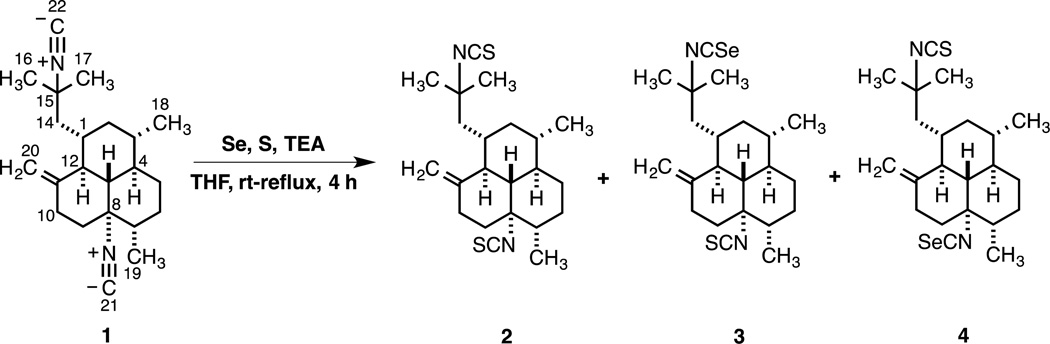
Since aliphatic isocyanides hardly react with elemental sulfur,15 the desired diisothiocyanate 2 was synthesized via the isothiocyanation of 1 as outlined in Scheme 1. Thus, treatment of diisocyanide 1 with S, Et3N, and catalytic amounts of Se in refluxing THF following a synthetic protocol previously described by Fujiwara and co-workers, afforded 8,15- diisothiocyano-11(20)-amphilectene (2) in 18% yield.16,17 Surprisingly, the desired product was accompanied by large amounts of unreacted 1 along with smaller quantities of congeners 3 and 4 (53%), formed as a 2:3 mixture of regioisomers that was inseparable by chromatography (the integration of selected signals in the 1H NMR spectra of the reaction products provided the isomer ratio). Addition of 2.5 mol% of S or increasing the refluxing time up to 16 h failed to afford full conversion to 2 or to preclude the formation of 3 and 4. These results suggest that in this case the reaction might exhibit a low catalytic activity of Se (i.e. the rate determining step appears to be the reaction between 1 and elemental Se and not the Se–S exchange) and that perhaps the amount of Se catalyst to isocyanide should be increased to >10 mol% (vide infra).18 Even though the reaction was very sluggish, we were delighted to have these compounds at hand since their biological evaluation was at this point of outmost interest to us. As the only differences between 3 an 4 were a result of the –NCS and –NCSe functionalities switching positions, these isomers have nearly identical 13C NMR shifts, apart from those at C-8 and C-15 (and their substituents). Nevertheless, we were able to distinguish the terpene isothiocyanate groups from its isoselenocyanate counterparts in 3 (minor) and 4 (major) by the 13C chemical shift of the –NCS (129–132 ppm) vs –NCSe (121–125) group. Although these signals are typically of low intensity in the 13C NMR spectra (during 1D spectroscopic acquisition an extended delay time (>5 s) and a 90° pulse angle are usually required to enhance their intensity) their detection was easily accomplished with a 700 MHz spectrometer. These noticeable differences in 13C NMR spectroscopic data, in combination with 2D NMR experiments (HSQC and HMBC spectra), allowed us to assign the structure of each isomer unambiguously.
Scheme 1.
Synthesis of Isothiocyanate Analogs 2–4
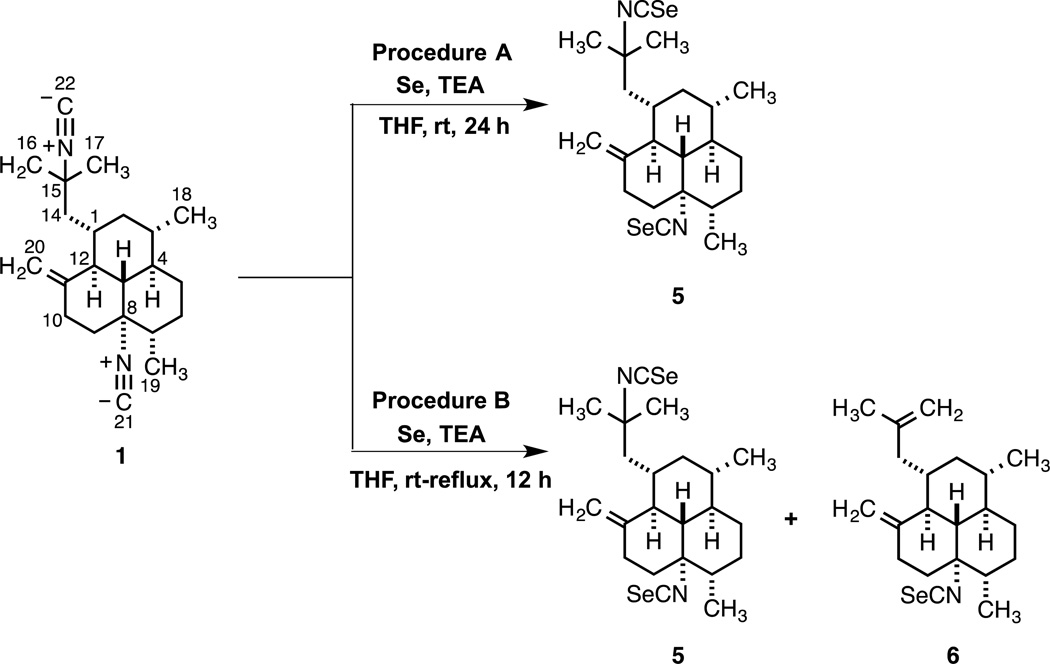
Concomitant with these efforts, we sought to achieve the isoselenocyanation of diisocyanide 1 with elemental selenium in the presence of TEA to give 8,15-diisoselenocyano-11(20)-amphilectene (5) in satisfactory yield.19 The synthesis and biological evaluation of 5 was very appealing to us since natural products bearing the isoselenocyanate moiety have never been isolated.20 Furthermore, synthetic isoselenocyanate-containing compounds apparently have never been investigated for potential antiplasmodial or antimycobacterial activity.21 Thus, insertion of two selenium atom equivalents at C-21 and C-22 of diisocyanide 1 via an isoselenocyanation reaction with Se using TEA in THF at 25 °C led cleanly to 5 (78% yield). Gratifyingly, when the reaction was conducted in refluxing THF diisoselenocyanate 5 (obtained in 50% yield) was accompanied by lesser quantities of isoselenocyanate 6 as a single regioisomer following purification by flash- and HPLC chromatography. In this fashion, the reaction proceeded with selective base-mediated decomposition of 5 at the more reactive C-15 isoselenocyanate group to give 6, albeit in modest yield (33% yield). We welcome the formation of 6 as it provided an opportunity to scrutinize its potential anti-infective properties.22 The results of these Se insertion reactions are portrayed in Scheme 2.
Scheme 2.
Synthesis of Isoselenocyanate Analogs 5 and 6
As already mentioned above, transformation of 1 to 8,15-diisothiocyano-11(20)-amphilectene (2) via Se-catalyzed isothiocyanation was characterized by poor yields of the expected product (≤18%) and the recovery of starting material. In principle, a more efficient one-pot pathway could be based on diisoselenocyanation of 1 to 5 with Se (Scheme 2, Method A) followed by facile Se–S exchange in the presence of TEA to give 2 along lines demonstrated in Scheme 1. Indeed, the TEA-mediated Se-S exchange of 5 proceeds efficiently in THF upon refluxing for 4 h, and affords the expected diisothiocyanide 2 (71% yield). The results show that the Se-S exchange for 5 is considerably faster than the rate of C-Se bond insertion at the C-8 and C-15 isocyanides of 1.23
With characterization data provided for all of the natural product hybrids, the synthesized compounds were evaluated in an in vitro growth inhibition assay against two P. falciparum Dd2 (drug resistant) and 3D7 (chloroquine-sensitive) malaria parasite lines, using the antimalarial drug chloroquine as reference standard. Concomitantly, compounds 1–6 were assayed against a laboratory strain of Mtb H37Rv, using the antimycobacterial drug rifampicin as the control in the determination of the MIC value of each compound (Table 1). Active compounds were then assessed for potential cytotoxicity to human cells through the use of cultured Vero cells (Table 2). The values for cytotoxicity (IC50) are calculated and compared to the IC50 of antiparasitic activity and MIC of antimycobacterial activity values through calculation of a Selectivity Index (SI) for each compound through the following formulae: SI = IC50/IC50 of antiparasitic activity and SI = IC50/MIC of antimycobacterial activity (shown in the far right columns of Table 2). A higher value indicates a higher degree of selectivity to P. falciparum and Mtb than to mammalian cells.
Table 1.
In vitro antiplasmodial and antimycobacterial activity of compounds 1–6
| Compound | IC50 ± SEM (µM) Dd2 |
IC50 ± SEM (µM) 3D7 |
MABA MIC (µM)a |
|---|---|---|---|
| 1 | 0.0031 ± 0.0001 | 0.0012 ± 4.12E-05 | 9.8 |
| 2 | 11.5863 ± 0.4784 | 11.7669 ± 0.3711 | 99.1 |
| 3 and 4b | 0.1433 ± 0.0064 | 0.3084 ± 0.0175 | 26.8 |
| 5 | 0.0066 ± 0.0004 | 0.0025 ± 0.0002 | 3.9 |
| 6 | 0.1490 ± 0.0089 | 0.1885 ± 0.0155 | 2.1 |
| CQ | 0.0519 ± 0.0039 | 0.0109 ± 0.0009 | – |
| RMP | – | – | 0.09 |
Values are means of two experiments.
Tested as a 2:3 mixture of regioisomers.
CQ = chloroquine and RMP = rifampicin (+Ctrls).
Table 2.
Comparison of selectivity indexes of compounds 1–6 with CQ and RMP
| Compound | IC50 Vero cell µM | SIa | SIb | SIc |
|---|---|---|---|---|
| 1 | 99.74 | 32174 | 83117 | 10.2 |
| 2 | >100 | >9 | >8 | >1.0 |
| 3 and 4d | 78.14 | 545 | 253 | 2.9 |
| 5 | 48.55 | 7356 | 19420 | 12.4 |
| 6 | 95.22 | 639 | 505 | 45.3 |
| CQ | 234.47e | 4518 | 21511 | – |
| RMP | >100 | – | – | >1100 |
Selectivity index (SI) defined by the ratio: IC50 (in mammalian Vero cell lines)/IC50 of antiparasitic activity against Dd2 (CQ-resistant strain) cell line.
Selectivity index (SI) defined by the ratio: IC50 (in mammalian Vero cell lines)/IC50 of antiparasitic activity against 3D7 (CQ-sensitive strain) cell line.
Selectivity index (SI) defined by the ratio: IC50 (in mammalian Vero cell lines)/MIC of antimycobacterial activity against M. tuberculosis H37Rv cell line.
Tested as a 2:3 mixture of regioisomers.
Value obtained from Ref. 24.
CQ = chloroquine and RMP = rifampicin (+Ctrls).
Except for the diisothiocyanate-functionalized amphilectane diterpene 2, all of the isoselenocyanate hybrids (3–6) showed sub-micro molar in vitro antiplasmodial activity (0.0025–0.3084 µM) against the two malaria parasite lines screened. Among these hybrids, only compound 5 having two isoselenocyanate functionalities showed more activity with Dd2 IC50 = 0.0066 and 3D7 IC50 = 0.0025 µM when compared to the standard drug chloroquine (Dd2 IC50 = 0.0519 µM, 3D7 IC50 = 0.0109 µM). Remarkably, hybrid 5 showed less toxicity (SI = 7356) than chloroquine (SI = 4518) against the drug resistant P. falciparum Dd2 strain. In the end, however, starting scaffold 1 with two isocyanide groups proved to be the most promising compound of the series (Dd2 IC50 = 0.0031 µM, 3D7 IC50 = 0.0012 µM), which was manifold times more active and less toxic than the standard drug (Tables 1 and 2). Interestingly, previous work by König et. al. has demonstrated that as for inhibition against P. falciparum the exchange of the isocyanide against the isocyanate group always results in a more significant drop in potency when compared to the –NC ⇒ –NCS exchange.12a On the other hand, our data suggest that switching the isocyanide for the isoselenocyanate functionality leads to no significant loss in antiparasitic activity.25 Altogether, the most notable results obtained from this limited series of compounds are those for 3–6. To our knowledge, this is the first report of isoselenocyanate-functionalized inhibitors of P. falciparum.
When screened for in vitro activity against Mtb H37Rv in a microbroth dilution assay, the best (lowest) MIC values of 3.9 and 2.1 µM, respectively, were determined for isoselenocyanate-functionalized hybrids 5 and 6. On the other hand, hybrids with an isothiocyanate moiety (2–4) had the worst (highest) MIC’s (26.8–99.1 µM) (Table 1). Interestingly, amphilectane-based diterpene 6 with a single isoselenocyanate moiety was identified as both the most potent (MIC = 2.1 µM) and the least toxic (the highest SI value of 45.3 was determined for 6) of the series (Table 2). Given its good MIC and SI, analog 6 is a potential candidate for efficacy studies in mice, and should future collaborations demonstrate that this isoselenocyanate-functionalized amphilectane diterpene has good pharmacokinetic properties, it could become a new anti-TB drug.
When compared to 1, the observation that the two most promising selenium containing compounds of the series, 5 and 6, could be more toxic raises the question as to whether this new class of anti-infective agents should be considered an avenue for further development. Despite the high toxicity of many selenium compounds, organic derivatives of selenium have been previously synthesized for medical applications.13a As a result, selenium-containing compounds are of increasing interest because of their chemical properties and biological activities.26 While based on a very limited library of hybrid compounds, this investigation demonstrates for the first time that isoselenocyanate-functionalized amphilectane diterpenes could become important antimalarial and anti-TB pharmacophores.
Supplementary Material
Acknowledgments
We thank E. Avilés and J. Marrero heartily for the initial isolation and characterization of (−)-8,15-diisocyano-11(20)-amphilectene (1). Mass spectral determinations were provided by the Mass Spectrometry Laboratory of the University of Illinois at Urbana–Champaign. We are grateful to Y. Wang and B. Wan (ITR-UIC) for their excellent technical assistance during the anti-TB and cytotoxicity bioassays. Financial support to K. Nieves was provided by the UPR-RISE Fellowship Program (2R25GM061151-09). This research was supported by the NIH Grant 1SC1GM086271-01A1 awarded to A. D. Rodríguez.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data (experimental details for the synthesis, molecular structure characterization, biological evaluation, and copies of the 1H NMR, 13C NMR and HR-EIMS spectra of new compounds 2–6) associated with this article can be found, in the online version, at http://dx.doi.org/.......
References and notes
- 1.WHO report. 2013 Available at http://www.who.int/whr/en>.
- 2.WHO. WHO Malaria Report. 2013
- 3.Baird JK. Trends Parasitol. 2007;23:533. doi: 10.1016/j.pt.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 4.(a) Builders MI. Int. J. Pharm. 2013;3:40. [Google Scholar]; (b) Biamonte MA, Wanner J, Le Roch KG. Bioorg. Med. Chem. Lett. 2013;23:2829. doi: 10.1016/j.bmcl.2013.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. WHO Global Tuberculosis Report. 2014 Available at http://www.who.int/tb/publications/global_report/en/>.
- 6.(a) Nguta JM, Appiah-Opong R, Nyarko AK, Yeboah-Manu D, Addo PGA. Int. J. Mycobacteriol. 2015;4:165. doi: 10.1016/j.ijmyco.2015.05.004. [DOI] [PubMed] [Google Scholar]; (b) D’Ambrosio L, Centis R, Sotgiu G, Pontali E, Spanevello A, Migliori GB. ERJ Open Res. 2015;1 doi: 10.1183/23120541.00010-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Dartois V, Barry CE., 3rd Bioorg. Med. Chem. Lett. 2013;23:4741. doi: 10.1016/j.bmcl.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Green KD, Garneau-Tsodikova S. Front Microbiol. 2013;4:208. doi: 10.3389/fmicb.2013.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Wratten SJ, Faulkner DJ, Hirotsu K, Clardy J. Tetrahedron Lett. 1978:4345. [Google Scholar]; (b) Ciavatta ML, Fontana A, Puliti R, Scognamiglio G, Cimino G. Tetrahedron. 1999;55:12629. [Google Scholar]; (c) Ciavatta ML, Gavagnin M, Manzo E, Puliti R, Mattia CA, Mazzarella L, Cimino G, Simpson JS, Garson MJ. Tetrahedron. 2005;61:8049. [Google Scholar]
- 9.Avilés E, Rodríguez AD. Org. Lett. 2010;12:5290. doi: 10.1021/ol102351z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Mayer AMS, Avilés E, Rodríguez AD. Bioorg. Med. Chem. 2012;20:279. doi: 10.1016/j.bmc.2011.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Avilés E, Prudhomme J, Le Roch KG, Rodríguez AD. Tetrahedron. 2015;71:487. doi: 10.1016/j.tet.2014.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Avilés E, Prudhomme J, Le Roch KG, Franzblau SG, Chandrasena K, Mayer AMS, Rodríguez AD. Bioorg. Med. Chem. Lett. 2015;25:5339. doi: 10.1016/j.bmcl.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattorusso E, Taglialatela-Scafati O. Mar. Drugs. 2009;7:130. doi: 10.3390/md7020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) König GM, Wright AD, Angerhofer CK. J. Org. Chem. 1996;61:3259. [Google Scholar]; (b) Wright AD, Wang H, Gurrath M, König GM, Kocak G, Neumann G, Loria P, Foley M, Tilley L. J. Med. Chem. 2001;44:873. doi: 10.1021/jm0010724. [DOI] [PubMed] [Google Scholar]; (c) Wright AD, McCluskey A, Robertson MJ, MacGregor KA, Gordon CP, Guenther J. Org. Biomol. Chem. 2011;9:400. doi: 10.1039/c0ob00326c. [DOI] [PubMed] [Google Scholar]; (d) Avilés E, Rodríguez AD, Vicente J. J. Org. Chem. 2013;78:11294. doi: 10.1021/jo401846m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The growing interest in isoselenocyanates has been inspired by the potential application of organoselenium compounds in organic synthesis as well as new knowledge of the role of selenium in biochemical processes.
- 14.(a) Parnham MJ, Graf E. Prog. Drug Res. 1991;36:9. doi: 10.1007/978-3-0348-7136-5_1. [DOI] [PubMed] [Google Scholar]; (b) May SW. Expert Opin. Investig. Drugs. 2002;11:1261. doi: 10.1517/13543784.11.9.1261. [DOI] [PubMed] [Google Scholar]; (c) Block E, Bird S, Tyson JF, Uden PC, Zhang X, Denoyer E. Phosphorus Sulphur Silicon Relat. Elem. 1998;136:1. [Google Scholar]
- 15.(a) Boyer JH, Ramakrishnan VT. J. Org. Chem. 1972;37:1360. [Google Scholar]; (b) Tanaka S, Uemura S, Okano M. Bull. Chem. Soc. Jpn. 1977;50:2785. [Google Scholar]
- 16.Fujiwara S, Shin-Ike T, Sonoda N, Aoki M, Okada M, Miyoshi N, Kambe N. Tetrahedron Lett. 1991;32:3503. [Google Scholar]
- 17.Terpene isothiocyanates can be readily distinguished from their thiocyanate counterparts by the 13C chemical shift of the –NCS (126–132 ppm) vs –SCN (112–114 ppm) group; see: He H-Y, Faulkner DJ, Shumsky JS, Hong K, Clardy J. J. Org. Chem. 1989;54:2511. Pham AT, Ichiba T, Yoshida WY, Scheuer PJ, Uchida T, Tanaka J, Higa T. Tetrahedron Lett. 1991;32:4843. Fusetani N, Wolstenholme HJ, Shinoda K, Asai N, Matsunaga S, Onuki H, Hirota H. Tetrahedron Lett. 1992;33:6823.
- 18.Typically isothiocyanates are obtained from thermal decomposition of dithiocarbamates or dithiocarbamic acid salts which in turn can be prepared by mixing carbon disulfide or thiophosgene with amines; see Wong R, Dolman SJ. J. Org. Chem. 2007;72:3969. doi: 10.1021/jo070246n. and references therein Hodgkins JE, Ettlinger MG. J. Org. Chem. 1956;21:404. Hodgkins JE, Ettlinger MG. J. Org. Chem. 1964;29:3098. Itoh K, Lee IK, Matsuda I, Sakai S, Ishii Y. Tetrahedron Lett. 1967:2667. Shibanuma T, Shiono M, Mukaiyama T. Chem. Lett. 1977:573. Blotny G. Liebigs Ann. Chem. 1982:1927.
- 19.Sonoda N, Yamamoto G, Tsutsumi S. Bull. Chem. Soc. Jnp. 1972;45:2937. [Google Scholar]
- 20.Garud DR, Koketsu M, Ishihara H. Molecules. 2007;12:504. doi: 10.3390/12030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Interestingly the selenium-induced cytotoxicity of P. falciparum with sodium selenite has been investigated, see: Taguchi N, Hatabu T, Yamaguchi H, Suzuki M, Sato K, Kano S. Exp. Parasitol. 2004;106:50. doi: 10.1016/j.exppara.2004.01.005. Suradji EW, Hatabu T, Kobayashi K, Yamazaki C, Abdulah R, Nakazawa M, Nakajima-Shimada J, Koyama H. Parasitology. 2011;138:1852. doi: 10.1017/S0031182011001399.
- 22.Since an adequate supply of isoselenocyanate 6 for the proposed biological studies was at hand, a decision was made at this point not to pursue further optimization.
- 23.Conceivably this minor modification to the original procedure reported by Fujiwara and co-workers (Ref. 16) should yield isothiocyanates in higher yields and shorter reaction times.
- 24.Tyagi V, Khan S, Shivahare R, Srivastava K, Gupta S, Kidwai S, Srivastava K, Puri SK, Chauhan PMS. Bioorg. Med. Chem. Lett. 2013;23:291. doi: 10.1016/j.bmcl.2012.10.101. [DOI] [PubMed] [Google Scholar]
- 25.While a detailed investigation into the structure–activity relationships of active isoselenocyanate 3–6 is currently lacking a recent investigation into the antiplasmodial mechanism of action for marine isocyanides, isothiocyanates, and isocyanates has revealed that these metabolites inhibit heme crystallization thus inhibiting the growth of P. falciparum, see: Young RM, Adendorff MR, Wright AD, Davies-Coleman MT. Eur. J. Med. Chem. 2015;93:373. doi: 10.1016/j.ejmech.2015.02.011.
- 26.(a) Patrick L. Altern. Med. Rev. 2004;9:239. [PubMed] [Google Scholar]; (b) Sharma AK, Sharma A, Desai D, Madhunapantula SV, Huh SJ, Robertson GP, Amin S. J. Med. Chem. 2008;51:7820. doi: 10.1021/jm800993r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.