Abstract
Objectives
We sought to determine the benefit of secondary cytoreductive surgery (SCRS) in patients with low-grade serous ovarian or peritoneal carcinoma, and whether cytoreduction to no gross residual disease affects survival.
Methods
A single institution retrospective chart review was conducted in patients with recurrent low-grade serous carcinoma who underwent SCRS between 1995–2012. Data including demographics, survival, chemotherapy, disease characteristics at the time of surgery, residual disease, and operative complications were collected. Overall survival (OS) and progression-free survival (PFS) were calculated. Kaplan-Meier and log-rank tests were used to examine survival outcomes.
Results
Forty-one patients met inclusion criteria. The median time between primary tumor debulking and SCRS was 33.2 months. Of 41 eligible patients who underwent SCRS, 32 (78%) had gross residual disease at the completion of secondary surgery. The median PFS for patients with no gross residual disease after SCRS was 60.3 months, compared to 10.7 months for patients with gross residual disease (p=0.008). Median OS from diagnosis for patients with no gross residual disease after SCRS was 167.5 months compared to 88.9 months (p=0.10). Median OS from the time of SCRS for patients with no gross residual disease was 93.6 months compared to 45.8 months (p=0.04). Complications occurred in 61% of patients after SCRS; there were no deaths directly attributable to surgery.
Conclusion
Our results suggest a benefit to SCRS in patients with recurrent low-grade serous carcinoma. Efforts to maximally cytoreduce patients should be made as patients with no gross residual disease had a better PFS and a trend toward better OS.
Keywords: Low-grade serous ovarian cancer, secondary cytoreduction, optimal cytoreduction
Introduction
High-grade serous ovarian carcinomas comprise the majority of the estimated 22,000 new cases of ovarian cancer per year in the United States (1). Low-grade serous carcinoma constitutes a smaller (5–10%), yet significant proportion of serous carcinoma cases (2, 3). It is accepted that high-grade and low-grade serous carcinoma arise from molecularly discrete pathways and exhibit divergent clinical behavior (4–7). For example, while the overall five-year survival for patients with low-grade SOC is longer compared to high-grade serous carcinoma (8), low-grade serous carcinoma are relatively chemoresistant (9, 10). Emerging targeted therapies have shown promise in low-grade serous carcinoma (11); however, the role of secondary surgery remains unclear.
In the setting of recurrent high-grade serous carcinoma, most patients are offered chemotherapy or hormonal therapy, and a small subset of patients may benefit from secondary cytoreductive surgery (SCRS). Retrospective reviews suggest that secondary cytoreduction confers a survival advantage in a highly-selected group of patients with recurrent epithelial histology, particularly patients with platinum-sensitive disease with a single site of recurrence (12, 13). Ongoing prospective clinical trials such as GOG 213 are attempting to more clearly define the role of secondary cytoreduction (14). While some studies have included low-grade serous histology (15), none have specifically focused on secondary cytoreduction in this particular patient population. We therefore sought to determine the benefit of secondary cytoreduction in low-grade serous carcinoma, whether cytoreduction to no gross residual disease had an impact on progression-free and overall survival (PFS, OS), and whether certain patient characteristics could identify ideal candidates for SCRS.
Methods
After obtaining approval from the Institutional Review Board at the University of Texas MD Anderson Cancer Center, women with a diagnosis of low-grade serous carcinoma who underwent secondary cytoreduction for disease progression/recurrence between 1995–2011 were identified. Patients who met the following inclusion criteria were selected: 1) pathologically confirmed low-grade serous histology at the time of initial and secondary cytoreduction, 2) SCRS performed at MD Anderson Cancer Center, or at another institution if complete operative reports and follow-up notes were available; and 3) any stage disease by International Federation of Gynecology and Obstetrics (FIGO) criteria with documented recurrence. Seventy-seven women with low-grade serous ovarian or peritoneal carcinoma were identified as potentially having had SCRS. Of those 77 women, 36 were excluded from analysis due to the following reasons: 1) Patients did not have adequate information from medical records (n = 29), 2) Patients underwent second-look surgery and not a true secondary debulking (n = 3), 3) Patients developed progressive disease during primary therapy (n = 2), or 4) Patients had recurrent borderline tumors without a diagnosis of low-grade serous carcinoma (n = 2).
Exclusion criteria included patients who had undergone SCRS at an outside institution with inadequate information in their medical records, or non-low-grade serous carcinoma histology. Patients were included if they had undergone surgery for low malignant potential (LMP) tumors in the past, but both primary and secondary cytoreduction efforts had to demonstrate low-grade serous carcinoma histology. Second-look surgery was not counted as secondary cytoreduction. Interval tumor reductive surgery after initial neoadjuvant chemotherapy was also not considered secondary cytoreductive surgery.
Patient demographics and details of initial diagnosis, including stage and treatment with surgical resection and chemotherapy, were collected. Information was documented regarding relapse, progression-free survival (PFS), mode of treatment at the time of relapse (chemotherapy versus immediate surgery), serum CA-125 and CT findings at the time of relapse, presence of ascites or symptoms, and physical exam findings. At the time of secondary surgery, number of lesions, amount of residual disease, estimated blood loss (EBL), postoperative complications and length of hospital stay were recorded. Data regarding post-operative treatment, PFS after surgery, survival after surgery, and overall survival were also documented.
Outcomes of interest were overall survival (OS) and progression-free survival (PFS). OS was calculated from the date of diagnosis to the date of death or last known contact and from the date of SCRS to date of death or last known contact. PFS was calculated from the date of SCRS to the date of recurrence/progression or date of death (whichever occurred first). Cox proportional hazards regression was used to evaluate the impact of clinical variables on PFS. Variables with p<0.10 by univariate analysis were included in a multivariate model. The following variables were examined by univariate Cox regression to determine whether they were associated with PFS: presence of ascites at the time of progression/recurrence, CA 125 levels at the time of progression/recurrence, age at SCRS, platinum status at the time of progression/recurrence (resistant versus sensitive, using standard definition), treatment at the time of progression/recurrence (chemotherapy vs SCRS directly), residual disease upon completion of SCRS, and the number of tumor nodules noted at the time of SCRS (< 3 vs ≥ 3). Variables with p-values <0.10 were included in a multivariate analysis. Kaplan-Meier and log-rank tests were used to examine survival outcomes. Chi-square tests were used to examine differences between categorical variables. P-values of <0.05 were considered statistically significant.
Results
A total of 41 patients who met inclusion criteria comprised our study cohort. Table 1 displays patient characteristics. Median age at time of initial diagnosis was 41.3 years (range 21–74). Six patients (14.6%) were initially diagnosed with serous LMP tumors prior to their diagnosis of low-grade serous carcinoma; the remaining 35 (85.4%) had low-grade serous carcinoma at the time of initial diagnosis. Most patients were white (76%), and most patients (85.4%) had FIGO stage III or IV disease.
Table 1.
Patient characteristics (N=41)
| Variable | Median (Range) |
|---|---|
| Age at diagnosis (years) | 41.3 (21.0, 73.7) |
| n (%) | |
| Ethnicity | |
| White | 31 (75.6) |
| Black | 4 (9.8) |
| Hispanic | 3 (7.3) |
| Other | 3 (7.3) |
| FIGO stage at initial diagnosis | |
| I or II | 3 (7.3) |
| III or IV | 35 (85.4) |
| Unknown | 3 (7.3) |
| Disease status at end of primary cytoreductive surgery | |
| No gross residual | 7 (17.0) |
| Gross residual | 24 (58.5) |
| Unknown | 10 (24.4) |
| Adjuvant therapy | |
| Surveillance | 4 (9.8) |
| Chemotherapy | |
| Platinum + taxane | 26 (63.4) |
| Platinum, single agent | 5 (12.2) |
| Platinum + cyclophosphamide | 3 (7.3) |
| Platinum + taxane + bevacizumab | 1 (2.4) |
| Platinum + hormonal agent | 1 (2.4) |
| Hormonal therapy | |
| Letrozole | 1 (2.4) |
| Maintenance therapy | |
| Chemotherapy | |
| Platinum | 2 (4.9) |
| Hormonal therapy | |
| Letrozole | 4 (9.8) |
| Tamoxifen | 5 (12.2) |
| Leuprolide acetate | 1 (2.4) |
At the end of primary tumor reductive surgery, seven (17.1%) patients had no remaining gross evidence of cancer (hereafter noted as “residual disease”), 24 (58.5%) had gross residual disease, and 10 (24.4%) had unknown residual disease status. Following initial surgery, thirty-six patients (87.8%) received a median of 6 cycles of chemotherapy as adjuvant treatment at the time of primary diagnosis (range 3–15 cycles), the majority of which consisted of combination platinum and taxane regimens. Of the five patients who did not receive adjuvant chemotherapy, one patient received hormonal therapy, and four underwent surveillance. Eleven patients also received hormonal therapy for maintenance purposes. Details of primary adjuvant and maintenance therapies are included in Table 1. Upon completion of primary treatment (surgery with or without postoperative systemic therapy), 23 (56%) patients were considered without evidence of disease, 16 (39%) women had persistent disease, and two (4.9%) patients had unknown disease status.
The median time between primary cytoreductive surgery and SCRS was 33.2 months. Prior to SCRS, 16 (39%) women received systemic therapy for progressive/recurrent disease in an effort to reduce tumor burden, while 25 (61%) proceeded immediately to SCRS. Table 2 details systemic treatments immediately prior to and following SCRS. The median time from initial recurrence to SCRS was 2.4 months (range, 0.40–76.1). Twenty-seven patients had evidence of cancer on physical examination, including three with ascites, and two had chest x-ray evidence of metastases.
Table 2.
Chemotherapy and hormonal regimens for patients prior to and after undergoing secondary cytoreductive surgery (SCRS) (N=41)
| Regimen or agent given prior to SCRS | n (%) |
| Chemotherapy | |
| Paclitaxel | 4 (9.8) |
| Platinum/taxane | 4 (9.8) |
| Carboplatin1 | 2 (4.9) |
| Pegylated liposomal doxorubicin | 1 (2.4) |
| High dose chemotherapy with peripheral stem cell transplant | 1 (2.4) |
| Ifosfamide and etoposide | 1 (2.4) |
| Vinorelbine | 1 (2.4) |
| Hormonal treatment | |
| Letrozole | 1 (2.4) |
| Tamoxifen | 1 (2.4) |
| Regimen or agent given after SCRS | n (%) |
| Chemotherapy | |
| Platinum + taxane | 8 (19.5) |
| Platinum + pegylated lipopsomal doxorubicin | 2 (4.9) |
| Pegylated liposomal doxorubicin | 2 (4.9) |
| Taxane, single agent | 2 (4.9) |
| Topotecan | 2 (4.9) |
| Platinum + taxane + bevacizumab | 1 (2.4) |
| Platinum, single agent | 1 (2.4) |
| Ifosofamide + etoposide | 1 (2.4) |
| Hexamethylmelanamine | 1 (2.4) |
| Gemcitabine | 1 (2.4) |
| Chemotherapy, not otherwise specified | 1 (2.4) |
| Hormonal treatment | |
| Letrozole | 5 (12.2) |
| Tamoxifen | 4 (9.8) |
| Anastrozole | 1 (2.4) |
| Leuprolide acetate | 1 (2.4) |
One patient who received carboplatin also received concurrent tamoxifen.
Table 3 highlights information at the time of SCRS, including complications. At the completion of secondary cytoreductive surgery, 9 (22.0%) patients had no gross residual disease, and 32 (78.0%) were left with gross residual disease. Twenty-five patients (61%) experienced complications. After SCRS, 22 patients (53.7%) received a median number of 6 cycles of chemotherapy for recurrent disease (range, 2–19), and 11 patients received hormonal therapy. Eight patients received no initial postoperative therapy.
Table 3.
Characteristics of patients undergoing secondary cytoreductive surgery (SCRS) (N=41)
| Variable | n (%) |
|---|---|
| Initial treatment at time of first progression/recurrence | |
| Chemotherapy | 16 (39.0) |
| Surgery | 25 (61.0) |
| Platinum status at the time of SCRS | |
| Resistant | 17 (41.5) |
| Sensitive | 19 (46.3) |
| Did not receive any platinum-based chemotherapy | 5 (12.2) |
| Disease status at end of SCRS | |
| No gross residual | 9 (22.0) |
| Gross residual | 32(78.0) |
| Complications | |
| Hemorrhage requiring transfusion | 11 (26.8) |
| Pneumonia | 2 (4.9) |
| Abscess | 1 (2.4) |
| Anastomotic leak | 1 (2.4) |
| Bacteremia | 1 (2.4) |
| Cystotomy | 2 (4.9) |
| Enterotomy | 1 (2.4) |
| ICU admission | 2 (2.4) |
| Pancreatitis | 1 (2.4) |
| Urinary tract infection | 1 (2.4) |
| Wound infection | 1 (2.4) |
| Readmission for small bowel obstruction | 1 (2.4) |
The median overall survival (OS) from the date of diagnosis of low-grade serous carcinoma for the entire group was 102.0 months (95%CI, 74.0, 130.0). Median PFS after secondary cytoreduction for the entire study population was 15 months (95%CI, 3.7, 26.3). The exact dates of disease progression were not specified for three patients. Consequently, these patients were excluded from PFS calculations. The median OS from the date of SCRS was 64.1 (95%CI, 39.8, 88.5).
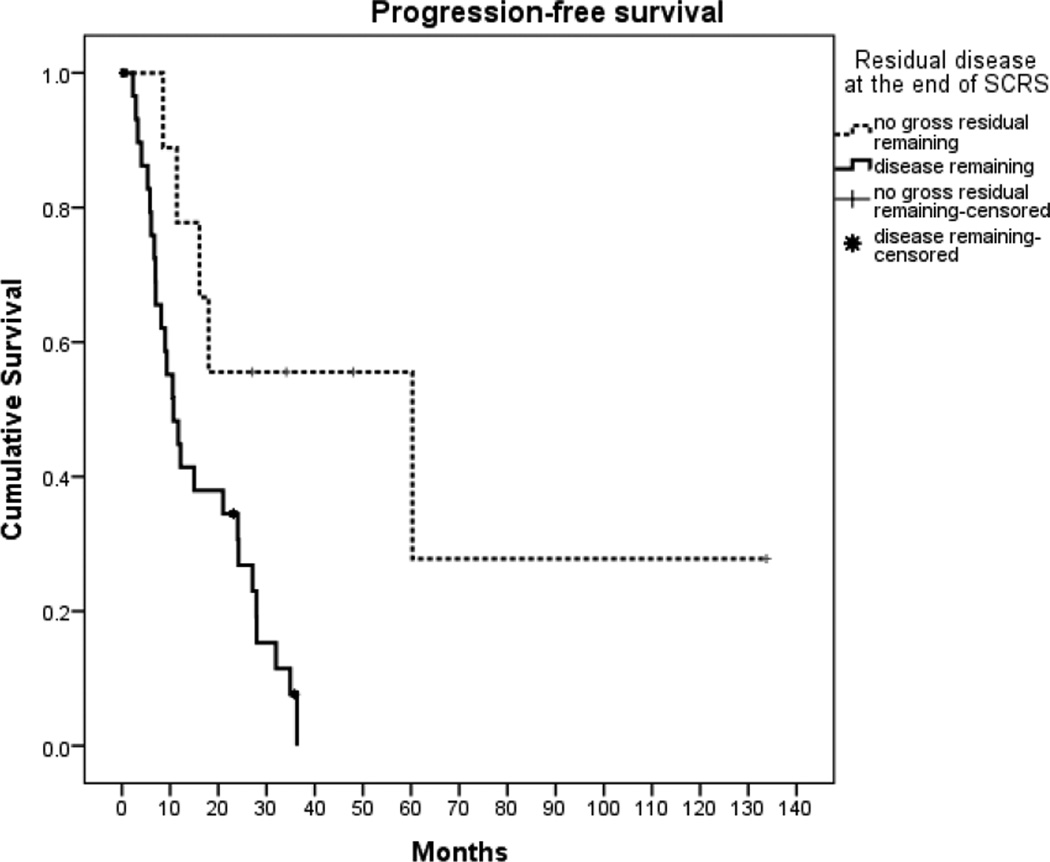
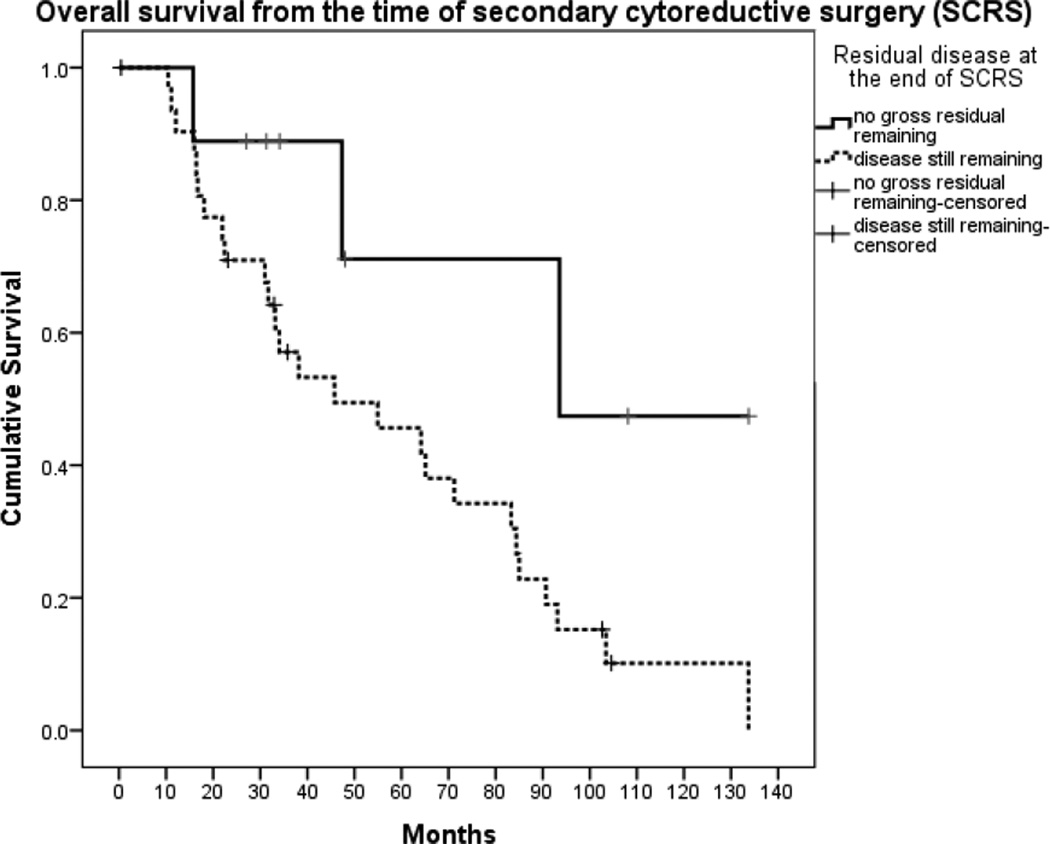
When analyzed according to residual disease at the completion of SCRS, women with no gross residual cancer at the conclusion of SCRS had better PFS of 60.3 months (95% CI, 0.0, 123.9) compared with just 10.7 months (95% CI, 6.5, 14.9) for patients with residual disease (p=0.008) (Figure 1). Women with no residual disease at the conclusion of SCRS also had a trend towards longer OS of 167.5 months (95% CI, 72.5, 262.5) from the date of initial diagnosis compared with 88.9 months (95% CI, 69.6, 108.2) for patients with residual disease (p=0.10), although this did not reach statistical significance. When calculated from date of SCRS, the trend remained; women without gross residual disease had a longer median OS of 93.6 months compared to 45.8 months in women who had residual disease after surgery (p=0.04) (Figure 2). Women who proceeded directly to SCRS at the time of progression/recurrence had a trend towards a longer median OS of 83.3 months (95% CI, 57.3, 109.3) compared to 33.2 months (95% CI, 70.0, 67.5) for women who initially received chemotherapy for progressive/recurrent disease before proceeding to SCRS, although this was not statistically significant (p=0.09).
Figure 1.
Progression-free survival from the time of secondary cytoreductive surgery (p=0.008)
Figure 2.
Overall survival from the time of secondary cytoreductive surgery (p=0.04)
Based on results of the univariate analysis, treatment strategy at the time of progression/recurrence, residual disease at completion of SCRS, and the number of tumor nodules met criteria for inclusion in the multivariate analysis for PFS. In multivariate Cox regression, the only variables that remained were whether patients received chemotherapy and then underwent SCRS or proceeded directly to SCRS, and the number of tumor nodules at the time of surgery (Table 4). Proceeding directly to SCRS resulted in a hazard ratio of 0.43 (95% CI, 0.20, 0.93); p=0.03. The presence of 3 or more tumor nodules at the time of surgery conferred a hazard ratio of 5.29 (95% CI, 0.71, 39.35); p=0.10).
Table 4.
Univariate and multivariate results for progression-free survival (N=41)
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variable | N | HR [95% CI] | p | HR; [95% CI] | p |
| Ascites1 | |||||
| No (reference) | 37 | - | |||
| Yes | 3 | 0.91 [0.28, 3.02] | 0.88 | ||
| Residual disease | |||||
| No gross residual (reference) | 9 | - | |||
| Gross residual | 32 | 3.83 [1.32, 11.19] | 0.01 | ||
| Treatment strategy at recurrence | |||||
| Systemic therapy (reference) | 16 | - | - | ||
| Secondary cytoreductive surgery | 25 | 0.36 [0.17, 0.77] | 0.009 | 0.43 [0.20, 0.93] | 0.03 |
| CA125 at time of progression/recurrence2 | |||||
| < median, (57 U/mL) (reference) | 18 | - | |||
| ≥ median, (57 U/mL) | 16 | 1.71 [0.77, 3.82] | 0.19 | ||
| Age at progression/recurrence | |||||
| < median, (45.6 years) (reference) | 20 | - | |||
| ≥ median, (45.6 years) | 21 | 0.58 [0.28, 1.22] | 0.16 | ||
| Number of tumor nodules at progression/ recurrence3 | |||||
| < 3 (reference) | 4 | - | - | ||
| ≥ 3 | 36 | 6.20 [0.84, 45.7] | 0.07 | 5.29 [0.71, 39.35] | 0.10 |
Ascites information missing for 1 patient
CA 125 values missing for 7 patients
Number of tumor nodules missing for 1 patient
Discussion
This study demonstrates that patients with low-grade serous carcinoma who underwent secondary cytoreductive surgery to no gross residual disease experienced a 50-month gain in PFS and a 47.8-month gain in OS from the time of SCRS compared to patients with gross residual disease.
Our findings mirror that of other studies examining SCRS in epithelial ovarian cancer, most of which are comprised of high-grade serous histology. Al Rawahi et al performed a meta-analysis on 1194 women who underwent SCRS and concluded that cytoreduction to no gross residual disease was associated with significantly improved survival (13). In 2009, Bristow et al examined 2019 patients undergoing secondary cytoreduction and found that the degree of cytoreduction correlated directly with survival (12). There was a 3-month improvement in survival for each 10% increase in the proportion of women undergoing cytoreduction to no residual tumor. In these cited studies, discrepancies existed in defining the definition of “optimal” cytoreduction. Additionally, those authors acknowledged that selection bias in the surgical cohort may have over-inflated the benefit of SCRS. Current prospective clinical trials including DESKTOPIII, GOG 213, and the Dutch SoCceR may mitigate bias and better define the true benefit of secondary cytoreduction in recurrent ovarian cancer.
Several reports have attempted to identify ideal candidates for SCRS. A pooled international analysis examined 1100 patients undergoing SCRS for epithelial ovarian cancer (15). This group also experienced longer OS of 57.5 months after SCRS with complete resection of tumor compared to 27 months for the group with 0.1–1.0 cm tumor and only 15.6 months in the group with > 1.0 cm residual tumor. They also found that patients with a longer progression-free interval, without ascites at the time of recurrence, and with localized (versus diffuse) disease had improved survival and were perhaps better surgical candidates. Similarly, the DESKTOP OVAR trial attempted to identify patients who might benefit from SCRS and found that performance status, absence of ascites, and initial FIGO stage were predictors of complete resection (16). Other smaller studies have reached similar conclusions. In general, ascites, number and size of implants, performance status, and progression free interval all determine resectability and survival (17–20). Frederick et al suggested that a preoperative serum CA-125 level less than 250 U/ml was predictive of SCRS to no residual disease (21), although the predictive role of serum CA-125 in surgical outcomes remains controversial. However, it is uncertain if these findings in patients with high-grade serous carcinoma are applicable to patients with low-grade serous carcinoma.
We attempted to identify predictors of resectability and survival. Initial evaluation of PFS suggested that the strongest predictor was the number of tumor nodules at the time of recurrence. Therefore, we assessed the correlation between number of nodules documented on preoperative CT at the time of recurrence and intraoperative findings. Interestingly, when the number of tumor nodules as determined by CT was included in the Cox regression analysis, this variable was less predictive (HR=1.49 (95% CI, 0.60, 3.69) p= 0.39) of PFS compared to number of tumor nodules determined from the operative report. Based on our data, there was an association between CT findings and intra-operative findings when there were three or fewer tumor nodules noted intraoperatively. However, findings on CT were not associated with presence of residual disease at the time of surgery (OR=4.0; 95% CI, (0.73–21.84); p= 0.10). Small overall and subgroup numbers limited our ability to draw solid conclusions about other preoperative factors such as serum CA-125 levels, or the presence or absence of ascites.
Generally, low-grade serous carcinoma has an indolent clinical course, and patients with this disease have a longer OS than high-grade serous carcinoma patients, but their tumors are relatively less chemosensitive (4, 9, 10). In fact, the role of surgery may be more important in the subgroup of patients with low-grade serous carcinoma. A few small retrospective studies have focused on SCRS in low-grade serous carcinoma (22, 23). Crispens and colleagues examined 49 patients with recurrent or progressive low-grade serous carcinoma and borderline tumors and found an association between optimal cytoreduction and overall survival (22). Another group reviewed their experience with 26 patients with recurrent micropapillary serous carcinoma, and found that patients who underwent optimal debulking had an OS of 61 months from the date of recurrence versus 25.5 months for patients who underwent suboptimal resection (23). While our patients experienced a longer overall survival from the time of recurrence (167.5 vs 88.9 months), the same trend was observed with regard to cytoreductive effort.
As low-grade serous histology is relatively rare in epithelial ovarian carcinoma, our study sample was inherently small and limited by the biases of retrospective medical record review. As a single-institution review, selection and referral biases could have also influenced outcomes. For example, patients who underwent SCRS directly after their diagnosis of progression/recurrence may have had a better performance status and fewer co-morbidities than those who first received chemotherapy and then SCRS. The wide array of systemic therapies administered both before and after SCRS also likely influence PFS and OS outcomes. Additionally, tumor biology could have affected surgical outcomes and response to chemotherapy, thereby confounding conclusions. It is notable that patients who had experienced an initial progression or recurrence after primary treatment were included in the analysis. Importantly, the tumor biology of low-grade serous carcinoma is distinctly different from that of high-grade serous carcinoma. Whereas patients with high-grade histology may have a 70–80% chance of being clinically disease-free following primary surgery and chemotherapy, patients with low-grade serous carcinoma are somewhat less likely to be clinically disease-free after primary treatment. In our study cohort, only 56% of patients were without evidence of disease after primary therapy. Similarly, in our previous report of 112 women with stage II–IV low-grade serous carcinoma of the ovary, only 52% were clinically disease-free at the completion of primary treatment (4). A subset of 42 patients underwent second-look surgery, and only two (5%) of these patients had microscopically negative findings (4). Thus, the reference to “progression” or “recurrence” is directly related to the disease status at completion of primary treatment and simply may reflect that true nature of low-grade serous carcinoma. Furthermore, the majority of patients with low-grade serous carcinoma who have persistent tumor at completion of primary therapy have actually responded to treatment and will only develop progressive disease at some future date. The relatively low rate of resection to no macroscopic residual disease in this patient cohort is notable. It is conceivable that the degree of desmoplasia, calcifications, and infiltrative nature of low-grade serous carcinoma could have accounted for this low respectability rate. In addition, the conduct of this study underscored the difficulty in accurately evaluating and documenting residual tumor. We chose to dichotomize patients in this study based on presence or absence of macroscopic residual disease at completion of SCRS rather than “optimal” versus “suboptimal” for two principal reasons: 1) the definition of “optimal” varied from < 1 cm to < 2 cm during the study period, and 2) adequate documentation on residual cancer was lacking in several instances.
In summary, secondary cytoreduction should be considered in select patients with low-grade serous carcinoma, as this particular histology is less responsive to chemotherapy and women with this histologic subtype may have fewer therapeutic alternatives. The major challenge remains the optimal selection criteria for this procedure. If SCRS is recommended, our results emphasize the importance of a maximal surgical cytoreductive effort, as patients with no residual disease enjoyed a longer progression-free and overall survival than those patients left with macroscopic residual disease.
Highlights.
In patients with recurrent low grade serous carcinoma, secondary cytoreduction to no gross residual disease was associated with a significantly longer progression-free survival and overall survival compared to outcomes of women with gross residual disease.
At the time of progression or recurrence, patients who proceeded directly to secondary cytoreductive surgery had a better progression-free survival compared to patients who initially received systemic therapy in an effort to reduce tumor burden prior to secondary cytoreductive surgery.
Secondary cytoreductive efforts should be considered in select patients with recurrent low grade serous carcinoma.
Acknowledgments
This work was supported in part by NCI-DHHS-NIH T32 Training Grant (T32 CA101642) (EKC) and by The Sara Brown Musselman Fund for Serous Ovarian Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors of this manuscript declare no conflicts of interest
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Plaxe SC. Epidemiology of low-grade serous ovarian cancer. Am J Obstet Gynecol. 2008;198(4):459 e1–458 e1. doi: 10.1016/j.ajog.2008.01.035. discussion e8-9. [DOI] [PubMed] [Google Scholar]
- 3.Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23(1):41–44. doi: 10.1097/01.pgp.0000101080.35393.16. [DOI] [PubMed] [Google Scholar]
- 4.Gershenson DM, Sun CC, Lu KH, Coleman RL, Sood AK, Malpica A, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108(2):361–368. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 5.Shvartsman HS, Sun CC, Bodurka DC, Mahajan V, Crispens M, Lu KH, et al. Comparison of the clinical behavior of newly diagnosed stages II-IV low-grade serous carcinoma of the ovary with that of serous ovarian tumors of low malignant potential that recur as low-grade serous carcinoma. Gynecol Oncol. 2007;105(3):625–629. doi: 10.1016/j.ygyno.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Singer G, Kurman RJ, Chang HW, Cho SK, Shih Ie M. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160(4):1223–1228. doi: 10.1016/s0002-9440(10)62549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gershenson DM. The life and times of low-grade serous carcinoma of the ovary. Am Soc Clin Oncol Educ Book. 2013;2013:195–199. doi: 10.14694/EdBook_AM.2013.33.e195. [DOI] [PubMed] [Google Scholar]
- 8.Fader AN, Java J, Ueda S, Bristow RE, Armstrong DK, Bookman MA, et al. Survival in women with grade 1 serous ovarian carcinoma. Obstet Gynecol. 2013;122(2 Pt 1):225–232. doi: 10.1097/AOG.0b013e31829ce7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gershenson DM, Sun CC, Bodurka D, Coleman RL, Lu KH, Sood AK, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol. 2009;114(1):48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Schmeler KM, Sun CC, Bodurka DC, Deavers MT, Malpica A, Coleman RL, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2008;108(3):510–514. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Farley J, Brady WE, Vathipadiekal V, Lankes HA, Coleman R, Morgan MA, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14(2):134–140. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol. 2009;112(1):265–274. doi: 10.1016/j.ygyno.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Al Rawahi T, Lopes AD, Bristow RE, Bryant A, Elattar A, Chattopadhyay S, et al. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev. 2013;2:CD008765. doi: 10.1002/14651858.CD008765.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NCT00565851. A Phase III Randomized Controlled Clinical Trial of Carboplatin and Paclitaxel (or Gemcitabine) Alone or in Combination With Bevacizumab (NSC #704865, IND #113912) Followed by Bevacizumab and Secondary Cytoreductive Surgery in Platinum-Sensitive, Recurrent Ovarian, Peritoneal Primary and Fallopian Tube Cancer. NCI-Supplied Agents: Bevacizumab (NSC #704865, IND #113912).
- 15.Zang RY, Harter P, Chi DS, Sehouli J, Jiang R, Trope CG, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105(7):890–896. doi: 10.1038/bjc.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harter P, du Bois A, Hahmann M, Hasenburg A, Burges A, Loibl S, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13(12):1702–1710. doi: 10.1245/s10434-006-9058-0. [DOI] [PubMed] [Google Scholar]
- 17.Tian WJ, Chi DS, Sehouli J, Trope CG, Jiang R, Ayhan A, et al. A risk model for secondary cytoreductive surgery in recurrent ovarian cancer: an evidence-based proposal for patient selection. Ann Surg Oncol. 2012;19(2):597–604. doi: 10.1245/s10434-011-1873-2. [DOI] [PubMed] [Google Scholar]
- 18.Park JY, Eom JM, Kim DY, Kim JH, Kim YM, Kim YT, et al. Secondary cytoreductive surgery in the management of platinum-sensitive recurrent epithelial ovarian cancer. J Surg Oncol. 2010;101(5):418–424. doi: 10.1002/jso.21470. [DOI] [PubMed] [Google Scholar]
- 19.Bae J, Lim MC, Choi JH, Song YJ, Lee KS, Kang S, et al. Prognostic factors of secondary cytoreductive surgery for patients with recurrent epithelial ovarian cancer. J Gynecol Oncol. 2009;20(2):101–106. doi: 10.3802/jgo.2009.20.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salani R, Santillan A, Zahurak ML, Giuntoli RL, 2nd, Gardner GJ, Armstrong DK, et al. Secondary cytoreductive surgery for localized, recurrent epithelial ovarian cancer: analysis of prognostic factors and survival outcome. Cancer. 2007;109(4):685–691. doi: 10.1002/cncr.22447. [DOI] [PubMed] [Google Scholar]
- 21.Frederick PJ, Ramirez PT, McQuinn L, Milam MR, Weber DM, Coleman RL, et al. Preoperative factors predicting survival after secondary cytoreduction for recurrent ovarian cancer. Int J Gynecol Cancer. 2011;21(5):831–836. doi: 10.1097/IGC.0b013e31821743f9. [DOI] [PubMed] [Google Scholar]
- 22.Crispens MA, Bodurka D, Deavers M, Lu K, Silva EG, Gershenson DM. Response and survival in patients with progressive or recurrent serous ovarian tumors of low malignant potential. Obstet Gynecol. 2002;99(1):3–10. doi: 10.1016/s0029-7844(01)01649-0. [DOI] [PubMed] [Google Scholar]
- 23.Bristow RE, Gossett DR, Shook DR, Zahurak ML, Tomacruz RS, Armstrong DK, et al. Recurrent micropapillary serous ovarian carcinoma. Cancer. 2002;95(4):791–800. doi: 10.1002/cncr.10789. [DOI] [PubMed] [Google Scholar]