Abstract
BACKGROUND
Atherosclerotic renal-artery stenosis is a common problem in the elderly. Despite two randomized trials that did not show a benefit of renal-artery stenting with respect to kidney function, the usefulness of stenting for the prevention of major adverse renal and cardiovascular events is uncertain.
METHODS
We randomly assigned 947 participants who had atherosclerotic renal-artery stenosis and either systolic hypertension while taking two or more antihypertensive drugs or chronic kidney disease to medical therapy plus renal-artery stenting or medical therapy alone. Participants were followed for the occurrence of adverse cardiovascular and renal events (a composite end point of death from cardiovascular or renal causes, myocar-dial infarction, stroke, hospitalization for congestive heart failure, progressive renal insufficiency, or the need for renal-replacement therapy).
RESULTS
Over a median follow-up period of 43 months (interquartile range, 31 to 55), the rate of the primary composite end point did not differ significantly between participants who underwent stenting in addition to receiving medical therapy and those who received medical therapy alone (35.1% and 35.8%, respectively; hazard ratio with stenting, 0.94; 95% confidence interval [CI], 0.76 to 1.17; P = 0.58). There were also no significant differences between the treatment groups in the rates of the individual components of the primary end point or in all-cause mortality. During follow-up, there was a consistent modest difference in systolic blood pressure favoring the stent group (−2.3 mm Hg; 95% CI, −4.4 to −0.2; P = 0.03).
CONCLUSIONS
Renal-artery stenting did not confer a significant benefit with respect to the prevention of clinical events when added to comprehensive, multifactorial medical therapy in people with atherosclerotic renal-artery stenosis and hypertension or chronic kidney disease. (Funded by the National Heart, Lung and Blood Institute and others; ClinicalTrials.gov number, NCT00081731.)
Renal-artery stenosis, which is present in 1 to 5% of people with hyper-tension,1,2 often occurs in combination with peripheral arterial or coronary artery disease.3,4 Results of community-based screening suggest that the prevalence among persons older than 65 years of age may be as high as 7%.5 Renal-artery stenosis may result in hypertension, ischemic nephropathy, and multiple long-term complications.6 Uncontrolled studies performed in the 1990s suggested that renal-artery angioplasty or stenting resulted in significant reductions in systolic blood pressure7,8 and in the stabilization of chronic kidney disease.9,10 Subsequently, there were rapid increases in the rate of renal-artery stenting among Medicare beneficiaries, with the annual number of procedures increasing 364% between 1996 and 2000.11 However, three randomized trials of renal-artery angioplasty failed to show a benefit with respect to blood pressure.12-14 Two subsequent randomized trials of stenting did not show a benefit with respect to kidney function.15,16 To our knowledge, no studies to date have been designed specifically to assess clinical outcomes.
Given the prevalence of atherosclerotic renal-artery stenosis, this condition is an important public health issue. If stenting prevents the progression of chronic kidney disease and lowers blood pressure, it has the potential to prevent serious health consequences, including adverse cardiovascular and renal events. In contrast, if stenting confers neither of these benefits, it is likely to incur substantial cost without a public health advantage. Therefore, we performed a randomized clinical trial to determine the effects of renal-artery stenting on the incidence of important cardiovascular and renal adverse events.17
METHODS
STUDY OVERSIGHT
The Cardiovascular Outcomes in Renal Athero-sclerotic Lesions (CORAL) study was a multi-center, open-label, randomized, controlled trial that compared medical therapy alone with medical therapy plus renal-artery stenting in patients with atherosclerotic renal-artery stenosis and elevated blood pressure, chronic kidney disease, or both. The methods have been described previously.17 The trial protocol was developed by the steering committee (see the Supplementary Appendix, available with the full text of this article at NEJM.org) and was approved by the institutional review board at each participating center. The members of the steering committee vouch for the accuracy and completeness of the data and analyses and for the fidelity of this report to the trial protocol, which is available at NEJM.org.
Funding was provided by the National Heart, Lung, and Blood Institute. Medications were donated by AstraZeneca and Pfizer. The short-tip Angioguard device was donated by Cordis, and supplemental financial support was provided by both Cordis and Pfizer. None of the funders had any role in the design of the trial protocol, in the collection, analysis or interpretation of the data, or in the decision to submit the manuscript for publication. The trial was conducted under the guidance of an independent data and safety monitoring board convened by the National Heart, Lung, and Blood Institute.
STUDY POPULATION
Before entry into the trial, all participating sites were required to qualify in a roll-in phase. Qualification involved approval of the expertise of the lead onsite interventionalist by the angiographic core laboratory. The details of this approval process are described in the Supplementary Appendix.
Trial enrollment began on May 16, 2005. All participating patients provided written informed consent. According to the original trial protocol, persons with severe renal-artery stenosis were eligible if they had hypertension with a systolic blood pressure of 155 mm Hg or higher while receiving two or more antihypertensive medications. Severe renal-artery stenosis was defined angiographically as stenosis of at least 80% but less than 100% of the diameter or stenosis of at least 60% but less than 80% of the diameter of an artery, with a systolic pressure gradient of at least 20 mm Hg. All angiograms were analyzed by the angiographic core laboratory at the University of Virginia with the use of a validated computerized quantitative vascular analysis program (Medis QVA 6.0).
A number of subsequent changes were made in the enrollment criteria during the course of the trial but before the trial concluded or the data were unblinded. The threshold of 155 mm Hg for defining systolic hypertension was no longer specified. Patients who did not have systolic hypertension but who had renal-artery stenosis could be enrolled if they had chronic kidney disease, which was defined as an estimated glomerular filtration rate (GFR) of less than 60 ml/min/1.73 m2 of body-surface area, as calculated with the use of the modified Modification of Diet in Renal Disease (MDRD) formula.18 Severe renal-artery stenosis could be identified with the use of duplex ultrasonography, magnetic resonance angiography, or computed tomographic angiography.
Exclusion criteria were renal-artery stenosis due to fibromuscular dysplasia, chronic kidney disease from a cause other than ischemic nephropathy or associated with a serum creatinine level higher than 4.0 mg per deciliter (354 μmol per liter), kidney length of less than 7 cm, and a lesion that could not be treated with the use of a single stent. Complete inclusion and exclusion criteria are listed in the Supplementary Appendix.
RANDOMIZATION AND INTERVENTIONS
We assigned participants, in a 1:1 ratio, to either medical therapy alone or stenting plus medical therapy. Randomization was performed by means of an interactive voice randomization system with the use of a permuted block design. All participants in both treatment groups received antiplatelet therapy and other protocol-driven medical therapies to control blood pressure and glucose and lipid levels in accordance with guidelines.19,20
Unless otherwise contraindicated, the following medications were mandated by the protocol: the angiotensin II type-1 receptor blocker candesartan (Atacand, AstraZeneca), with or without hydrochlorothiazide, and the combination agent amlodipine–atorvastatin (Caduet, Pfizer), with the dose adjusted on the basis of blood pressure and lipid status. Participants received voucher cards that allowed them to obtain the medications (candesartan, hydrochlorothiazide, and atorvastatin–amlodipine) from their local pharmacies at no personal cost. The target blood pressure was less than 140/90 mm Hg in patients without coexisting conditions and less than 130/80 mm Hg in patients with diabetes or chronic kidney disease. Medications were adjusted until the blood-pressure goal was reached.17 Blood pressure was measured three times, 2 minutes apart, in each participant, with the use of an oscillometric device. The measurements were made while participants were sitting quietly, and the mean of the last two measurements was used.
Participants in the stent group underwent placement of a Palmaz Genesis stent (Cordis); predilation was performed at the discretion of the investigator. All renal arteries with stenoses of 60% or more were treated. In patients with multiple stenoses, stenting could be performed as a single procedure or in intervals of 2 to 4 weeks. Before August 2006, the use of the short-tip Angioguard device was required for embolic protection; after this date, an embolic protection device approved by the Food and Drug Administration (FDA) was used at the operator's discretion.
Crossovers from the medical therapy group to the stent group were reviewed by a designated crossover committee. Crossovers were not approved unless a qualifying outcome event had occurred or all of the following conditions were met: acute anuric renal failure, complete occlusion of all renal arteries, and at least one kidney more than 8 cm in length.
STUDY END POINTS
The primary end point was the occurrence of a major cardiovascular or renal event — a composite of death from cardiovascular or renal causes, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or the need for permanent renal-replacement therapy. Myocardial infarction was adjudicated on the basis of the presence of clinical symptoms or electrocardiographic changes and elevated cardiac markers. Hospitalization for congestive heart failure was included in the analysis if the patient was hospitalized for 12 hours or longer because of documented signs and symptoms of heart failure and received intravenous therapy (vasodilators, diuretics, or inotropes) during the hospital stay. Progressive renal insufficiency was defined as a reduction from baseline of 30% or more in the estimated GFR, with the reduction sustained for 60 days or longer and not attributable to other causes. Secondary clinical end points included the individual components of the primary end point (with death from cardiovascular causes and death from renal causes as separate end points), as well as all-cause mortality. Complete definitions of the study end points are provided in the Supplementary Appendix. A single end-point committee whose members were unaware of the group assignments adjudicated all end points.
The definitions of end points were modified on March 12, 2012, by the CORAL steering committee, and the modifications were approved by the data and safety monitoring board and the FDA. These modifications, which were made before the data were unblinded and with the steering committee unaware of event rates in the study groups, were intended to bring the definitions of end points into alignment with clinical event definitions that had evolved during the course of the study. Details of the changes in end-point definitions are provided in Table S1 in the Supplementary Appendix.
STATISTICAL ANALYSIS
We originally calculated that 1080 participants would need to be enrolled for the study to have 90% power to test the hypothesis that stenting would reduce the incidence of the primary end point by 25% (hazard ratio, 0.75) at 2 years, at a two-sided type I error rate of 0.05. Because the recruitment was slower than anticipated, the data and safety monitoring board recommended termination of recruitment on January 30, 2010 (at which point 947 participants had undergone randomization), and follow-up was extended through September 28, 2012, to preserve the statistical power.
All the analyses were performed on an intention-to-treat basis. All participants who underwent randomization were included in the intention-to-treat analyses with the exception of the 16 participants (8 in each group) who were enrolled at a single site at which scientific integrity issues were identified; an administrative decision was made to exclude the data from these participants from the intention-to-treat analysis (see additional information below). Continuous variables are expressed as means and standard deviations and were compared with the use of Student's t-tests. Medians are presented with interquartile ranges. Categorical variables are expressed as proportions and were compared with the use of the chi-square test or Fisher's exact test, as appropriate. Time-to-event outcomes (including the primary end point) are expressed as Kaplan–Meier estimates and were compared between the treatment groups with the use of the log-rank statistic. The Cox proportional-hazards model was used to estimate the hazard ratios and associated 95% confidence intervals. Prespecified secondary analyses included tests for interaction effects between the primary end point and sex, race, presence or absence of diabetes, and presence or absence of global renal ischemia (defined as stenosis of 60% or more of the diameter of all arteries supplying both kidneys or stenosis of 60% or more of the diameter of all arteries supplying a single functioning kidney). The effect of treatment on systolic blood pressure over time was estimated with the use of a repeated-measures analysis.
The primary composite end point was tested at the 0.0497 level to adjust for a single interim analysis. All other analyses were performed at the 0.05 level without adjustment for multiple comparisons.
RESULTS
STUDY POPULATION AND TREATMENT
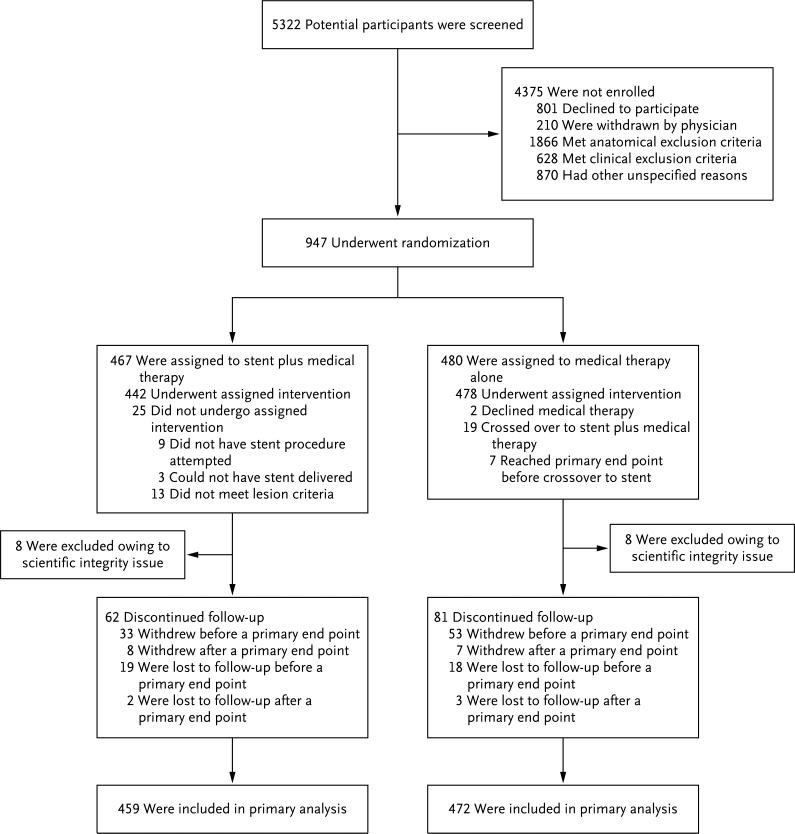
Between May 16, 2005, and January 30, 2010, a total of 5322 patients were screened, and 947 were randomly assigned to stenting plus medical therapy (467 patients) or medical therapy alone (480 patients) (Fig. 1). The reasons for nonenrollment of screened patients are shown in Figure 1 and in Table S2 in the Supplementary Appendix. One center was found during monitoring to have obtained consent from some participants after study procedures were initiated. That site was terminated from the study, and the 16 participants at that site were withdrawn from the study, owing to issues of scientific integrity relating to informed consent and the eligibility of participants. All study data are reported for the remaining 931 trial participants.
Figure 1. Screening, Randomization, and Follow-up.
All the participants who underwent randomization were included in the primary analysis, with the exception of the 16 participants (8 in each group) enrolled at a single site at which concerns regarding scientific integrity related to informed consent and eligibility of enrolled participants were raised during monitoring. The 19 patients who crossed over from medical therapy alone to stent plus medical therapy were included in the intention-to-treat analysis in the medical therapy–alone group.
The two groups were well matched at baseline (Table 1). Among the 472 patients in the medical-therapy group, 19 crossed over to stenting. A total of 12 crossovers were not approved by the crossover committee; 7 were approved because they occurred after the patients had had a primary end-point event. Participants were followed for a median of 43 months (interquartile range, 31 to 55).
Table 1.
Baseline Characteristics of the Study Population, According to Treatment Group.*
| Characteristic | Stenting plus Medical Therapy (N = 459) | Medical Therapy Only (N = 472) |
|---|---|---|
| Age (yr) | 69.3±9.4 | 69.0±9.0 |
| Male sex (%) | 51.0 | 48.9 |
| Race (%)† | ||
| Black | 7.0 | 7.0 |
| Other | 93.0 | 93.0 |
| Body-mass index‡ | 28.2±5.3 | 28.7±5.7 |
| Systolic blood pressure (mm Hg) | 149.9±23.2 | 150.4±23.0 |
| Blood pressure at target level (%)§ | 29.2 | 25.3 |
| Estimated GFR (ml/min/1.73 m2)¶ | 58.0±23.4 | 57.4±21.7 |
| Stage ≥3 chronic kidney disease (%) | 49.6 | 50.4 |
| Method of identification of stenosis (%) | ||
| Angiography | 68.4 | 68.6 |
| Duplex ultrasonography | 25.5 | 24.2 |
| Computed tomographic angiography | 4.4 | 5.3 |
| Magnetic resonance angiography | 1.7 | 1.9 |
| Medical history and risk factors (%) | ||
| Diabetes | 32.4 | 34.3 |
| Prior myocardial infarction | 26.5 | 30.2 |
| History of heart failure | 12.0 | 15.1 |
| Smoking in past yr | 28.0 | 32.2 |
| Hyperlipidemia | 89.4 | 90.0 |
| Angiographic findings∥ | ||
| % Stenosis, as assessed by core laboratory | 67.3±11.4 | 66.9±11.9 |
| % Stenosis, as assessed by investigator | 72.5±14.6 | 74.3±13.1 |
| Global ischemia (%)** | 20.0 | 16.2 |
| Bilateral disease (%)†† | 22.0 | 18.1 |
Plus-minus values are means ±SD. There were no significant differences between the groups in any of the characteristics listed here (P>0.05).
Race was self-reported. Other included white (91.5% in the stent group and 90.9% in the medical therapy-only group), as well as American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The target level of blood pressure was less than 140/90 mm Hg for patients without coexisting conditions and less than 130/80 mm Hg for patients with diabetes or chronic kidney disease.
The estimated glomerular filtration rate (GFR) was calculated with the use of the modified Modification of Diet in Renal Disease formula.
Angiographic data are shown for patients who underwent invasive angiography.
Global ischemia was defined as stenosis of 60% or more of the diameter of all arteries supplying both kidneys or stenosis of 60% or more of the diameter of all arteries supplying a single functioning kidney.
Bilateral disease was defined as stenosis of 60% or more of the diameter of at least one artery supplying each kidney.
STENTING AND PERIPROCEDURAL EVENTS
Stents were placed in 434 of the 459 patients in the stent group (94.6%) and resulted in a mean (±SD) reduction of the stenosis from 68±11% to 16±8% (P<0.001) (Fig. 1). The most common angiographic complication was arterial dissection, which occurred in 11 patients (details of stent treatment, including procedural complications, are provided in Table S3 in the Supplementary Appendix). No one in the stent group (or in the medical therapy–only group) required dialysis within 30 days after randomization. One person (0.2%) in the stent group initiated dialysis between 30 and 90 days after randomization. A patient randomly assigned to medical therapy alone had a fatal stroke on the day of randomization.
CLINICAL OUTCOMES
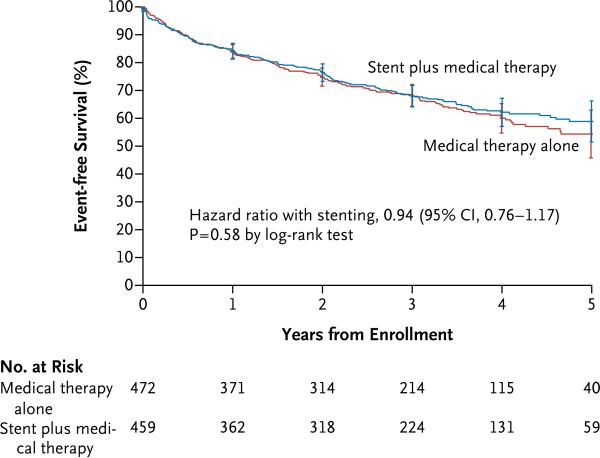
There was no significant difference in the occurrence of the primary composite end point between the stent group and medical therapy–only group (35.1% and 35.8%, respectively; hazard ratio, 0.94; 95% confidence interval [CI], 0.76 to 1.17; P = 0.58) (Table 2 and Fig. 2). In addition, no significant between-group differences were observed in the rates of the components of the primary end point (Table 2, and Fig. S1 through S6 in the Supplementary Appendix). We also observed no significant difference in all-cause mortality during the follow-up period (Table 2).
Table 2.
Clinical End Points.*
| End Point | Stenting plus Medical Therapy (N = 459) | Medical Therapy Only (N = 472) | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| no. (%) | ||||
| Primary end point: death from cardiovascular or renal causes, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or permanent renal-replacement therapy† | 161 (35.1) | 169 (35.8) | 0.94 (0.76-1.17) | 0.58 |
| Components of primary end point‡ | ||||
| Death from cardiovascular or renal causes | 20 (4.4) | 20 (4.2) | ||
| Stroke | 12 (2.6) | 16 (3.4) | ||
| Myocardial infarction | 30 (6.5) | 27 (5.7) | ||
| Hospitalization for congestive heart failure | 27 (5.9) | 26 (5.5) | ||
| Progressive renal insufficiency | 68 (14.8) | 77 (16.3) | ||
| Permanent renal-replacement therapy | 4 (0.9) | 3 (0.6) | ||
| Secondary clinical end points§ | ||||
| Death from any cause | 63 (13.7) | 76 (16.1) | 0.80 (0.58-1.12) | 0.20 |
| Death from cardiovascular causes | 41 (8.9) | 45 (9.5) | 0.89 (0.58-1.36) | 0.60 |
| Death from renal causes | 2 (0.4) | 1 (0.2) | 1.89 (0.17-20.85) | 0.60 |
| Stroke | 16 (3.5) | 23 (4.9) | 0.68 (0.36-1.28) | 0.23 |
| Myocardial infarction | 40 (8.7) | 37 (7.8) | 1.09 (0.70-1.71) | 0.70 |
| Hospitalization for congestive heart failure | 39 (8.5) | 39 (8.3) | 1.00 (0.64-1.56) | 0.99 |
| Progressive renal insufficiency | 77 (16.8) | 89 (18.9) | 0.86 (0.64-1.17) | 0.34 |
| Permanent renal-replacement therapy | 16 (3.5) | 8 (1.7) | 1.98 (0.85-4.62) | 0.11 |
The hazard ratios were calculated with the use of multivariable proportional-hazards regression. P values were calculated with the use of the log-rank statistic.
Only the first event per participant is included in the composite.
Components of the composite are included only if it was the first event contributing to the primary end point.
The first event for each component of the primary composite end point is included as a secondary end point.
Figure 2. Kaplan–Meier Curves for the Primary Outcome.
Survival curves are truncated at 5 years owing to instability of the curves because few participants remained in the study after 5 years.
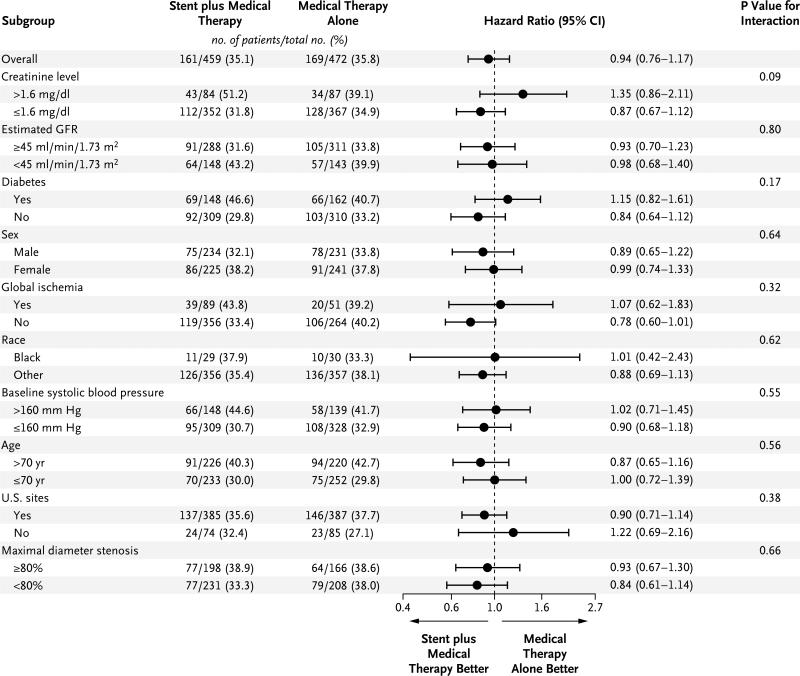
No interactions were observed between treatment and the four prespecified subgroups — those defined according to sex, race (black vs. others), presence or absence of global ischemia, and presence or absence of diabetes — with respect to the occurrence of a primary end-point event (Fig. 3). In addition, no significant differences in the treatment effect were observed in other subgroups.
Figure 3. Forest Plot of Treatment Effects within Subgroups.
Hazard ratios for stenting plus medical therapy versus medical therapy alone include all available follow-up data for the primary composite end point. None of the tests for treatment and subgroup interaction were significant (P>0.05). To convert the values for creati-nine to micromoles per liter, multiply by 88.4. The estimated glomerular filtration rate (GFR) was calculated with the use of the modified Modification of Diet in Renal Disease formula. Global ischemia was defined as stenosis of 60% or more of the diameter of all arteries supplying both kidneys or stenosis of 60% or more of the diameter of all arteries supplying a single functioning kidney. For the subgroup of blacks versus others, the analysis was limited to U.S. sites. SBP denotes systolic blood pressure.
BLOOD PRESSURE OVER TIME
At baseline, participants were taking a mean of 2.1±1.6 antihypertensive medications. At the end of the study, the number of medications increased in both the stent group and the medical therapy–only group but did not differ significantly between the two groups (3.3±1.5 and 3.5±1.4 medications, respectively; P = 0.24). Systolic blood pressure declined in both the medical therapy–only group (by 15.6±25.8 mm Hg) and the stent group (by 16.6±21.2 mm Hg). In the longitudinal analysis, the systolic blood pressure was modestly lower in the stent group than in the medical therapy–only group (−2.3 mm Hg; 95% CI, −4.4 to −0.2 mm Hg; P = 0.03), and the difference persisted throughout the follow-up period (Fig. S7 in the Supplementary Appendix).
DISCUSSION
The CORAL trial was designed to test whether renal-artery stenting, when added to protocol-driven contemporary medical therapy, improves clinical outcomes in persons with atherosclerotic renal-artery stenosis. We found no benefit of stenting with respect to the rate of the composite primary end point or any of its individual components, including death from cardiovascular or renal causes, stroke, myocardial infarction, congestive heart failure, progressive renal insufficiency, and the need for renal-replacement therapy. This result was consistent across all prespecified subgroups, including patients with global renal ischemia and patients with other high-risk characteristics. We did observe a modest, but statistically significant, reduction of 2 mm Hg in systolic blood pressure with stenting, but this reduction did not translate into a reduction in clinical events.
Other randomized trials, including the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial15 and the Stent Placement and Blood Pressure and Lipid-Lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,16 assessed the usefulness of renal-artery stenting with respect to kidney function and showed no significant difference in this key measure. These studies have been criticized for enrolling some participants who did not have clinically significant renal-artery stenosis and for not having their findings confirmed by core laboratories.21 In addition, none of the previous studies were designed specifically cally to detect a benefit with respect to clinical events. We sought to address these concerns in CORAL.
A key issue in the interpretation of our results is whether the medical therapy that was given to CORAL participants can be replicated in clinical practice. The medical therapy in our study included the use of an angiotensin-receptor blocker, with or without a thiazide-type diuretic, with the addition of amlodipine for blood-pressure control. In addition, participants received anti-platelet therapy and atorvastatin for management of lipid levels, and diabetes was managed according to clinical practice guidelines.19,20 With this regimen, patients who received medical treatment alone had remarkably good cardiovascular and renal outcomes, despite their advanced age and the high rates of hypertension, diabetes, chronic kidney disease, and other coexisting cardiovascular conditions.
Renal-artery stenting remains a common procedure in current clinical practice. The CORAL study shows that, when added to a background of high-quality medical therapy, contemporary renal-artery stenting provides no incremental benefit. From this result, it is clear that medical therapy without stenting is the preferred management strategy for the majority of people with atherosclerotic renal-artery stenosis.
The CORAL trial had some limitations. First, patients could be enrolled in the trial with renal-artery stenosis of 60% or more, and there is debate about the severity of stenosis that is necessary to justify intervention.22 However, we were unable to show a benefit among participants with renal-artery stenosis of more than 80%, as measured by the enrolling investigators. Second, we did not include patients with fibromuscular dysplasia, and several studies suggest that angioplasty alone may improve blood-pressure control and even cure hypertension in young persons.23 Third, although the inclusion criteria for CORAL were intentionally broad, some patients who were screened and deemed to be eligible were not enrolled in the trial, including patients who were not enrolled because of the preference of their physician. Some of these patients may have been treated by means of stenting by physicians who were convinced of the clinical benefit of the procedure. Nonetheless, the baseline clinical and angiographic characteristics of the study population, as well as the response with respect to systolic blood pressure, were remarkably similar to those in patients enrolled in previous single-group, FDA-approval trials of renal stents.24-26
In summary, renal-artery stenting did not confer a significant benefit with respect to the prevention of clinical events when added to comprehensive, multifactorial medical therapy in people with atherosclerotic renal-artery stenosis and hypertension or chronic kidney disease.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supported by grants (U01HL071556, U01HL072734, U01HL072735, U01HL072736, and U01HL072737) from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Dr. Murphy reports holding equity interest in Summa Therapeutics. Dr. Cutlip reports that his institution holds research contracts with Medtronic, Boston Scientific, and Abbott Vascular. Dr. Cohen reports receiving personal fees from Abbott, AstraZeneca, Eli Lilly, and Medtronic, and grant support from Abbott, AstraZeneca, Eli Lilly, Medtronic, Boston Scientific, Biomet, and Janssen. Dr. Matsumoto reports receiving consulting fees from Boston Scientific and the Medicines Company and grant support from Medtronic, Cook and W.L. Gore. Dr. Jaff reports receiving consulting fees from AstraZeneca and serving as an uncompensated advisor to Cordis, Boston Scientific, Abbott, Covidien, and Medtronic. Dr. Lewis reports receiving grant support from Novartis, Amgen, and Sanofi Aventis. Dr. Tuttle reports receiving consulting fees and grant support from Eli Lilly. Dr. Rundback reports receiving fees for board membership from VIVA Physicians, personal fees from Covidien, Biotronik, and St. Jude, and lecture fees from Covidien and CSI. No other potential conflict of interest relevant to this article was reported.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Derkx FH, Schalekamp MA. Renal artery stenosis and hypertension. Lancet. 1994;344:237–9. doi: 10.1016/s0140-6736(94)93002-3. [DOI] [PubMed] [Google Scholar]
- 2.Ram CV. Renovascular hypertension. Curr Opin Nephrol Hypertens. 1997;6:575–9. doi: 10.1097/00041552-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Olin JW, Melia M, Young JR, Graor RA, Risius B. Prevalence of atherosclerotic renal artery stenosis in patients with atherosclerosis elsewhere. Am J Med. 1990;88:46N–51N. [PubMed] [Google Scholar]
- 4.Harding MB, Smith LR, Himmelstein SI, et al. Renal artery stenosis: prevalence and associated risk factors in patients undergoing routine cardiac catheterization. J Am Soc Nephrol. 1992;2:1608–16. doi: 10.1681/ASN.V2111608. [DOI] [PubMed] [Google Scholar]
- 5.Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36:443–51. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 6.Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–79. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum U, Krumme B, Flügel P, et al. Treatment of ostial renal-artery stenoses with vascular endoprostheses after unsuccessful balloon angioplasty. N Engl J Med. 1997;336:459–65. doi: 10.1056/NEJM199702133360702. [DOI] [PubMed] [Google Scholar]
- 8.Burket MW, Cooper CJ, Kennedy DJ, et al. Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J. 2000;139:64–71. doi: 10.1016/s0002-8703(00)90310-7. [DOI] [PubMed] [Google Scholar]
- 9.Harden PN, MacLeod MJ, Rodger RS, et al. Effect of renal-artery stenting on progression of renovascular renal failure. Lancet. 1997;349:1133–6. doi: 10.1016/s0140-6736(96)10093-3. [DOI] [PubMed] [Google Scholar]
- 10.Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC. Effect of renal artery stenting on renal function and size in patients with atherosclerotic reno-vascular disease. Circulation. 2000;102:1671–7. doi: 10.1161/01.cir.102.14.1671. [DOI] [PubMed] [Google Scholar]
- 11.Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery interventions by Medicare ben eficiaries, 1996-2000. AJR Am J Roentgenol. 2004;183:561–8. doi: 10.2214/ajr.183.3.1830561. [DOI] [PubMed] [Google Scholar]
- 12.van Jaarsveld BC, Krijnen P, Pieterman H, et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. N Engl J Med. 2000;342:1007–14. doi: 10.1056/NEJM200004063421403. [DOI] [PubMed] [Google Scholar]
- 13.Plouin PF, Chatellier G, Darné B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Hypertension. 1998;31:823–9. doi: 10.1161/01.hyp.31.3.823. [DOI] [PubMed] [Google Scholar]
- 14.Webster J, Marshall F, Abdalla M, et al. Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. J Hum Hypertens. 1998;12:329–35. doi: 10.1038/sj.jhh.1000599. [DOI] [PubMed] [Google Scholar]
- 15.The ASTRAL Investigators Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–62. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 16.Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with ath erosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150:840–8. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 17.Cooper CJ, Murphy TP, Matsumoto A, et al. Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J. 2006;152:59–66. doi: 10.1016/j.ahj.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function — measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Foy A, Ruggiero NJ II, Filippone EJ. Revascularization in renal artery stenosis. Cardiol Rev. 2012;20:189–93. doi: 10.1097/CRD.0b013e31824a72e9. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Zhang D, Haller S, et al. Determinants of renal function in patients with renal artery stenosis. Vasc Med. 2011;16:331–8. doi: 10.1177/1358863X11419998. [DOI] [PubMed] [Google Scholar]
- 23.Trinquart L, Mounier-Vehier C, Sapoval M, Gagnon N, Plouin PF. Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: a systematic review and meta-analysis. Hypertension. 2010;56:525–32. doi: 10.1161/HYPERTENSIONAHA.110.152918. [DOI] [PubMed] [Google Scholar]
- 24.Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol. 2005;46:776–83. doi: 10.1016/j.jacc.2004.11.073. [DOI] [PubMed] [Google Scholar]
- 25.Jaff MR, Bates M, Sullivan T, et al. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv. 2012;80:343–50. doi: 10.1002/ccd.24449. [DOI] [PubMed] [Google Scholar]
- 26.Rocha-Singh K, Jaff MR, Lynne Kelley E. Renal artery stenting with noninvasive duplex ultrasound follow-up: 3-year results from the RENAISSANCE renal stent trial. Catheter Cardiovasc Interv. 2008;72:853–62. doi: 10.1002/ccd.21749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.