Abstract
IMPORTANCE
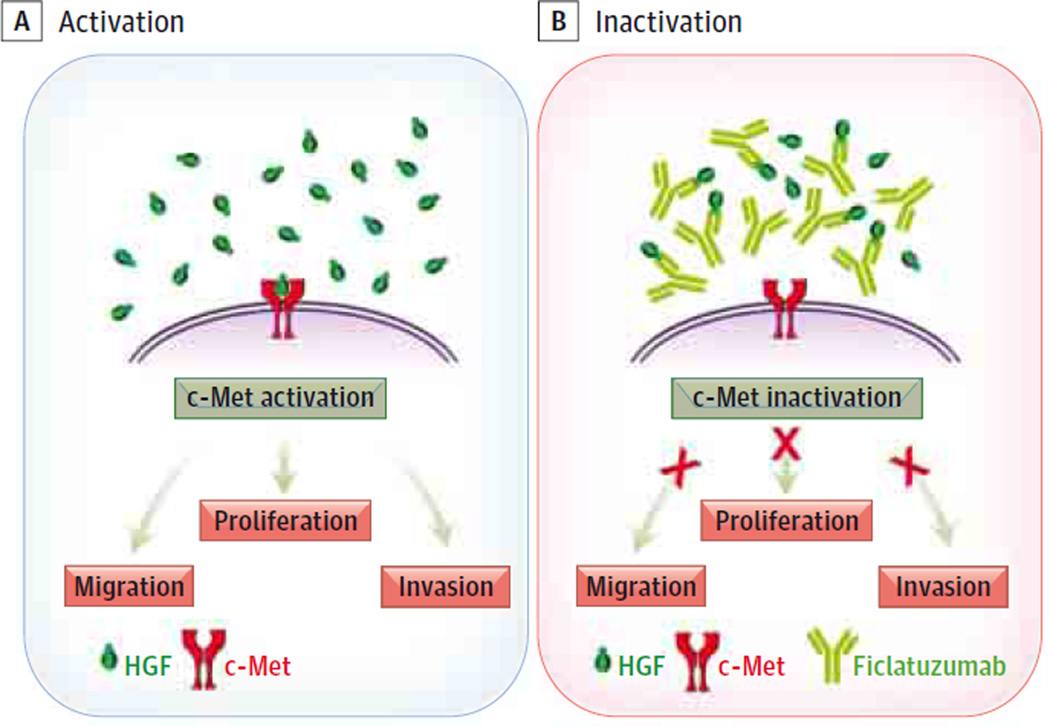
Ficlatuzumab can be used to treat head and neck squamous cell carcinoma (HNSCC) by inhibiting c-Met receptor-mediated cell proliferation, migration, and invasion.
OBJECTIVE
To understand the effect of ficlatuzumab on HNSCC proliferation, migration, and invasion.
DESIGN, SETTING, AND PARTICIPANTS
The effects of ficlatuzumab on HNSCC proliferation, invasion, and migration were tested. Mitigation of c-Met and downstream signaling was assessed by immunoblotting. The tumor microenvironment has emerged as an important factor in HNSCC tumor progression. The most abundant stromal cells in HNSCC tumor microenvironment are tumor-associated fibroblasts (TAFs). We previously reported that TAFs facilitate HNSCC growth and metastasis. Furthermore, activation of the c-Met tyrosine kinase receptor by TAF-secreted hepatocyte growth factor (HGF) facilitates tumor invasion. Ficlatuzumab is a humanized monoclonal antibody that sequesters HGF, preventing it from binding to and activating c-Met. We hypothesized that targeting the c-Met pathway with ficlatuzumab will mitigate TAF-mediated HNSCC proliferation, migration, and invasion. Representative HNSCC cell lines HN5, UM-SCC-1, and OSC-19 were used in these studies.
EXPOSURES FOR OBSERVATIONAL STUDIES
The HNSCC cell lines were treated with ficlatuzumab, 0 to 100 µg/mL, for 24 to 72 hours.
MAIN OUTCOMES AND MEASURES
Ficlatuzumab inhibited HNSCC progression through c-Met and mitogen-activated protein kinase (MAPK) signaling pathway.
RESULTS
Ficlatuzumab significantly reduced TAF-facilitated HNSCC cell proliferation (HN5, P< .001; UM-SCC-1, P< .001), migration (HN5, P = .002; UM-SCC-1, P = .01; and OSC-19, P = .04), and invasion (HN5, P = .047; UM-SCC-1, P = .03; and OSC-19, P = .04) through a 3-dimensional peptide-based hydrogel (PGmatrix). In addition, ficlatuzumab also inhibited the phosphorylation of c-Met at Tyr1234/1235 and p44/42 MAPK in HNSCC cells exposed to recombinant HGF.
CONCLUSIONS AND RELEVANCE
We demonstrate that neutralizing TAF-derived HGF with ficlatuzumab effectively mitigates c-Met signaling and decreases HNSCC proliferation, migration, and invasion. Thus, ficlatuzumab effectively mitigates stromal influences on HNSCC progression.
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide, with approximately 40 000 new cases per year in the United States and 500 000 worldwide.1 HNSCC arises from the epithelial lining of the upper aerodigestive tract, and the 5-year mortality rate from this disease is still close to 50%.2 Historically, surgery and radiation have been the mainstays of treatment. Traditionally, the role of chemotherapy has been enhancing the effects of radiation therapy. There are only 6 US Food and Drug Administration–approved drugs for the treatment of HNSCC, and only 2 that have been approved since 1978. However, the survival rates continue to be very low, highlighting the need for improved therapeutic approaches.
c-Met is a proto-oncogene and encodes tyrosine kinase activity, which is overexpressed in several cancers, including HNSCC3; HNSCC tumors are associated with various stromal cells, and these cells are active contributors to neoplastic transformation, tumor invasion, and metastasis. The tumor microenvironment has emerged as an important factor in HNSCC tumor progression.4 The molecular crosstalk has not been fully elucidated and continues to be studied. The most abundant stromal cells in the HNSCC tumor microenvironment are tumor-associated fibroblasts (TAFs). We previously reported that TAFs facilitate HNSCC growth and metastasis.5 In addition, we reported that TAFs, but not HNSCC cell lines, secrete hepatocyte growth factor (HGF); HGF was initially discovered as a mitogen that promoted growth of hepatocytes, epithelial tissues, endothelial cells, and melanocytes.6 In addition, fibroblast-secreted HGF was found to dissociate epithelial cells and to induce a more invasive phenotype in several carcinoma cell lines.7,8 Hepatocyte growth factor is present in higher serum concentrations in patients with HNSCC compared with healthy individuals.9 Also, HGF is present in higher concentrations locally in HNSCCs that have metastasized, compared with normal oral cavity epithelium, and nonmetastatic lesions.9 An elevated HGF level in the tumor is an indicator of poor prognosis in non–small-cell lung cancer (NSCLC) and breast cancer.10,11 We reported that both HGF and c-Met levels are increased in HNSCC compared with normal mucosa and that HGF acts in a paracrine manner to facilitate HNSCC cell proliferation and invasion.12
Activation of the c-Met triggers various signaling pathways that drive several tumorigenic properties.12 Ligand binding activates signaling cascades, including the mitogen-activated protein kinases (MAPKs), phosphatidylinositide 3-kinases (PI3Ks), signal transducer and activator of transcription 3 (STAT3), RAS, and notch pathways, resulting in cell morphogenesis, motility, growth, and survival. Inhibition of the HGF c-Met axis is an attractive target in the treatment of HNSCC. Ficlatuzumab is a humanized IgG1 HGF-inhibitory monoclonal antibody that binds HGF with a high affinity and specificity. Preclinical trials have shown that ficlatuzumab does effectively bind HGF and has antitumor effects on NSCLC and glioma preclinical models.13,14 It has a half-life of approximately 7 to 10 days and has a low systemic clearance. A phase 1 trial has shown it to be well tolerated, with the most common reported adverse effects including fatigue, peripheral edema, headache, diarrhea, and rash.15 Herein, we demonstrate that ficlatuzumab effectively inhibits TAF-facilitated HNSCC invasion and migration in several HNSCC cell lines. Furthermore, we demonstrate the efficacy of ficlatuzumab in inhibiting TAF-facilitated HNSCC proliferation and c-Met signaling. Together, these data indicate that ficlatuzumab may be effective in mitigating stroma-facilitated HNSCC progression.
Methods
Tissue Culture and Reagents
Previously described and well-characterized HNSCC cell lines that are human papilloma virus (HPV) negative were used in this study. HNSCC cell lines HN5, UM-SCC-1, and OSC-19 were established from an oral cavity tumor, a floor-of-mouth tumor and metastatic tongue HNSCC, respectively. Cells were cultured and maintained in Dulbecco modified Eagle medium (DMEM)with 10% heat-inactivated fetal bovine serum (Invitrogen). The TAFs were isolated from primary HNSCC explants as previously described.12 Primary HNSCC specimens were collected under the auspices of the biospecimen repository core at the University of Kansas Cancer Center with written informed consent from patients, using protocols approved by the institutional review boards at the University of Kansas Medical Center. Antibodies to phospho–c-Metandphospho-p44/42 MAPK were purchased from Cell Signaling; vimentin, from Abcam; and β-tubulin, Sigma-Aldrich. Ficlatuzumab was obtained under a material transfer agreement from AVEO Pharmaceuticals Inc.
Conditioned Media
The TAF cells were grown to 90% to 100% confluence. Growth media on confluent cultures were replaced with serum-free DMEM for 48 hours. Supernatants were clarified by centrifugation at 4700 rpm for 10 minutes, aliquoted, and stored at −80°C. Conditioned media were used within 1 month of collection.
Migration Assay
Cell migration was evaluated in vitro using semipermeable modified Boyden inserts with a pore size of 8 µm (Coaster). The HNSCC cells were plated in triplicate at a density of 2 to 3 × 104 per well in serum-free media or TAF-conditioned media in the insert. Outer wells contained vehicle control (DMEM), TAF-conditioned media with or without ficlatuzumab, or with ficlatuzumab alone. HNSCC cells (2000 per well) were simultaneously plated in 96-well plates to assess cell viability. After 24 hours at 37°C in a 5% carbon dioxide incubator, the cells that migrated through the insert were fixed and stained with Hema 3 (Fisher Scientific) per the manufacturer's instructions. Cells plated in 96-well plates were subjected to the CyQuant cell proliferation assay (Life Technologies). The number of migrating cells was normalized to the cell viability. Fold change in migration relative to the vehicle control (DMEM) was determined.
Invasion Assay
Cell invasion was evaluated in vitro through synthetic matrix-coated semipermeable modified Boyden inserts with a pore size of 8 µm (Coaster). The PGmatrix kit (PepGel LLC) consists of self-assembling peptide nanofiber hydrogel solution (PGMatrix) and a gel trigger solution (PGworks). Inserts were coated with PGmatrix (1:0.2 dilution of gel in 12 DMEM) and allowed to set at 37°C for 1 hour per the manufacturer’s instructions. HNSCC cells were plated in triplicate at a density of 2 × 104 per well in serum-free media or TAF-conditioned media on top of the synthetic gel in the insert. Outer wells contained vehicle control (DMEM) or TAF-conditioned media with or without ficlatuzumab or with ficlatuzumab alone. HNSCC cells (2000 per well) were simultaneously plated in 96-well plates to assess cell viability. After 24 hours at 37°C in a 5%carbon dioxide incubator, the cells in the insert were removed by wiping gently with a cotton swab. Cells that invaded the matrix and passed through the membrane pores of insert were fixed and stained with Hema 3 (Fisher Scientific) according to the manufacturer’s instructions. Cells plated in 96-well plates were subjected to a CyQuant cell proliferation assay (Life Technologies). The number of invading cells was normalized to the cell viability. Fold change in invasion relative to the vehicle control (DMEM) was determined.
Immunofluorescence
HN5 and UM-SCC-1 cells (20 000 per well) were cultured in 4-chamber slides, and serum was starved for 48 hours. Cells were treated with vehicle control or ficlatuzumab (20 µg/mL) with or without TAF-conditioned media for 24 hours. Cells were washed, fixed with 2% paraformaldehyde, permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS), and blocked with 2% bovine serum albumin in PBS for 45 minutes at room temperature. Cells were incubated with primary antibody (1:500) at 4°C overnight, followed by the appropriate DyLight 488-conjugated secondary antibodies (1:1000) (Thermo Fisher). The cells were imaged by confocal microscopy (using a Nikon Eclipse E2000-U inverted with heated environmental chamber).
Proliferation Assay
The HN5 and UM-SCC-1 cells were seeded in 96-well plates (1500 cells per well), and after 12 hours cells were treated with DMEM alone or TAF-conditioned media with or without ficlatuzumab at various doses. After 72 hours of treatment, media were discarded and cells subjected to a freeze-thaw cycle at −80°C for 10 minutes followed by 37°C for 10 minutes to completely lyse the cells. Cell viability was determined using the CyQuant proliferation kit (Life Technologies) according to the manufacturer’s instructions.
Immunoblotting
Whole-cell lysates were extracted using lysis buffer and a mixture of protease and phosphatase inhibitors as previously described.5 Lysates were sonicated on ice, centrifuged for 30 minutes at 13 000 rpm, and the supernatants stored at −80°C. Equal amounts of proteins (50–60 µg of total protein)were separated through a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Nitrocellulose blots were blocked with Odyssey blocking buffer (LI-COR) 1:1 in PBS 1% Tween 20 (PBS-T) and incubated with primary antibody in blocking buffer overnight at 4°C. Immunoblots were washed 3 times with PBS-T followed by 1 to 2 hours of incubation with DyLight 488-conjugated secondary antibody. Protein bands were detected using the LI-COR Odyssey protein imaging system (LI-COR Biotechnology) and quantified using Image J software (version 1.48).
Statistical Analysis
All in vitro assays were repeated 3 times in triplicate. Data are reported as the mean (standard error of mean [SEM]). Statistical analyses were conducted using GraphPad Prism 6 software (version 6.03). Significance in treatment responses was obtained using analysis of variance with unpaired 2-tailed t test. P < .05 was considered statistically significant.
Results
Inhibition of TAF-Facilitated HNSCC Migration and Invasion
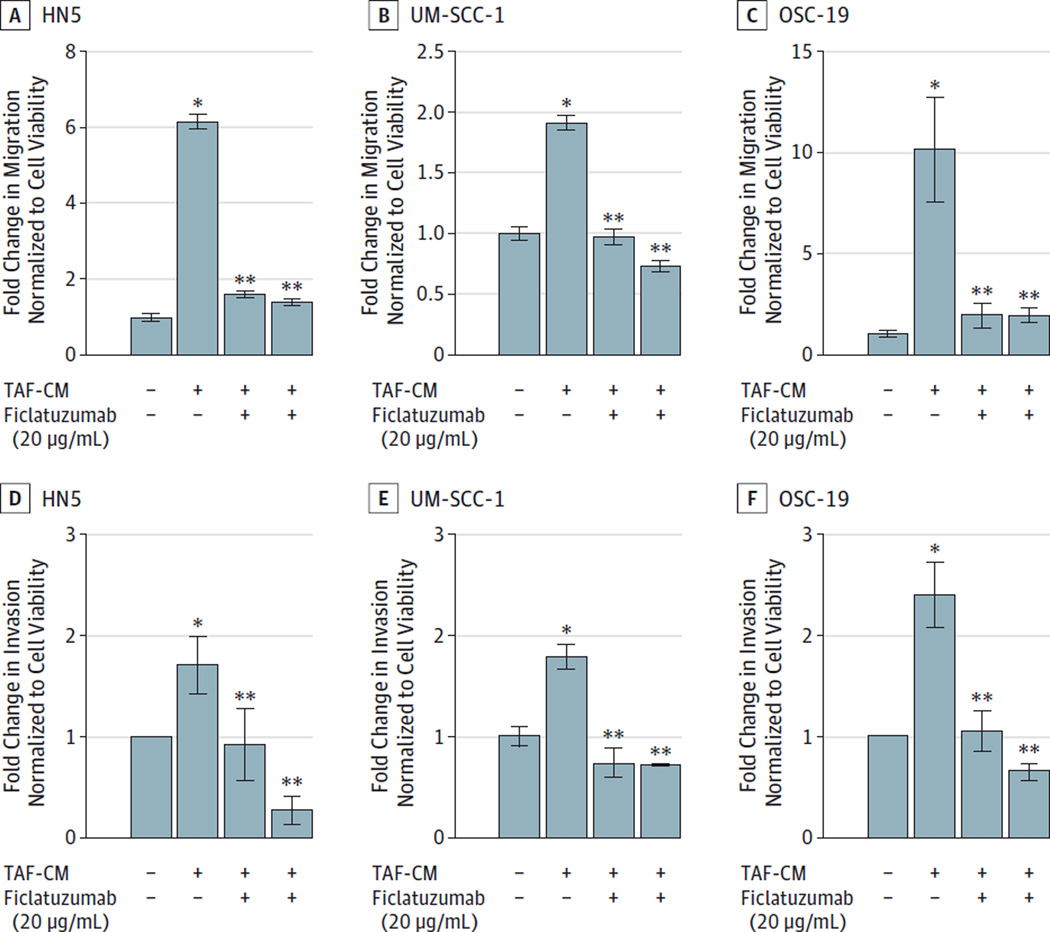
Metastatic spread is a major concern in HNSCC.16 The metastatic process involves cell migration and invasion through locoregional tissue. Migrations is a process whereby cells move across the surface of the tissue or basement membrane. Invasion encompasses migration and also tissue penetration mediated by proteolytic degradation surrounding the matrix. Tumor-secreted factors, including matrix metalloproteases, actively degrade the surrounding extracellular matrix (ECM).17 We previously reported that the TAFs increase invasion and migration of HNSCC cells via activation of c-Met.5,12 We tested the efficacy of ficlatuzumab in mitigating TAF-facilitated HNSCC invasion through PGmatrix, a synthetic hydrogel matrix. The synthetic matrix does not contain growth factors present in commercially available ECM, such as Matrigel (Corning Life Sciences). To determine whether HGF inhibitor ficlatuzumab abrogates TAF-facilitated migration and HNSCC invasion, HNSCC cells were treated with vehicle control (DMEM), TAF-conditioned media with or without ficlatuzumab, or with ficlatuzumab alone. Ficlatuzumab significantly reduced TAF-mediated HNSCC migration (Figure 1A–C) (HN5, P = .002; UM-SCC-1, dP = .01; and OSC-19, P = .04) and invasion (Figure 1D–F) (HN5, P = .047; UM-SCC-1, P = .03; and OSC-19, P = .04). These results suggest that ficlatuzumab effectively decreased TAF-conditioned media facilitated HNSCC migration and invasion.
Figure 1. Inhibition of TAF-Induced HNSCC Migration and Invasion.
Head and neck squamous carcinoma (HNSCC) cells were treated with Dulbecco modified Eagle medium alone, tumor-associated fibroblast (TAF)-conditioned medium (TAF-CM) with or without ficlatuzumab (20 µg/mL) or ficlatuzumab alone in transwell migration chambers for 24 hours. Invasion of HNSCC cells through a synthetic hydrogel matrix (PGMatrix) was assessed over 24 hours. Ficlatuzumab significantly inhibited TAF-CM induced migration (A–C) in HN5 (P= .002), UM-SCC-1 (P= .09), and OSC-19 (P= .04) cells and invasion (D–F) in HN5 (P= .047), UM-SCC-1 (P= .03), and OSC-19 (P= .04) cells. The data represent cumulative results from 3 independent experiments. Error bars represent the mean ± SEM
Inhibition of Epithelial to Mesenchymal Marker Vimentin Expression in HNSCC Cells
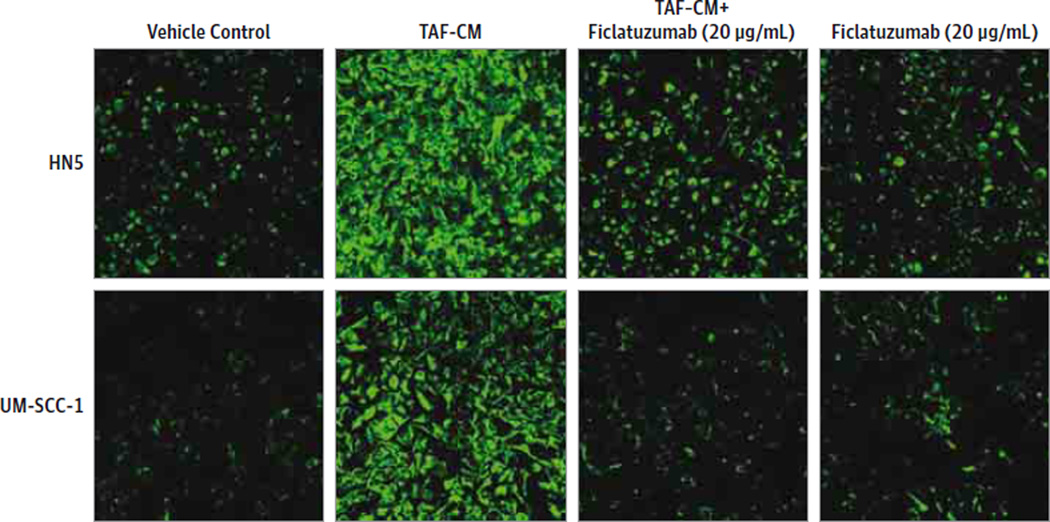
Vimentin is an established marker for epithelial-to-mesenchymal transition (EMT) in cancer cells. The acquisition of a mesenchymal phenotype facilitates epithelial cell motility. To determine if TAF-conditioned media induced EMT in HNSCC via c-Met, we carried out immunofluorescence analyses for vimentin. The TAF-conditioned media increased vimentin levels in HNSCC cell lines (Figure 2). Furthermore, ficlatuzumab inhibited TAF-conditioned media-induced vimentin expression in HNSCC. Thus, ficlatuzumab effectively mitigates TAF-facilitated EMT in HNSCC cells.
Figure 2. Mitigation of TAF-Induced Vimentin Expression in HNSCC Cells.
Head and neck squamous carcinoma (HNSCC) cells treated with Dulbecco modified Eagle medium alone, tumor-associated fibroblast (TAF)-conditioned medium (TAF-CM) with or without ficlatuzumab (20 µg/mL) or ficlatuzumab alone were analyzed by immunofluorescence for vimentin (green) expression. TAF-CM induced vimentin expression was effectively reduced in the presence of ficlatuzumab.
Inhibition of TAF-Facilitated HNSCC Proliferation
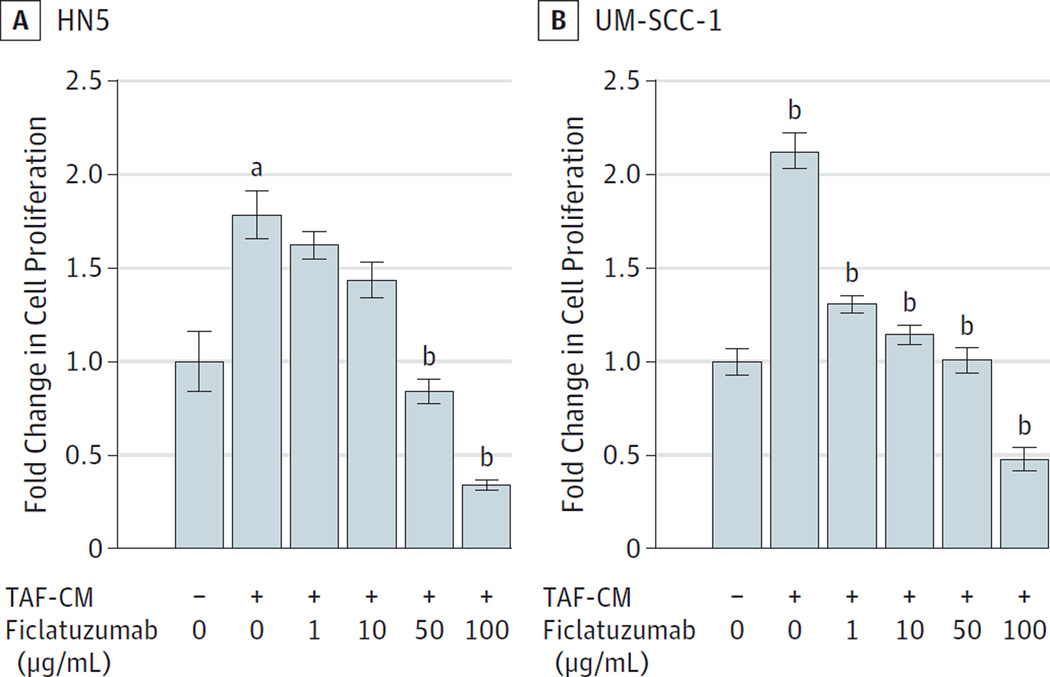
We previously reported5 that TAF-secreted HGF facilitated HNSCC cell proliferation. To determine the antitumor efficacy of ficlatuzumab, HN5 and UM-SCC-1 cells were treated with vehicle control (DMEM), TAF-conditioned media with or without ficlatuzumab, or ficlatuzumab alone for 72 hours. Ficlatuzumab significantly reduced HNSCC viability (HN5, P < .001; UM-SCC-1, P < .001) despite stimulation with TAF-conditioned medium (Figure 3A and B). We used 5 doses of ficlatuzumab (0, 1, 10, 50, and 100 µg/mL) for the treatment of HNSCC cells exposed to TAF-conditioned medium. We found that, even at the lowest dose of 1 µg/mL, there was an 80% reduction in TAF-conditioned, medium-facilitated HNSCC proliferation. This result strongly suggests that ficlatuzumab effectively reduces proliferation induced by TAF-secreted HGF in HNSCC cells.
Figure 3. Inhibition of TAF-Induced HNSCC Cell Proliferation.
HNSCC cells were treated with Dulbecco’s modified Eagle medium alone (−), TAF-conditioned medium (TAF-CM) (+), with or without ficlatuzumab (0, 1, 10, 50, 100 µg/mL) for 72 hours. Ficlatuzumab significantly inhibited TAF-facilitated (A) HN5 (P< .0001) and (B) UM-SCC-1 (P< .0001) proliferation. Cumulative results from a minimum of 2 independent experiments are graphed. Error bars represent the mean ± standard error of the mean.
a P= .005.
b P< .001.
Inhibition of HGF-Mediated Signaling in HNSCC Cells
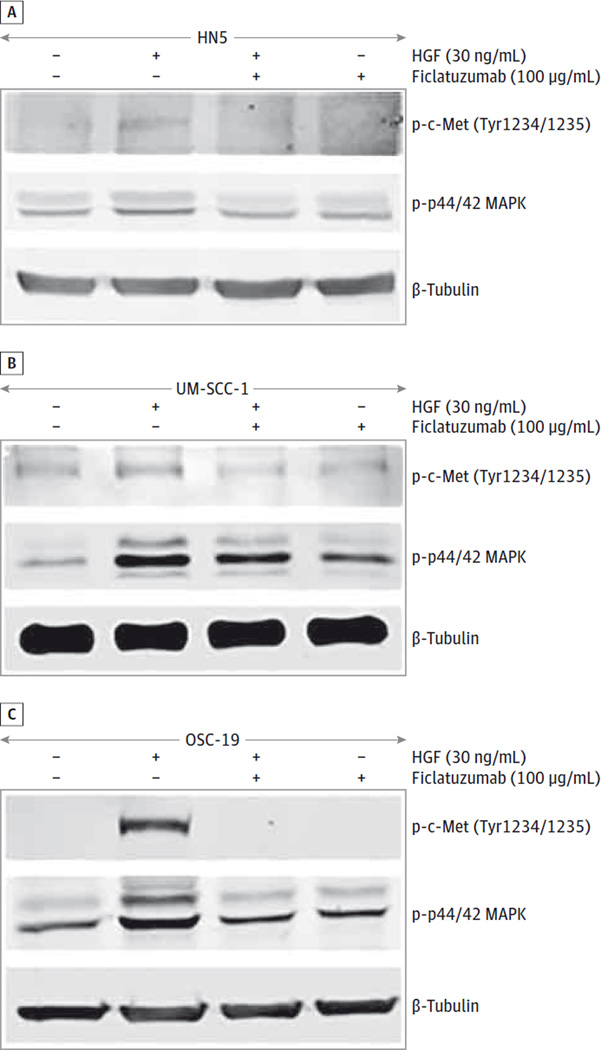
Hepatocyte growth factor stimulation results in activation of c-Met and downstream signal transduction pathways.12 Activation of c-Met and downstream p44/42 mitogen-activated protein kinase (MAPK) has been reported to facilitate HNSCC progression.12 We assessed the ability of ficlatuzumab to mitigate HGF-stimulated phosphorylation of c-Met and p44/42 MAPK. After 72 hours of serum starvation, HNSCC cells were exposed to recombinant HGF (30 ng/mL) with or without ficlatuzumab for 10 minutes. Hepatocyte growth factor triggered phosphorylation of c-Met at Tyr1234/1235 and p44/42 MAPK in UM-SCC-1 cells. Surprisingly, we found less phosphorylation of c-Met at Tyr1234/1235 and p44/42 MAPK in HN5 cells after exposing them to recombinant HGF (30 ng/mL). Furthermore, ficlatuzumab reduced the phosphorylation of c-Met and p44/42 MAPK in HNSCC cells compared with cells treated with recombinant HGF alone (Figure 4). These data strongly suggest that inhibition of HGF by ficlatuzumab can deregulate MAPK signaling implicated in the regulation of HNSCC cell proliferation.
Figure 4. Inhibition of TAF-Induced c-Met and p44/42 MAPK Phosphorylation in HNSCC Cells.
Head and neck squamous carcinoma (HNSCC) cells were serum starved for 72 hours and treated with Dulbecco modified Eagle medium alone and recombinant hepatocyte growth factor (HGF) (30 ng/mL) with or without ficlatuzumab (100 µg/mL) for 10 minutes. Cell lysates were analyzed for phospho-c-Met and p44/42 mitogen-activated protein kinase (MAPK) by immunoblotting. Ficlatuzumab inhibited HGF-mediated phosphorylation of c-Met and p44/42 MAPK in (A) HN5, (B) UM-SCC-1, and (C) OSC-19 cells.
Discussion
The proto-oncogene c-MET encodes a receptor tyrosine kinase that is activated by the primary ligand, HGF. Ligand binding activates signaling cascades, including MAPK, RAS, PI3K, STAT3, and NOTCH, resulting in cell morphogenesis, motility, growth, and survival. c-Met and HGF are reported to be overexpressed in over 80% of HNSCCs and increased c-Met copy numbers in 13% of HNSCC tumor samples.12,18 c-Met expression is a prognostic biomarker in HPV-negative HNSCC, with overexpression correlating with reduced disease-free and overall survival.19,20 Furthermore, c-Met expression has been implicated in resistance to radiation, cisplatin, and cetuximab therapy.21–23 c-Met overexpression results in enhanced cell motility, angiogenesis, and invasion/metastases, and therefore is an important therapeutic target.
The c-Met receptor is unique among receptor tyrosine kinases in which a single amino acid sequence (Y1349VHVNATY1356VNV) forms a docking region for several SH-2 domain-containing signal transducers.24 It has been shown that replacement of Tyr1349 and Tyr1356 within this multifunctional docking site by phenylalanines blocks HGF-mediated cell proliferation, migration, and invasion.25,26 Furthermore, phosphorylation of these tyrosine residues is critical for binding of most transducers.26 Even though the tyrosine kinase domain of the c-Met receptor is critical for the actual activation of downstream transducers, binding of ligand to the receptor is probably necessary to bring the transducers in position to be activated. Ligand binding to c-Met recruits ATP to the tyrosine kinase domain of the receptor and leads to phosphorylation of tyrosine residues 1230, 1234, and 1235, which is indispensable for downstream kinase activity.27 Accordingly, in most studies, prevention of ligand binding effectively abrogates the c-Met-mediated signaling.28
Ficlatuzumab is a humanized monoclonal IgG1 antibody with a high affinity and specificity for HGF. In a preclinical trial13 using a murine model and glioblastoma cells, ficlatuzumab provided an important survival advantage compared with the control group as a single-agent therapy, and also when combined with a traditional chemotherapy agent, temozolomide. In a phase 1 trial15,29 in advanced solid tumors and multiple myeloma, ficlatuzumab was fairly well tolerated, with the most common adverse effects of peripheral edema, fatigue, and nausea up to a maximum dose of 20 mg/kg, administered every 2 weeks. In a phase 2 trial30 in Asian patients with adenocarcinoma of the lung, ficlatuzumab was tested in combination with the tyrosine kinase inhibitor gefitinib. No benefit in progression-free survival was seen in the overall group.29,30 In retrospective analyses of patients with activating mutations of EGFR, there was a significant benefit in progression-free survival and overall survival seen with ficlatuzumab and gefitinib compared with gefitinib alone.29–31 A phase 2, global, randomized, double-blind clinical trial evaluating ficlatuzumab in combination with erlotinib (Tarceva) in patients with NSCLC with activating EGFR mutations is currently under way (NCT02318368).
An ongoing phase 1 clinical trial combining ficlatuzumab with chemoradiotherapy is also underway (Ficlatuzumab, Cisplatin and IMRT in Locally Advanced HNSCC [NCT02277184]). Resistance to the epidermal growth factor receptor (EGFR) has been reported to be mediated by c-Met activation.32 A combination of ficlatuzumab and the EGFR inhibitor cetuximab is being tested in a phase 1 trial in patients with recurrent/metastatic HNSCC (NCT02277197). There are several receptor tyrosine kinase inhibitors and monoclonal antibodies that are currently being tested against c-Met and HGF in early-phase clinical trials.
Conclusions
Herein, we have demonstrated for the first time, to our knowledge, that the HGF-targeted antibody ficlatuzumab inhibits TAF-facilitated HNSCC cell migration, invasion, and proliferation (Figure 5). Furthermore, ficlatuzumab mitigated the effects of TAF-secreted HGF-mediated c-Met phosphorylation and downstream signaling through MAPK. Together, these findings strongly suggest that ficlatuzumab may be efficacious in treating HNSCC.
Figure 5. Model Summarizing the Antitumor Effects of Ficlatuzumab on Paracrine Stimulation of HNSCC by TAF-Secreted HGF.
Hepatocyte growth factor (HGF) binds to the tyrosine kinase receptor. Activation of downstream signal molecules facilitates head and neck squamous carcinoma (HNSCC) cell proliferation, migration, and invasion. Ficlatuzumab, a monoclonal antibody binds HGF, downregulating c-Met and downstream targets, inhibiting HNSCC proliferation, migration and invasion. TAF indicates tumor-associated fibroblast.
Acknowledgments
Funding/Support: This study was funded by the Department of Otolaryngology, University of Kansas Medical Center and University of Kansas Cancer Center’s CCSG (1-P30-CA168524-02).
Role of the Funder/Sponsor: The funding souces had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We acknowledge support from the University of Kansas Cancer Center’s Biospecimen Repository Core Facility staff for helping obtain human specimens. We thank Shrikant Anant, PhD, for access to the microplate reader.
Footnotes
Author Contributions: Drs Kumar and Kandl contributed equally to this work. Dr Kumar had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hamilton, Shnayder, Kakarala, Ledgerwood, Sun, Girod, Thomas.
Acquisition, analysis, or interpretation of data: Kumar, Kandl, Hamilton, Tsue, Ledgerwood, Sun, Huang, Thomas.
Drafting of the manuscript: Kumar, Kandl, Hamilton, Shnayder, Thomas.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Kumar.
Obtained funding: Thomas.
Administrative, technical, or material support: Kandl, Hamilton, Kakarala, Sun, Girod.
Study supervision: Thomas.
Conflict of Interest Disclosures: None reported.
Drs Shnayder, Tsue, Kakarala, and Ledgerwood supervised the collection of tissue from which cells were isolated for the preclinical studies performed.
Previous Presentation: This article was presented at the Annual Meeting of the American Head and Neck Society; April 22, 2015; Boston, Massachusetts.
REFERENCES
- 1.Pignon JP, Bourhis J, Domenge C, Designé L MACH-NC Collaborative Group. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data: meta-analysis of chemotherapy on head and neck cancer. Lancet. 2000;355(9208):949–955. [PubMed] [Google Scholar]
- 2.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359(11):1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 3.Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol. 2011;3(1 suppl):S21–S35. doi: 10.1177/1758834011422557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leef G, Thomas SM. Molecular communication between tumor-associated fibroblasts and head and neck squamous cell carcinoma. Oral Oncol. 2013;49(5):381–386. doi: 10.1016/j.oraloncology.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler SE, Shi H, Lin F, et al. Enhancement of head and neck squamous cell carcinoma proliferation, invasion, and metastasis by tumor-associated fibroblasts in preclinical models. Head Neck. 2014;36(3):385–392. doi: 10.1002/hed.23312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kan M, Zhang GH, Zarnegar R, et al. Hepatocyte growth factor/hepatopoietin A stimulates the growth of rat kidney proximal tubule epithelial cells (RPTE), rat nonparenchymal liver cells, human melanoma cells, mouse keratinocytes and stimulates anchorage-independent growth of SV-40 transformed RPTE. Biochem Biophys Res Commun. 1991;174(1):331–337. doi: 10.1016/0006-291x(91)90524-b. [DOI] [PubMed] [Google Scholar]
- 7.Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990;111(5, pt 1):2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naldini L, Weidner KM, Vigna E, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10(10):2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida D, Kawamata H, Omotehara F, et al. Role of HGF/c-met system in invasion and metastasis of oral squamous cell carcinoma cells in vitro and its clinical significance. Int J Cancer. 2001;93(4):489–496. doi: 10.1002/ijc.1368. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita J, Ogawa M, Yamashita S, et al. Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res. 1994;54(7):1630–1633. [PubMed] [Google Scholar]
- 11.Siegfried JM, Weissfeld LA, Singh-Kaw P, Weyant RJ, Testa JR, Landreneau RJ. Association of immunoreactive hepatocyte growth factor with poor survival in resectable non-small cell lung cancer. Cancer Res. 1997;57(3):433–439. [PubMed] [Google Scholar]
- 12.Knowles LM, Stabile LP, Egloff AM, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15(11):3740–3750. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittra ES, Fan-Minogue H, Lin FI, et al. Preclinical efficacy of the anti-hepatocyte growth factor antibody ficlatuzumab in a mouse brain orthotopic glioma model evaluated by bioluminescence, PET, and MRI. Clin Cancer Res. 2013;19(20):5711–5721. doi: 10.1158/1078-0432.CCR-12-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Arcangelo M, Cappuzzo F. Focus on the potential role of ficlatuzumab in the treatment of non-small cell lung cancer. Biologics. 2013;7:61–68. doi: 10.2147/BTT.S28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patnaik A, Weiss GJ, Papadopoulos KP, et al. Phase I ficlatuzumab monotherapy or with erlotinib for refractory advanced solid tumours and multiple myeloma. Br J Cancer. 2014;111(2):272–280. doi: 10.1038/bjc.2014.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin CJ, Grandis JR, Carey TE, et al. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck. 2007;29(2):163–188. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 18.Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. Cancer invasion and metastasis: molecular and cellular perspective. In: Jandial R, editor. Metastatic Cancer: Clinical and Biological Perspectives. Baton Raton, FL: CRC Press; 2013. [Google Scholar]
- 19.Seiwert TY, Jagadeeswaran R, Faoro L, et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 2009;69(7):3021–3031. doi: 10.1158/0008-5472.CAN-08-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao D, Wang SH, Feng Y, Hua CG, Zhao J, Tang XF. Intratumoral c-Met expression is associated with vascular endothelial growth factor C expression, lymphangiogenesis, and lymph node metastasis in oral squamous cell carcinoma: implications for use as a prognostic marker. Hum Pathol. 2011;42(10):1514–1523. doi: 10.1016/j.humpath.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Lo Muzio L, Farina A, Rubini C, et al. Effect of c-Met expression on survival in head and neck squamous cell carcinoma. Tumour Biol. 2006;27(3):115–121. doi: 10.1159/000092716. [DOI] [PubMed] [Google Scholar]
- 22.De Bacco F, Luraghi P, Medico E, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst. 2011;103(8):645–661. doi: 10.1093/jnci/djr093. [DOI] [PubMed] [Google Scholar]
- 23.Sun S, Wang Z. Head neck squamous cell carcinoma c-Met+ cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011;129(10):2337–2348. doi: 10.1002/ijc.25927. [DOI] [PubMed] [Google Scholar]
- 24.Krumbach R, Schüler J, Hofmann M, Giesemann T, Fiebig HH, Beckers T. Primary resistance to cetuximab in a panel of patient-derived tumour xenograft models: activation of MET as one mechanism for drug resistance. Eur J Cancer. 2011;47(8):1231–1243. doi: 10.1016/j.ejca.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77(2):261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 26.Bardelli A, Longati P, Gramaglia D, Stella MC, Comoglio PM. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene. 1997;15(25):3103–3111. doi: 10.1038/sj.onc.1201561. [DOI] [PubMed] [Google Scholar]
- 27.Bardelli A, Longati P, Gramaglia D, et al. Uncoupling signal transducers from oncogenic MET mutants abrogates cell transformation and inhibits invasive growth. Proc Natl Acad Sci U S A. 1998;95(24):14379–14383. doi: 10.1073/pnas.95.24.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longati P, Bardelli A, Ponzetto C, Naldini L, Comoglio PM. Tyrosines1234–1235 are critical for activation of the tyrosine kinase encoded by the MET proto-oncogene (HGF receptor) Oncogene. 1994;9(1):49–57. [PubMed] [Google Scholar]
- 29.Blumenschein GR, Jr, Mills GB, Gonzalez-Angulo AM. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J Clin Oncol. 2012;30(26):3287–3296. doi: 10.1200/JCO.2011.40.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mok TSK, Geater SL, Agarwal S, et al. A randomized phase (PH) 2 study with exploratory biomarker analysis of ficlatuzumab (F) a humanized hepatocyte growth factor (HGF) inhibitory mab in combination with gefitinib (G) vs G in Asian patients (Pts) with lung adenocarcinoma (LA) Ann Oncol. 2012;23(9):2012–2023. [Google Scholar]
- 31.Mok TSK, Geater SL, Chang G-C, et al. Efficacy analysis of gefitinib ficlatuzumab in serum proteomic based subgroups of patients with previously untreated lung adenocarcinoma. Ann Oncol. 2014;25(70):iv58–iv84. [Google Scholar]
- 32.Mok TSK, Wu Y-L, Novello S, et al. Association between tumor EGFR and KRAS mutation status and clinical outcomes in NSCLC patients randomized to sorafenib plus best supportive care (BSC) or BSC alone: subanalysis of the Phase III Mission Trial. Ann Oncol. 2012;23(9):ixe1–ixe30. [Google Scholar]
- 33.Stabile LP, He G, Lui VW, et al. c-Src activation mediates erlotinib resistance in head and neck cancer by stimulating c-Met. Clin Cancer Res. 2013;19(2):380–392. doi: 10.1158/1078-0432.CCR-12-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]