Abstract
Well-balanced mitochondrial fission and fusion processes are essential for nervous system development. Loss of function of the main mitochondrial fission mediator, dynamin-related protein 1 (Drp1), is lethal early during embryonic development or around birth, but the role of mitochondrial fission in adult neurons remains unclear. Here we show that inducible Drp1 ablation in neurons of the adult mouse forebrain results in progressive, neuronal subtype-specific alterations of mitochondrial morphology in the hippocampus that are marginally responsive to antioxidant treatment. Furthermore, DRP1 loss affects synaptic transmission and memory function. Although these changes culminate in hippocampal atrophy, they are not sufficient to cause neuronal cell death within 10 weeks of genetic Drp1 ablation. Collectively, our in vivo observations clarify the role of mitochondrial fission in neurons, demonstrating that Drp1 ablation in adult forebrain neurons compromises critical neuronal functions without causing overt neurodegeneration.
In addition to their crucial importance in energy conversion, mitochondria serve many other housekeeping functions, including calcium buffering, amino-acid and steroid biosynthesis as well as fatty acids beta-oxidation and regulation of cell death. During the past decade, it has become increasingly clear that processes regulating mitochondrial morphology and ultrastructure are influenced by specific cellular requirements upon which mitochondria, in a precisely regulated manner, undergo fusion and division events.1 Maintaining this balance is especially important for highly energy-consuming, polarized cells such as neurons, where single organellar units sprouting from the mitochondrial network are transported along the cytoskeleton into dendrites and spines to meet local energy requirements.2 In addition, elaborate quality-control mechanisms also rely on mitochondrial dynamics: whereas defective organelles are sequestered by fission, enabling their removal from the mitochondrial network,3, 4 fusion supports qualitative homogeneity of the syncytium through complementation.5
Mitochondrial fusion and fission are mediated by large GTPases of the dynamin superfamily.6 The outer mitochondrial membrane mitofusins 1 (MFN1) and 2 (MFN2) tether mitochondrial membranes by homodimer or heterodimer formation,7 thereby initiating fusion of the organelles, a process that also involves the inner mitochondrial membrane-associated GTPase Optic Atrophy 1.8 In addition, MFN2 also mediates contacts between mitochondria and endoplasmic reticulum.9 The only known mammalian mitochondrial fission protein, Dynamin-Related Protein 1 (Drp1), translocates upon dephosphorylation by calcineurin10 to fission sites where it binds to mitochondrial fission factor.11 Drp1 translocation is preceded by ER membranes wrapping around mitochondria to constrict the organelles,12 thereby facilitating the formation of multimeric Drp1 complexes that, upon GTP hydrolysis, further tighten to complete the process of mitochondrial fission.13
Genetic evidence in mice and humans indicates that mitochondrial dynamics are crucially important in neurons: in humans, a sporadic dominant-negative DRP1 mutation caused a lethal syndromic defect with abnormal brain development;14 similarly, constitutive Drp1 knockout in the mouse brain leads to lethal neurodevelopmental defects.15, 16 Although the crucial role of Drp1 during brain development is undisputed, studies on Drp1 function in postmitotic (adult) neurons are scarce; likewise, Drp1 ablation studies in primary cultures have so far failed to yield a conclusive picture. In vitro, Drp1 ablation is reported to lead to a super-elongated neuroprotective17, 18, 19, 20, 21, 22, 23, 24 or an aggregated mitochondrial phenotype associated with neurodegeneration.15, 16, 25, 26, 27 These discrepancies are probably due to different experimental conditions: neuronal health is indeed influenced by the onset and duration of Drp1 inhibition, which varies considerably among the cited reports,28 and different types of neuronal cultures studied display different sensitivity to Drp1 inhibition. In vivo, Drp1 ablation in Purkinje cells results in oxidative stress and neurodegeneration,29 demonstrating that Drp1 is essential for postmitotic neurons' health. In contrast, transient pharmacological Drp1 inhibition is neuroprotective in several mouse ischemia models, indicating that temporarily blocking mitochondrial fission holds therapeutic potential.30, 31, 32
To elucidate the consequences of blocked mitochondrial fission in the central nervous system in vivo, we bypassed the critical role of Drp1 during brain development by generating Drp1flx/flx mice15 expressing tamoxifen-inducible Cre recombinase under the control of the CaMKIIα promoter.33 Upon induced Drp1 deletion in postmitotic adult mouse forebrain neurons, mice develop progressive, neuronal subtype-specific alterations in mitochondrial shape and distribution in the absence of overt neurodegeneration. In addition, respiratory capacity, ATP content, synaptic reserve pool vesicle recruitment as well as spatial working memory are impaired, demonstrating that severely dysregulated mitochondrial dynamics can compromise critical neuronal functions in vivo without causing neuronal cell death.
Results
Inducible Drp1 ablation in the adult mouse forebrain
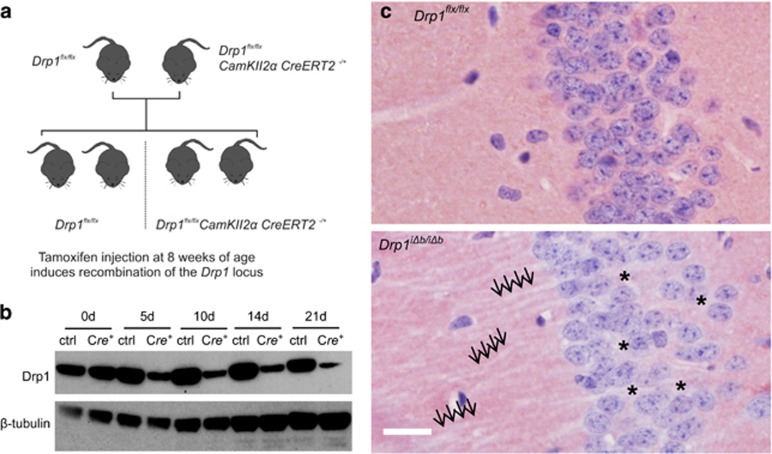
In order to study the effect of mitochondrial fission ablation in adult neurons, we generated a model of inducible Drp1 ablation in the forebrain by crossing mice with loxP sites inserted in the Drp1 gene (Drp1flx/flx)15 with mice expressing a gene for a tamoxifen-inducible Cre recombinase fusion protein under the control of the CaMKIIα promoter (Figure 1a),33 limiting recombination primarily to the forebrain. Eight-week-old offspring of these crossings were injected with tamoxifen on 5 consecutive days (Drp1 inducibly deleted in the brain, Drp1iΔb/iΔb), resulting in maximum DRP1 depletion in the hippocampus within 14 days (Figure 1b). Immunohistochemical DRP1 staining in Drp1iΔb/iΔb animals 4 weeks posttamoxifen injection (p.t.i.) confirmed that DRP1 was lacking specifically in hippocampal neurons (Figure 1c) and crosses of Drp1iΔb/iΔb mice with a reporter strain further confirmed the recombination following tamoxifen injection (Supplementary Figure S1A).
Figure 1.
Drp1 ablation in postmitotic forebrain neurons. (a) Drp1flx/flx and Drp1flx/flx CaMKIIα CreERT2−/+ mice were crossed, resulting in a Mendelian distribution of alleles. At 8 weeks of age, animals were injected with tamoxifen for 5 consecutive days to induce recombination of the floxed Drp1 locus. (b) Mice of the indicated genotype were killed at the indicated time points p.t.i. Hippocampal lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted using the indicated antibodies. (c) Immmunohistochemical staining (NovaRED (Vectorlabs, Burlingame, CA, USA), counterstain hematoxylin) for Drp1 was performed on hippocampal CA1 formalin-fixed, paraffin-embedded tissue sections of Drp1iΔb/iΔb mice 4 weeks p.t.i. Arrows indicate unstained hippocampal dendrites. Scale bar, 5 μm
Drp1 ablation causes progressive changes in mitochondrial morphology
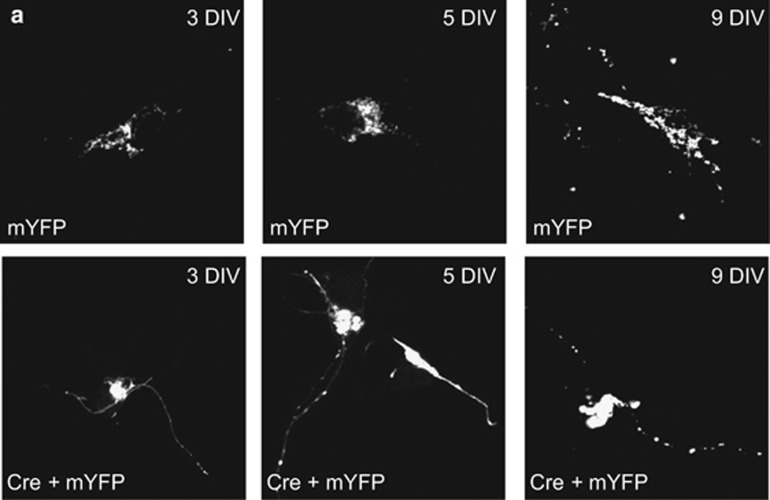
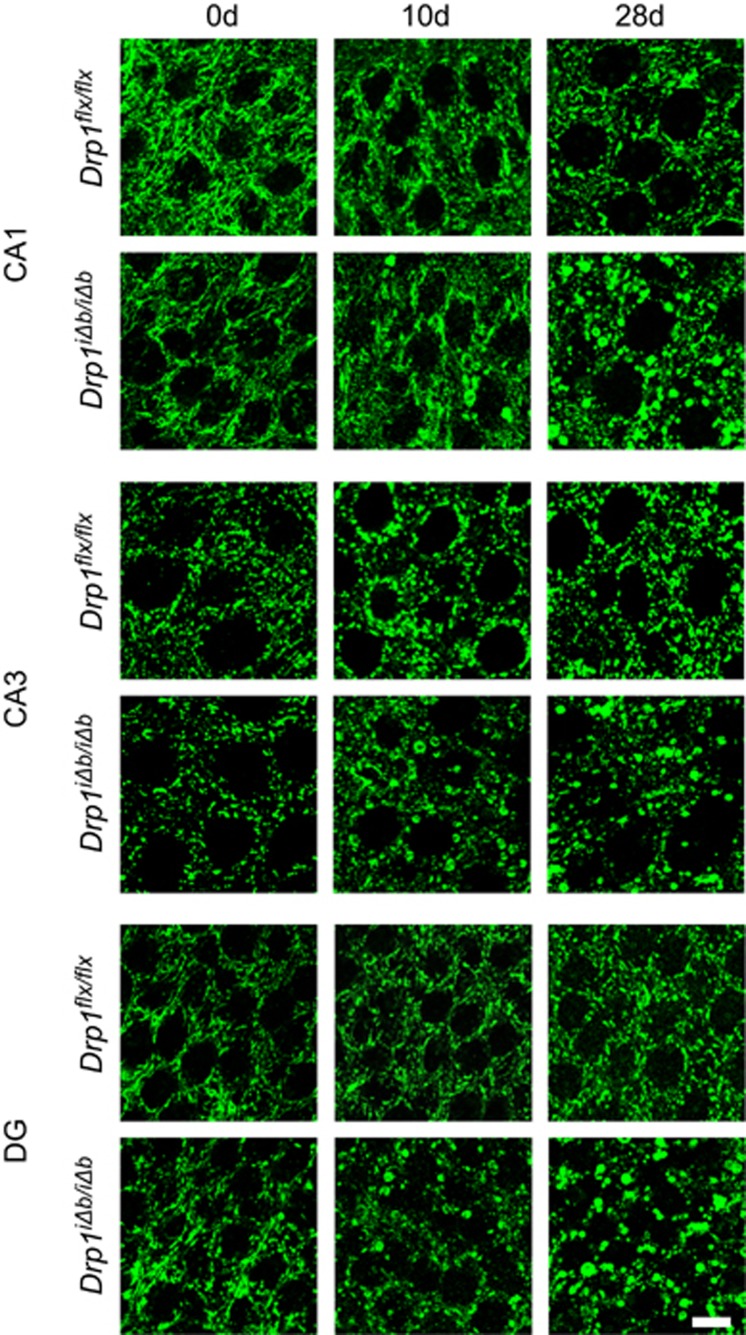
To characterize the consequences of inducible Drp1 ablation, we turned to an in vitro model of primary cortical neurons isolated from Drp1flx/flx mice where we could monitor by confocal microscopy the consequences of Cre recombinase-induced Drp1 ablation by co-transfecting it with a mitochondria-targeted yellow fluorescent protein (mtYFP). Starting at day 3 following in vitro recombination, mitochondria progressively clustered and aggregated in the soma. Fewer organelles were found within the neurite compartment, where mitochondria also appeared larger in comparison to sham-transfected controls (Figure 2). Comforted by these results, we moved to adult hippocampal neurons in vivo to study mitochondrial morphology by immunohistochemistry. At day 10 after Drp1 ablation, enlarged spherical mitochondria started to aggregate in the perikarya of hippocampal neurons. Whereas 10 days p.t.i. enlarged mitochondria clustered in the perikarya of approximately only 5% of CA1 neurons, in the dentate gyrus (DG) and CA3 hippocampal neurons, mitochondrial morphology changes occurred earlier after Drp1 ablation. Conversely, 4 weeks p.t.i., neuronal mitochondrial morphology was heavily altered in all three neuronal subpopulations, mitochondria of CA3 pyramidal neurons appearing considerably less filamentous and more fragmented compared with CA1 and DG neurons (Figure 3). These findings demonstrate that blocking fission alters mitochondrial morphology and distribution in a neuronal subtype-specific manner.
Figure 2.
Mitochondrial morphology in primary neuronal cultures after Drp1 ablation. (a) Primary cortical neurons isolated from Drp1flx/flx E17 embryos were co-electroporated with mtYFP- and Cre-expression constructs prior to seeding. Images were taken at the indicated time points of differentiation
Figure 3.
Mitochondrial morphology in the hippocampus after Drp1 ablation. Mice of the indicated genotype were killed at the indicated times p.t.i. Coronal sections of formalin-fixed, paraffin-embedded brains of Drp1iΔb/iΔb and control mice were immunostained for cytochrome c oxidase subunit 1. Images show CA1 and CA3 pyramidal neurons and DG granule cells. Scale bar, 10 μm
Synaptic transmission is impaired in Drp1-deficient CA1 pyramidal neurons
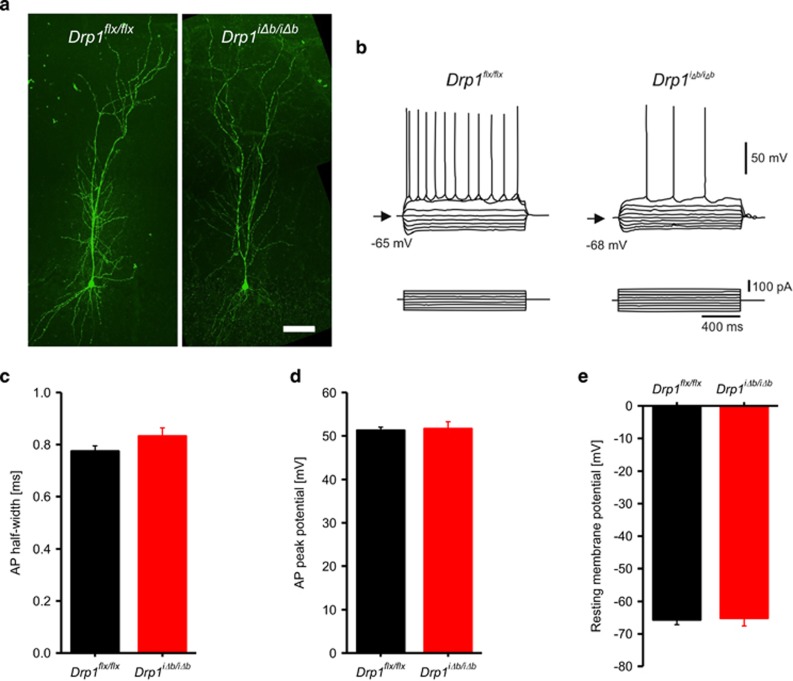
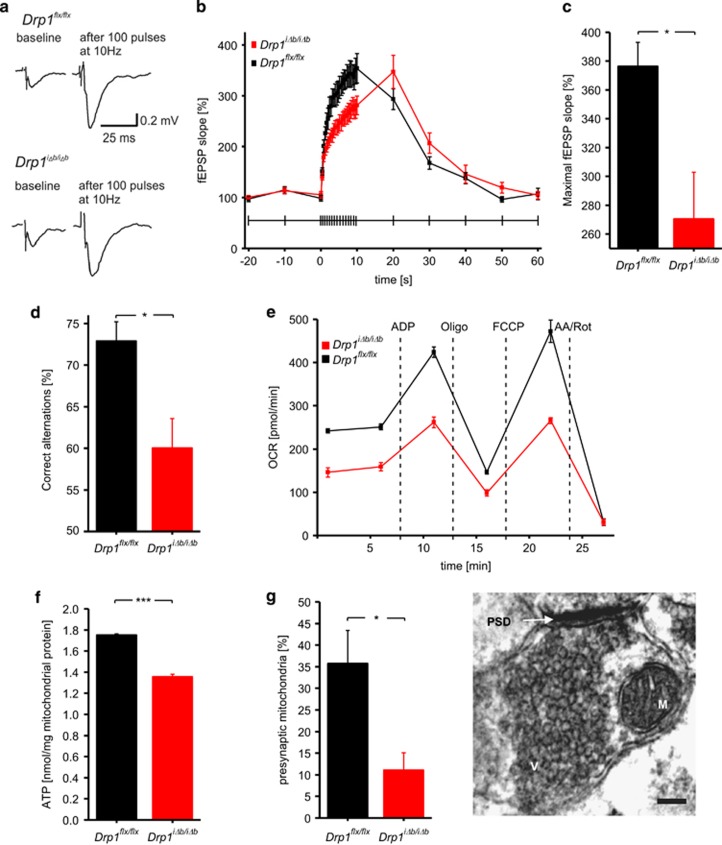
We next addressed whether the observed mitochondrial morphological defects resulted in any functional consequence by behavioral and electrophysiological tests. Biocytin filling indicated that dendritic morphology of hippocampal neurons was not altered 4 weeks p.t.i. (Figure 4a). Accordingly, whole-cell patch-clamp recordings revealed that CA1 pyramidal Drp1iΔb/iΔb neurons display normal resting membrane potential and action potential peak amplitudes as well as half-duration (Figures 4b and e). When we explored excitatory synaptic transmission by performing field potential recordings in the hippocampal Drp1iΔb/iΔb CA1 region, we observed that stimulation of the Schaffer collaterals evoked field excitatory postsynaptic potentials (fEPSPs) with normal paired-pulse facilitation (143%, n=10; Δt= 100 ms) similar to those observed in control mice (151%, n=6, P=0.52), indicating normal release probability. However, when synaptic transmission was challenged by application of 100 stimuli at either 10 or 100 Hz, frequency-dependent fEPSP facilitation was significantly reduced in Drp1iΔb/iΔb brain slices, where synaptic transmission also broke down more rapidly (Figures 5a and c; Supplementary Figures S1D and E). In addition, when subjected to a spontaneous alternation task (a behavioral assay for hippocampus-dependent working memory), short-term working memory was significantly impaired in Drp1iΔb/iΔb mice compared with their Drp1flx/flx littermates (Figure 5d), not because of impaired visual acuity and olfaction (Supplementary Figures S1F and G). These synaptic transmission deficits are similar to those observed in the neuromuscular junction of drp1-mutant Drosophila,34 which were attributed to a lack of synaptic ATP. We therefore measured oxygen consumption and ATP levels of hippocampal mitochondria and found oxygen consumption (Figure 5e) as well as ATP content (Figure 5f) to be reduced in Drp1-deficient samples. In addition, ultrastructural analysis by transmission electron microscopy (TEM) revealed a significant reduction in presynaptic mitochondria of hippocampal Drp1iΔb/iΔb neurons (Figure 5g). The combination of reduced mitochondrial content in presynaptic terminals and defective ATP production can explain the impaired synaptic function observed in Drp1iΔb/iΔb mice.
Figure 4.
Electrophysiological properties are not affected in Drp1-ablated neurons. (a) Acute hippocampal slices of Drp1iΔb/iΔb 4 weeks p.t.i. and control animals were prepared. CA1 pyramidal neurons were patch-clamped and infused with biocytin, which was revealed by immunofluorescence. Scale bar, 40 μm. Note the regular dendritic morphology. (b–d) One-second-long current steps of increasing amplitude were injected to induce action potentials in Drp1iΔb/iΔb 4 weeks p.t.i. and control CA1 pyramidal neurons; maximal amplitude and half width were plotted. Data represent average±S.E.M. of at least seven neurons. (e) Resting membrane potential of Drp1iΔb/iΔb 4 weeks p.t.i. and control CA1 pyramidal neurons. Data represent average±S.E.M. of at least seven neurons
Figure 5.
Impaired synaptic transmission in Drp1-ablated neurons. (a) Representative fEPSPs before and after 10 s of 10 Hz stimulation. fEPSPs were recorded in the CA1 stratum radiatum in Drp1iΔb/iΔb and control hippocampal slice cultures upon Schaffer collateral stimulation. (b) Mean slope of fEPSPs during the time course of a 10-s 10-Hz stimulation was plotted. Data represent average±S.E.M. of at least six neurons. (c) Maximal mean fEPSP slope after 10-s 10-Hz stimulation. Data represent average±S.E.M. of at least six neurons. (d) Drp1iΔb/iΔb 4 weeks p.t.i. and control animals were placed in an eight-arm radial maze, which they were left to systematically explore. Correct alternation of arm visits was scored. Data represent average±S.E.M. of at least six animals. (e) Oxygen consumption rate of isolated hippocampal mitochondria of Drp1iΔb/iΔb and control mice was measured with a Seahorse Bioscience XF24 Analyzer. Substances were injected at the indicated time points. Data represent average±S.E.M. of at least four animals whose hippocampi were pooled; measurements performed with at least six replicates. (f) ATP content of isolated hippocampal mitochondria of Drp1iΔb/iΔb and control mice as measured with a bioluminescence assay. Data represent average±S.E.M. of at least four animals whose hippocampi were pooled; measurements were performed with at least eight replicates. (g) Synapses in TEM images of Drp1iΔb/iΔb 4 weeks p.t.i. and control hippocampi were screened for presynaptic mitochondria. Data represent average±S.E.M. of at least 4 animals of which at least 100 synapses each were screened. TEM image shows a representative synaptic structure with a presynaptic mitochondrion (M), presynaptic vesicles (V) and a postsynaptic dense area (PSD). Scale bar, 150 nm. Asterisks denote P-values of an unpaired, two-tailed Student's t-test: *P<0.05
Forebrain-specific neuronal Drp1 ablation leads to hippocampal atrophy
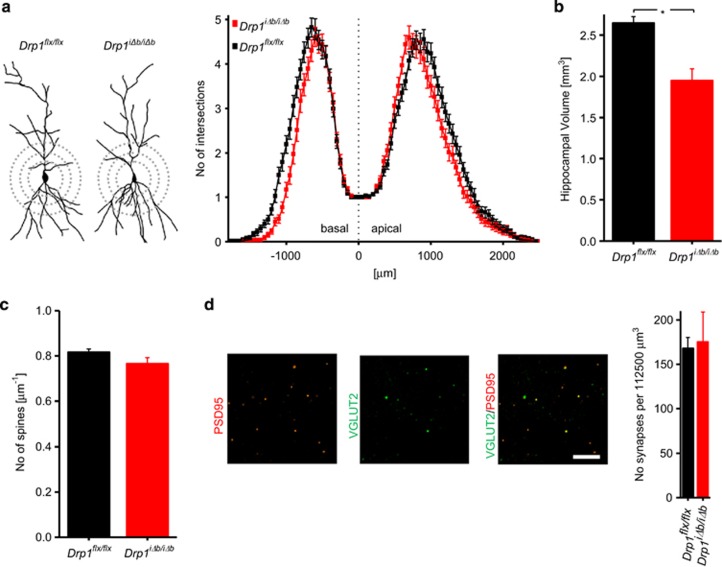
Several previous studies indicate that Drp1 ablation in vitro causes a reduction in the number of dendrites, spines, and synapses (reviewed by Oettinghaus et al.28). However, as no substantial changes in dendritic morphology were observed early after Drp1 ablation (4 weeks p.t.i.; Figure 4a), we performed Golgi silver impregnations on Drp1iΔb/iΔb brain sections 10 weeks p.t.i. Sholl analysis revealed that, although overall dendritic tree morphometry was unchanged, dendrites were shorter in Drp1iΔb/iΔb mice (Figure 6a), which was associated with decreased hippocampal volume (Figure 6b). In contrast, cortical or midbrain volumes did not change significantly (Supplementary Figure S1H). To test whether this hippocampal atrophy was associated with loss of spines, we quantified spine numbers on apical dendritic trees of Golgi-impregnated pyramidal CA1 neurons but did not find any difference (Figure 6c). Likewise, the number of synapses, as assessed by co-localization of fluorescent stains for presynaptic (VGLUT2) and postsynaptic (PSD95) markers in confocal Z-stacks, was normal (Figure 6d). Thus, the depletion of mitochondria from presynaptic hippocampal neuron terminals can account for the observed synaptic transmission impairments and the specific deficits in spatial working memory upon Drp1 ablation. Nevertheless, the maintenance of normal synapse and spine numbers 10 weeks following Drp1 ablation indicates that mature hippocampal neurons are able to cope with blocked mitochondrial fission with only mild functional alterations.
Figure 6.
Long-term Drp1 ablation causes hippocampal atrophy. (a) Representative camera lucida drawings and Sholl analysis of Golgi-stained Drp1iΔb/iΔb 10 weeks p.t.i. and control CA1 pyramidal neurons. Each data point represents the number of intersections of the dendritic tree with concentric perisomatic rings of increasing diameter (exemplified by dashed circles). Data represent average±S.E.M. of at least 100 neurons. (b) Hippocampal volume was recorded on H&E-stained, serial coronal cross-sections of Drp1iΔb/iΔb animals 8-weeks p.t.i. applying the Cavalieri principle. For cortical and midbrain volumes, only sections representing the coordinates bregma from 1.1 to −1.2 mm were considered. Data represent average±S.E.M. of at least five animals. (c) Number of spines visualized by Golgi staining per μm dendrite length (apical dendritic tree of CA1 pyramidal neurons). Data represent average±S.E.M. of at least 3 animals of which 1000 spines were counted. (d) Coronal cross-sections of Drp1iΔb/iΔb 10 weeks p.t.i. and control brains co-stained with presynaptic VGLUT2 (green) and postsynaptic marker PSD95 (red). A 150 × 150 × 5 μm3 confocal image stack in the CA1 stratum radiatum was recorded, and the number of overlapping puncta was determined. Data represent average±S.E.M. of at least five animals of which four stacks each were recorded. Scale bar, 5 μm. Asterisks denote P-values of an unpaired, two-tailed Student's t-test: *P<0.05
Adult forebrain neurons do not degenerate within 10 weeks of Drp1 ablation
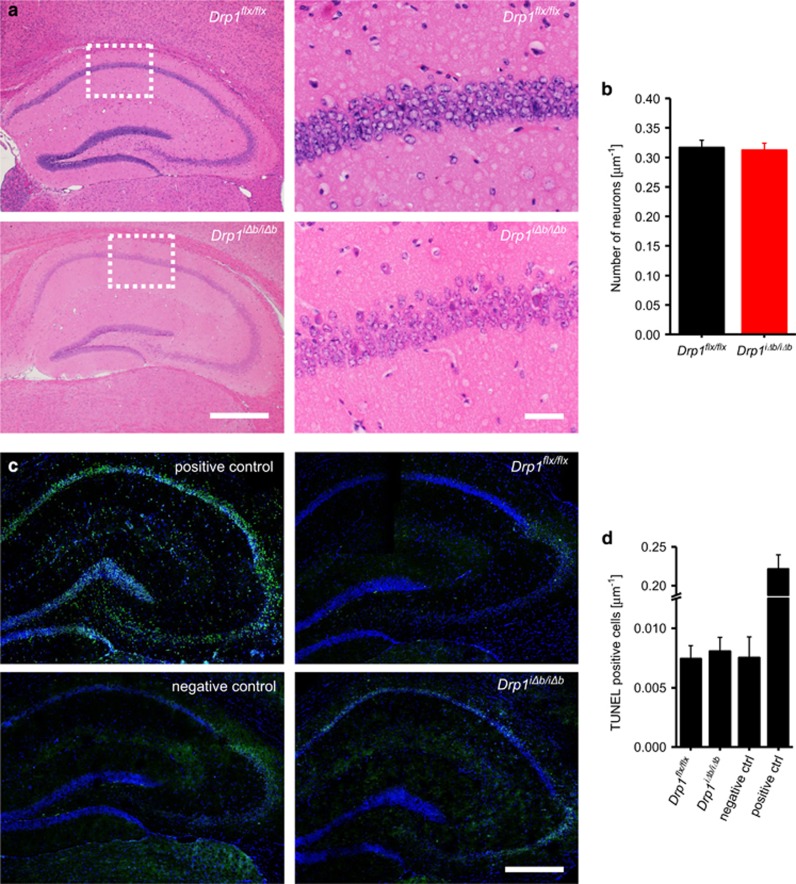
We next verified whether the above-reported changes caused by Drp1 ablation in the hippocampus led to neurodegeneration. Hippocampal neurons were qualitatively and quantitatively normal in hematoxylin–eosin (H&E)-stained hippocampal tissue sections (Figures 7a and b), with no signs of condensed eosinophilic neurons, chromatinolysis or apoptotic bodies. In addition, in situ TUNEL (TdT-mediated dUTP-biotin nick end labeling) did not detect any evidence of increased cell death in the hippocampus 10 weeks p.t.i. (Figures 7c and d). As neurons are believed to be especially dependent on ATP produced by oxidative phosphorylation, we wondered how they could compensate the reduced ATP production (Figure 5f) to avoid neurodegeneration. Comparative mass spectrometry analyses revealed that levels of glycolysis-related proteins were significantly changed, the rate-limiting enzyme Hexokinase 1 being markedly upregulated (4.37-fold; see Supplementary Table S1). These results indicate that neurons attempt to cope with the metabolic consequences of Drp1 ablation by upregulating glycolysis.
Figure 7.
Drp1 ablation does not lead to hippocampal neurodegeneration. (a) H&E staining of coronal cross-sections of formalin-fixed, paraffin-embedded Drp1iΔb/iΔb 10 weeks p.t.i. and control brains showing the hippocampus and a magnification of the CA1 region. Scale bars, 1 mm (left) and 50 μm (right). (b) Neuronal nuclei in the CA1 region were quantified manually. Data represent average±S.E.M. of at least 5 animals of which at least 200 nuclei each were counted. (c) In situ TUNEL of coronal cross-sections of frozen Drp1iΔb/iΔb 10 weeks p.t.i. and control brains showing hippocampus. Positive control was preincubated with DNAse I; negative control incubated without terminal deoxynucleotidyl transferase. Scale bar, 1 mm. (d) Neuronal nuclei in the CA1 region were quantified over a 250-μm length. Data represent average±S.E.M. of at least five animals
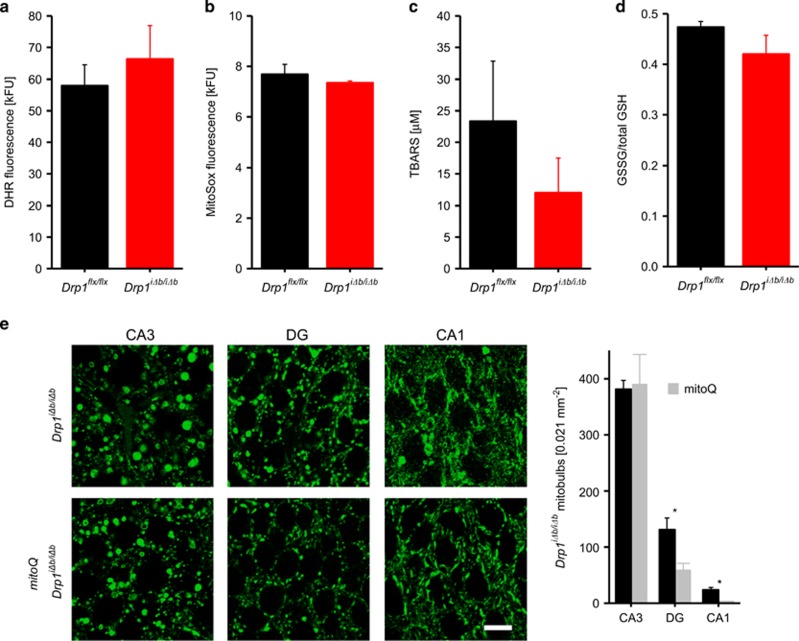
Although Drp1 ablation in Purkinje cells29 is accompanied by macroautophagy inhibition and reactive oxygen species (ROS) accumulation, extensive TEM analyses did not reveal significant autophagosome accumulation in the brain sections from Drp1iΔb/iΔb mice; of note, these consisted mostly of double-membrane structures measuring 500 nm (i.e., too small to accommodate mitochondria; Supplementary Figure S2). Accordingly, comparative mass spectrometry in hippocampal neurons did not reveal significant changes in autophagy-related proteins, including p62 and LC3 (Supplementary Table S2). Moreover, levels of ROS (measured by dihydrorhodamine and MitoSox fluorescence on isolated mitochondria), of thiobarbituric acid-reactive substances (TBARS), indicators of lipid peroxidation, as well as of oxidized (versus total) glutathione, were unchanged in mouse brain homogenates 4 weeks p.t.i. (Figures 8a–d). Indeed, our comparative mass spectrometry analyses revealed that 11 out of the 48 detected oxidative stress-associated proteins were upregulated in Drp1iΔb/iΔb brains 10 weeks p.t.i. (Supplementary Table S3). Ten of these proteins are regulated by the transcription factor nuclear factor erythroid 2 related factor (NRF2), whose inhibitor KEAP1 (Kelch-like ECH-associated protein 1) was significantly downregulated (fold change: 0.38; Q: 0.002; Supplementary Table S3). Among the upregulated proteins, we identified several glutathione S-transferases, participating in solubilizing peroxidized lipids and xenobiotics, and the multidrug resistance protein 1A capable of removing toxic components from the cytosol. In contrast, cytosolic and mitochondrial thioredoxins, NRF2 target genes that help reducing oxidized protein, were downregulated. These results indicate that Drp1 ablation in postmitotic neurons leads to a moderate activation of cellular antioxidant systems, possibly explaining why ROS levels were not increased but testifying that indeed also in forebrain neurons Drp1 ablation leads to ROS production. As in Purkinje cells ROS are involved in the formation of enlarged spherical mitochondrial bodies (mitobulbs),29 we decided to analyze whether treatment of mice with the mitochondrially targeted antioxidant mitoQ35 for 10 days p.t.i. could counteract the mitochondrial morphology phenotype. MitoQ significantly decreased mitobulbs in hippocampal CA1 and granule neurons of the DG (Figure 8e). However, in CA3 neurons transformation of mitochondria into mitobulbs is already complete at day 10 after Drp1 ablation (see Figure 3), and no mitoQ effect was observed, indicating that antioxidant treatment can merely delay the formation of mitobulbs but is not sufficient to suppress it.
Figure 8.
The antioxidant mitoQ ameliorates mitochondrial morphology in Drp1-deficient CA1 and DG hippocampal neurons. (a and b) Oxidative stress levels represented by dihydrorhodamine (DHR) and MitoSox fluorescence in cortical and hippocampal tissue homogenates of Drp1iΔb/iΔb 4 weeks p.t.i. and control mice. Data represent average±S.E.M. of at least four animals (tissue pooled), and measurements were performed with a minimum of four replicates. (c) Thiobarbituric acid-reactive substance (TBARS) levels, reflecting lipid peroxidation, in Drp1iΔb/iΔb 4 weeks p.t.i. and control cortical and hippocampal lysates measured by colorimetric assay. Data represent average±S.E.M. of at least six animals. (d) Ratio of oxidized to total glutathione in hippocampal lysates of Drp1iΔb/iΔb 4 weeks p.t.i. and control mice measured by colorimetric assay. Data represent average±S.E.M. of at least six animals. (e) Drp1iΔb/iΔb animals treated or not with daily mitoQ injections of 20 mg/kg i.p. were killed 10 days p.t.i.. and coronal cross-sections of formalin-fixed, paraffin-embedded brains immunostained for cytochrome c oxidase subunit 1. Mitobulbs of randomly selected areas within the indicated hippocampal subregions were quantified. Scale bar, 10 μm. Data represent average±S.E.M. of at least four animals. Asterisks denote P-values of an unpaired, two-tailed Student's t-test. *P<0.05
In conclusion, unlike Purkinje cells, adult forebrain neurons respond to the increased ROS formation caused by Drp1 ablation by upregulating the cellular antioxidant defenses that partly neutralize ROS accumulation.
Discussion
Mitochondrial fragmentation is a hallmark of apoptosis and accordingly of several neurodegenerative disorders. Conversely, the dynamin-related GTPase Drp1 is indispensable for neuronal maturation and brain development15, 16 and for cerebellar Purkinje cell survival.29 Our data conversely demonstrate that adult forebrain and hippocampal neurons display an increased functional reserve that allows them to survive Drp1 ablation.
In order to bypass the requirement for balanced mitochondrial dynamics during neuronal differentiation and brain development,15, 16 we devised a model of acute, inducible Drp1 ablation in adult forebrain, including hippocampal neurons. Upon tamoxifen-induced Drp1 ablation, mitochondria appeared enlarged and perinuclearly confined. These neurons were unexpectedly able to cope well with Drp1 ablation: changes in neuronal morphology, oxidative stress and cell death were negligible, whereas the reduction in localized ATP supply to presynaptic terminals impaired synaptic transmission, resulting in early memory defects.
The first visible phenotype of Drp1iΔb/iΔb mice, presenting as early as 4 weeks after tamoxifen administration, was a defect in spatial working memory, which could be traced back to the impairment in synaptic transmission upon sustained stimulation. Accordingly, and in line with the fact that defects in mitochondrial fission impair mitochondrial transport in neurites, presynaptic mitochondria were decreased. A similar electrophysiological phenotype associated with abnormal mitochondrial morphology was observed at neuromuscular junctions of drp1 mutant flies.34 Although that phenotype has primarily been attributed to ATP-dependent effects on reserve pool vesicle recycling owing to the lack of mitochondria at synaptic boutons and the overall reduction in oxidative phosphorylation, DRP1 may also be directly involved in synaptic vesicle formation, as shown more recently in mouse hippocampal neurons.36 Resting electrophysiological properties as well as paired-pulse facilitation, which were all unaffected, argue against a contribution of calcium-buffering defects to the overall phenotype (recently reviewed in Williams et al.37).
A predictable consequence of inhibiting mitochondrial fission was the change in mitochondrial network morphology, with predominantly enlarged spherical mitochondria that clustered around the nucleus. Of note, while previous reports in Purkinje cells29 attributed these ‘mitobulbs' to oxidative stress ensuing from defects in autophagy, we did not detect evidence suggesting a significant blockage of the latter in vivo, nor the presence of oxidative markers. Therefore, our data indicate that neurons in vivo are supported by the brain milieu to keep autophagy at a minimum level, even under stress conditions. This is consistent with reports that autophagy is an extremely rare event in neurons in vivo, even after 48 h of starvation.38 Even so, buffering of oxygen radicals by mitoQ administration slowed down the formation of ‘mitobulbs' in our mice, suggesting that an oxidative stress component is present in Drp1-ablated neurons, but efficiently managed by cellular defenses. A possible explanation for this discrepancy is that Purkinje cells represent an exceptionally large and extensively connected neuronal subtype, which might entail very high metabolic activity and associated ROS production (reviewed in Kern and Jones39), all of which could contribute to selective vulnerability of this neuronal subtype under certain stress conditions. It is important to note that Purkinje cell death in vivo started to occur already at around week 9 after Drp1 ablation.29 On the other hand, in line with the absence of oxidative stress, no neurodegeneration was observed upon 10-week-long Drp1 ablation in the hippocampus, the time frame when Drp1iΔb/iΔb animals had to be killed due to profound systemic-level metabolic changes (manuscript in preparation). Therefore, it cannot be excluded that the impaired neuronal mitochondrial functions might culminate in hippocampal neuronal death occurring 3 months beyond Drp1 ablation. Additionally, it is worth noting that murine Purkinje neuron differentiation continues throughout the first 3 weeks of life,40 the time when the L7/pcp2 promoter used by Kageyama et al.29 starts to be active. It may therefore be speculated that Drp1 ablation in these neurons may still overlap with the final stages of murine cerebellar development and thus also contribute to the observed Purkinje cell degeneration. We can conversely exclude neurodevelopmental defects caused by Drp1 ablation in our model, as recombination was induced at the age of 8 weeks and dendritic tree morphology as well as synapse and spine numbers were unaffected. Of note, a recent study reporting neurodegeneration upon Drp1 ablation in postmitotic dopaminergic neurons supports the notion that sensitivity to Drp1 ablation is neuronal subtype-dependent.41
Collectively, our results indicate that adult hippocampal neurons cope with profound impairment of mitochondrial dynamics and function by activating antioxidant and metabolic compensatory mechanisms. Predictably, certain neuronal functions, such as synaptic transmission, can be affected due to depletion of the organelles from presynaptic terminals, culminating in memory deficits. Conversely, these compensatory circuits are sufficient to maintain overall neuronal morphology, synapse and spine numbers and ultimately viability for as long as 3 months following Drp1 ablation. Our data therefore indicate that forebrain neurons can resist to mitochondrial dysmorphology and dysfunction, a critical feature to protect them and therefore the cognitive functions of higher mammals from mitochondriotoxic stimuli.
Materials and Methods
Mice
Drp1flx/flx mice15 were crossed with CaMKIIα CreERT2 (Cre+) animals, obtained from the European Mouse Mutant Archive (EMMA strain 02125).33 At 8 weeks of age, the resulting Drp1flx/flx Cre+ mice were injected i.p. with 1 mg tamoxifen (Sigma (Buchs, Switzerland); 10 mg/ml tamoxifen dissolved in a 9 : 1 ratio of sunflower seed oil to ethanol) twice daily on 5 consecutive days to induce recombination of the Drp1 locus. To check for recombination, Drp1flx/flx Cre+ mice were crossed with the RCE:loxP reporter mouse strain (Jackson Laboratories, Bar Harbor, ME, USA; strain 032037-JAX) harboring the R26R CAG-boosted Egfp (RCE) allele with a floxed STOP cassette upstream of the Egfp gene.
Histology
Serial coronal cross-sections of formalin-fixed, paraffin-embedded mouse brains were prepared, and sections representing the coordinates bregma (−1.34 mm)–(−2.46 mm) were selected and stained for COX subunit 1a (Abcam, Cambridge, UK, ab14705). Z-stacks were recorded with an inverted Zeiss (Feldbach, Switzerland) Axiovert 200M LSM 510 Meta confocal microscope with a × 100/1.4 Oil DIC objective using Enterprise (Zuerich, Switzerland) 405 nm and Argon 488 nm lasers. Z-stacks were projected onto a single plane using ImageJ (NIH, Bethesda, MD, USA). Synapse numbers were quantified as described previously.42 Golgi staining was performed on PBS-perfused, unfixed brains using a commercial kit (FD Neurotechnologies, Columbia, MD, USA).
Hippocampal volume was calculated from H&E-stained 100-μm-spaced, serial coronal cross-sections applying the Cavalieri principle. For cortical and midbrain volume, only sections representing coordinates bregma from 1.1 to −1.2 mm were considered, using corpus callosum as reference. TUNEL staining was performed on frozen, PBS-perfused, fixed brains using a commercial kit (Roche, Basel, Switzerland). Confocal images were stitched using a Fiji Plugin.43
Transmission electron microscopy
Semithin sections of osmium-stained hippocampi were prepared in order to identify hippocampal neurons, of which ultrathin sections were prepared. Imaging was done on a Phillips (Zürich, Switzerland) CM100 transmission electron microscope. Randomly selected TEM images were used to quantify presynaptic mitochondria of hippocampal synapses.
Electrophysiology
Transverse 350–400 μm-thick hippocampal brain slices were cut in a sucrose-based solution. During electrophysiological recordings, slices were continuously superfused with artificial cerebrospinal fluid maintained at 32–33 °C. During whole-cell patch-clamp recordings, hippocampal CA1 pyramidal neurons were filled with biocytin for subsequent morphological evaluation. fEPSPs were recorded with glass pipettes filled with 1 M NaCl placed in the stratum radiatum of the CA1 region. The stimulating electrode was placed ~500 μm away to stimulate Schaffer collaterals. Data analysis was performed offline using customized scripts written in python and Stimfit.
Behavioral analyses
Visual performance was tested in a water tank with a visible platform. Olfaction was checked using the cookie finding test. To score hippocampus-dependent working memory, the spontaneous alternation task was employed in an eight-arm radial maze, based on the spontaneous alternation paradigm.44
Western blotting
Proteins of brain lysates were separated on 4–12% BisTris SDS-PAGE gels, blotted onto PVDF membranes using the iBlot Dry Blotting System (Life Technologies, Carlsbad, CA, USA) and probed with the indicated primary antibodies and isotype-matched secondary antibodies conjugated to horseradish peroxidase. Signals were detected using ECL (GE Healthcare, Chalfont St Giles, UK).
Tissue culture
Cortical neurons from Drp1flx/flx mice were prepared from E16.5 embryos by modifying a previously described method.45 Prior to plating, cells were electroporated using the Neon Electroporation System (Life Technologies). For complete protocol, please refer to the Supplementary Information.
Quantitative proteomics
Brain tissue samples were lysed in 2% sodium deoxycholate, 100 mM ammonium bicarbonate buffer, reduced with 5 mM tris(2-chlorethyl)phosphate and alkylated with 10 mM iodoacetamide. Proteins were then digested by incubation with sequencing-grade modified trypsin (1/50, w/w; Promega, Madison, WI, USA) overnight at 37 °C. The digested samples were subsequently labeled with isobaric tag (TMT 6-plex, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. The TMT-labeled samples were re-solubilized to a final concentration of 1 mg/ml and separated on a 12-cm pH 3–10 IPG strip (GE Healthcare) with a 3100 OFFGEL fractionator (Agilent, Basel, Switzerland) as previously described.46
The setup of the μRPLC-MS system was as described previously.47 Chromatographic separation of peptides was carried out using an EASY nano-LC 1000 system (Thermo Fisher Scientific), equipped with a heated RP-HPLC column (75 μm × 50 cm) packed in-house with 1.9 μm C18 resin (Reprosil-AQ Pur, Dr. Maisch). Mass spectrometry analysis was performed on a dual pressure LTQ-Elite Orbitrap mass spectrometer equipped with a nanoelectrospray ion source (both Thermo Fisher Scientific).
Using the MASCOT algorithm (Matrix Science, Version 2.4.0), the resulting mgf files were searched against a decoy database containing normal and reverse sequences of the predicted SwissProt entries of Mus musculus (www.ebi.ac.uk, release date 16/05/2012) and commonly observed contaminants (in total 33 832 sequences for Mus musculus) generated using the SequenceReverser tool from the MaxQuant software (Version 1.0.13.13; www.maxquant.org). Next, database search results were imported to the Scaffold Q+ software (version 4.3.3, Proteome Software Inc., Portland, OR, USA), and the protein false identification rate was set to 1% based on the number of decoy hits. Protein probabilities were assigned by the Protein Prophet program.48 Acquired reporter ion intensities in the experiments were employed for automated quantification and statistically analyzed using a modified version of our in-house developed SafeQuant R script.47 In brief, reporter ion intensities were corrected for isotopic impurities according to the manufacturer's instructions. Intensities for each peptide and protein ID were summed, globally normalized across all acquisition runs and employed for ratio calculation and statistical analysis. For complete mass spectrometry materials and methods, please refer to the Supplementary Information.
Oxygen consumption and ATP levels
Mitochondria were isolated from hippocampus as previously described.49 Oxygen consumption rate was measured in isolated mitochondria from cortex and hippocampus using a Seahorse Bioscience (North Billerica, MA, USA) XF24Analyzer. ATP content from isolated mitochondria was determined by a bioluminescence assay (ViaLighTM HT; Cambrex Bio Science, Lonza, Basel, Switzerland).
Acknowledgments
We thank M Rüegg (Biozentrum, University of Basel) for helpful scientific discussions and advice; and C Lautenschlager, M Bänziger, S Ipsen (all from Institute of Pathology, Basel University Hospital) and M Dürrenberger (Microscopy Center Biozentrum, University of Basel) for their expert help with experimental procedures. MitoQ was kindly provided by M Murphy (MRC, Cambridge UK); pPGK-Cre was a kind gift from T Langer (Cologne). This work was supported by Swiss National Science Foundation grant 31003A_127308, the Novartis Foundation for Medical-Biological Research, the Desirée and Nils Yde Foundation (420-14) and the Nora van Meeuwen-Haefliger Foundation (to SF), a grant from the Forschungsfonds of Basel University (to BO) and Telethon Italy GGP12162, GPP10005, AIRC Italy, ERC ERMITO, FP7 CIG CristOpa, MIUR FIRB Automed (to LS). Publication costs are supported by the Neurex network (TriNeuron - Program Interreg IV Upper Rhine - European Regional Development Fund) www.neurex.org.
Author Contributions
BO, JMS, LMR, ML, CS, KS, AG and LM performed experiments. JB, AS, JH, AE, PD'A, PF and MT analyzed and interpreted experimental data. NI and KM provided reagents. LS and SF conceived the project, coordinated and supervised research. BO, LMR, LS and SF wrote the manuscript.
Glossary
- DG
dentate gyrus
- fEPSP
field excitatory postsynaptic potential
- H&E
hematoxylin–eosin
- iΔb
inducibly ablated in the brain
- p.t.i.
posttamoxifen injection
- TEM
transmission electron microscopy
- TUNEL
TdT-mediated dUTP-biotin nick end labeling
- ROS
reactive oxygen species
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by J-C Marine
Supplementary Material
References
- Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell 2012; 148: 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z-H. Mitochondrial trafficking and anchoring in neurons: New insight and implications. J Cell Biol 2014; 204: 1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland G-L, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J 2014; 33: 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 2008; 27: 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 2005; 280: 26185–26192. [DOI] [PubMed] [Google Scholar]
- Bliek AM, van der, Shen Q, Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol 2013; 5: a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science 2004; 305: 858–862. [DOI] [PubMed] [Google Scholar]
- Cipolat S, de Brito OM, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 2004; 101: 15927–15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008; 456: 605–610. [DOI] [PubMed] [Google Scholar]
- Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 2008; 105: 15803–15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol 2010; 191: 1141–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science 2011; 334: 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui HT, Shaw JM. Dynamin assembly strategies and adaptor proteins in mitochondrial fission. Curr Biol 2013; 23: R891–R899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med 2007; 356: 1736–1741. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 2009; 11: 958–966. [DOI] [PubMed] [Google Scholar]
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW et al. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol 2009; 186: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 2006; 25: 3900–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EC, McBride HM, Slack RS. Mitochondrial dynamics in the regulation of neuronal cell death. Apoptosis 2007; 12: 979–992. [DOI] [PubMed] [Google Scholar]
- Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D et al. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington's disease to apoptotic stimuli. EMBO Mol Med 2010; 2: 490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Merrill RA, Cribbs JT, Chen Y, Hell JW, Usachev YM et al. The spinocerebellar ataxia 12 gene product and protein phosphatase 2A regulatory subunit Bbeta2 antagonizes neuronal survival by promoting mitochondrial fission. J Biol Chem 2008; 283: 36241–36248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer K, Suppanz IE, Lingor P, Planchamp V, Goricke B, Fichtner L et al. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ 2007; 14: 651–661. [DOI] [PubMed] [Google Scholar]
- Tian C, Murrin LC, Zheng JC. Mitochondrial fragmentation is involved in methamphetamine-induced cell death in rat hippocampal neural progenitor cells. PLoS One 2009; 4: e5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KW, Pinon LG, Bampton ET, Nicotera P. Different pathways lead to mitochondrial fragmentation during apoptotic and excitotoxic cell death in primary neurons. J Biochem Mol Toxicol 2010; 24: 335–341. [DOI] [PubMed] [Google Scholar]
- Yuan H, Gerencser AA, Liot G, Lipton SA, Ellisman M, Perkins GA et al. Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons. Cell Death Differ 2007; 14: 462–471. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 2004; 15: 5001–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uo T, Dworzak J, Kinoshita C, Inman DM, Kinoshita Y, Horner PJ et al. Drp1 levels constitutively regulate mitochondrial dynamics and cell survival in cortical neurons. Exp Neurol 2009; 218: 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA et al. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 2009; 29: 9090–9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinghaus B, Licci M, Scorrano L, Frank S. Less than perfect divorces: dysregulated mitochondrial fission and neurodegeneration. Acta Neuropathol 2012; 123: 189–203. [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Zhang Z, Roda R, Fukaya M, Wakabayashi J, Wakabayashi N et al. Mitochondrial division ensures the survival of postmitotic neurons by suppressing oxidative damage. J Cell Biol 2012; 197: 535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohm J, Kim S-W, Mamrak U, Tobaben S, Cassidy-Stone A, Nunnari J et al. Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 2012; 19: 1446–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH et al. A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest Ophthalmol Vis Sci 2011; 52: 2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wang S, Li Y, Che L, Zhao Q. A selective inhibitor of Drp1, mdivi-1, acts against cerebral ischemia/reperfusion injury via an anti-apoptotic pathway in rats. Neurosci Lett 2013; 535: 104–109. [DOI] [PubMed] [Google Scholar]
- Erdmann G, Schutz G, Berger S. Inducible gene inactivation in neurons of the adult mouse forebrain. BMC Neurosci 2007; 8: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 2005; 47: 365–378. [DOI] [PubMed] [Google Scholar]
- Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells antioxidant and antiapoptotic properties. J Biol Chem 2001; 276: 4588–4596. [DOI] [PubMed] [Google Scholar]
- Li H, Alavian KN, Lazrove E, Mehta N, Jones A, Zhang P et al. A Bcl-xL–Drp1 complex regulates synaptic vesicle membrane dynamics during endocytosis. Nat Cell Biol 2013; 15: 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GSB, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. Mitochondrial calcium uptake. Proc Natl Acad Sci USA 2013; 110: 10479–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 2004; 15: 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Jones AM. Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B Crit Rev 2006; 9: 485–499. [DOI] [PubMed] [Google Scholar]
- Sudarov A, Joyner AL. Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev 2007; 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet A, Margolis EB, Zhang J, Hsieh I, Zhang J, Hnasko T et al. Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci 2014. [DOI] [PMC free article] [PubMed]
- Ippolito DM, Eroglu C. Quantifying Synapses: an immunocytochemistry-based assay to quantify synapse number. J Vis Exp 2010: 2270. [DOI] [PMC free article] [PubMed]
- Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009; 25: 1463–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci USA 1996; 93: 4693–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 2007; 27: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A et al. The quantitative proteome of a human cell line. Mol Syst Biol 2011; 7: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatter T, Ludwig C, Ahrné E, Aebersold R, Heck AJR, Schmidt A. Large-scale quantitative assessment of different in-solution protein digestion protocols reveals superior cleavage efficiency of tandem Lys-C/trypsin proteolysis over trypsin digestion. J Proteome Res 2012; 11: 5145–5156. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 2003; 75: 4646–4658. [DOI] [PubMed] [Google Scholar]
- Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F et al. Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc Natl Acad Sci USA 2009; 106: 20057–20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.