Abstract
In the central nervous system, tissue-type plasminogen activator (tPA) has been associated with both pro-death and prosurvival actions on neurons. In most cases, this has been related to exogenous tPA. In the present study, we addressed the influence of endogenous tPA. We first observed an increased transcription of tPA following either in vivo global brain ischemia in rats or in vitro oxygen glucose deprivation (OGD) on mice and rats hippocampal slices. Hippocampal slices from tPA-deficient mice were more sensitive to OGD than wild-type slices. Pharmacological approaches targeting the known receptors of tPA revealed that only the inhibition of phosphorylation of epidermal growth factor receptors (EGFRs) prevented the neuroprotective effect of endogenous tPA. This study shows that ischemic hippocampal neurons overproduce endogenous tPA as an intend to protect themselves from ischemic death, by a mechanism involving an activation of EGFRs. Thus, strategies contributing to promote either endogenous production of tPA or its associated EGFR-linked signaling pathway may have beneficial effects following brain injuries such as stroke.
In the circulation, the serine protease tissue-type Plasminogen Activator (tPA) is critical for the control of hemostasis. When secreted in the vascular lumen by endothelial cells, tPA promotes fibrinolysis via the conversion of fibrin-bound plasminogen into plasmin.1 Neurons and glial cells are also a source of tPA in the brain parenchyma,2 where it can act either as a plasminogen-dependent or -independent enzyme, a cytokine-like molecule or a neuromodulator to control neuronal plasticity, survival and death.3, 4, 5 Substrates, binding proteins or receptors for tPA, other than plasminogen, include the epidermal growth factor receptor (EGFR),6 the platelet derived growth factor-C,7 the low-density lipoprotein receptor-related protein (LRP),8 the annexin-II,9 the chemokine macrophage chemoattractant protein 110 and the N-methyl-d-aspartate receptor (NMDAR).11 In some cases, the recruitment of coreceptors might be necessary for tPA to exert its action.12
Several studies showed that tPA can promote neurotoxicity,5, 13 especially by an overactivation of NMDARs.8, 11, 14, 15, 16 However, tPA may also display prosurvival properties on neurons and oligodendrocytes4, 6, 17, 18, 19, 20 involving either an EGFR-dependent signaling,6 binding to Annexin-II18 or active NMDARs.20 Interestingly, although the proneurotoxic effects of tPA were reported for exogenous tPA11, 21 or based on data obtained from tPA-deficient mice,13 neurotrophic effects were mainly reported for endogenous tPA19 or from experiments performed on tPA overexpressing animals.20, 22 Interestingly, in vitro experiments showed that high doses of tPA (up to 60 nM) promote neuronal death induced by NMDA exposure11 or oxygen glucose deprivation (OGD),23 whereas lower doses of tPA (below 10 nM) are neuroprotective following OGD.20 Despite the heterogeneity of both the experimental models and the toxic paradigms used in these different studies, they all showed that the prosurvival effects of tPA can occur independently of its proteolytic activity,4, 17 through a so-called ‘cytokine' or ‘growth factor'-like effect.
In the present study, we combined studies in a model of global cerebral ischemia by circulatory arrest/resuscitation in rats and of OGD on hippocampal slices first, to investigate the role of endogenous tPA on the fate of hippocampal neurons challenged by ischemia, and second, to determine which molecular mechanism(s) might be involved. Our data reveal that global cerebral ischemia leads to an overexpression of tPA in hippocampal neurons, and that endogenous tPA is neuroprotective via an EGFR-dependent mechanism.
RESULTS
Global ischemia in rats and ODG on hippocampal slices promote neuronal tPA expression
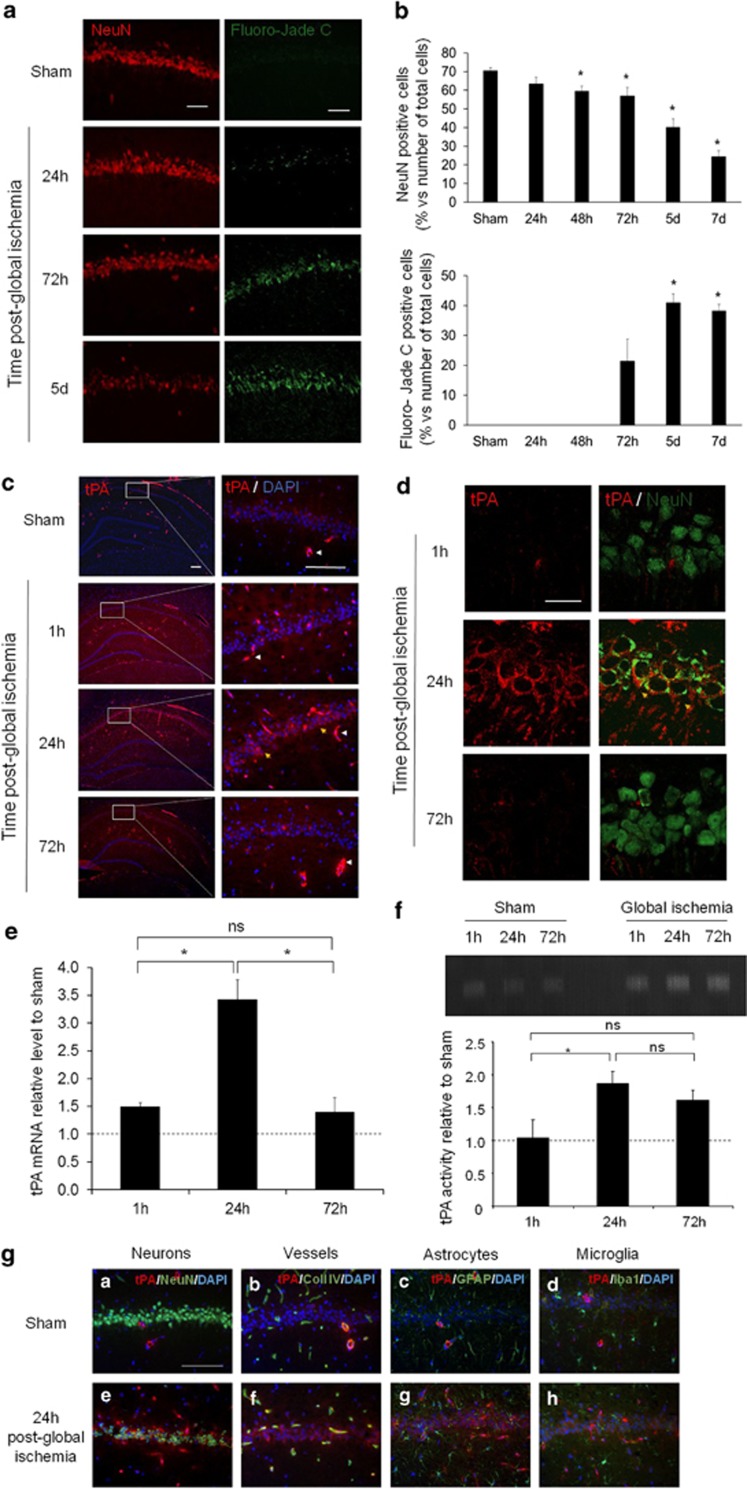
Global ischemia was induced in rats by a 3-min long circulatory arrest followed by resuscitation, as previously reported.24 Physiological parameters were recorded continuously throughout the surgery (Supplementary Figure 1). Hippocampi were then collected from 24 h and up to 7 days post onset for evaluation of neuronal death. NeuN and Fluoro-Jade C stainings performed to quantify the number of healthy and suffering/dying neurons revealed that cardiac arrest led to a time-dependent neuronal death in the hippocampal CA1 region beginning to be quantitatively evident between 48 (−15% of NeuN positive cells) and 72 h post injury and reaching a maximum at 7 days post injury (−65% of NeuN positive cells; Figures 1a and b). At 24 h post-global ischemia, as previously reported,25 we observed a NeuN staining outside of the neuronal nuclei, recovering at 72-h post-global ischemia (Figure 1d). Parallel immunostainings for tPA (Figures 1c and d) revealed increased levels of tPA as early as 1-h post injury, culminating at 24 h. Interestingly, whereas tPA was only detected in vessels in sham operated animals (Figures 1c and g), global ischemia increased tPA expression in neurons (yellow arrows; Figures 1c, d and g) and slightly in endothelial cells but not in astrocytes (GFAP-positive cells) and in microglia (Iba-1-positive cells; Figure 1g).
Figure 1.
Hippocampal neurons overexpress tPA after global cerebral ischemia. (a) Representative immunohistological stainings showing neurons (NeuN; TRITC) and degenerative cells (Fluoro-Jade C staining; FITC) in the CA1 layer of rat hippocampus subjected to global cerebral ischemia (GCI) induced by circulatory arrest (3 min) and resuscitation (sham, 24, 72 h and 5 days post-GCI) (scale bar: 50 μm) and (b) quantification of neuronal death in CA1 by counting of NeuN and Fluoro-jade C-positive cells (*P<0.05; n=3–6). (c) Representative immunohistological stainings for tPA (FITC) and DAPI nuclear staining in CA1 layer of hippocampus in sham and at 1, 24 and 72 h after GCI, white arrows show tPA-positive vessels and yellow arrows show tPA-positive neurons (scale bar: 100 μm). (d) Representative immunohistological stainings for tPA (TRITC) and neuron (NeuN; FITC) in CA1 layer of hippocampus at 1, 24 and 72 h after GCI (scale bar: 20 μm). (e) tPA mRNA expression relative to sham (*P<0.05; n=4) and (f) tPA activity relative to sham in hippocampus at 1, 24 and 72 h after GCI (*P<0.05; n=3). (g) Representative immunohistological staining for tPA (TRITC) (A–H) and neurons (NeuN; FITC) (A and E), vessels (type IV collagen; Coll IV; FITC) (B and F), astrocytes (GFAP; FITC) (C and G) or microglia (Iba1; FITC) (D and H), in addition of DAPI counterstaining (Blue) in sham and in ischemic animals (GCI) at 24-h post injury (scale bar: 50 μm)
These qualitative observations were first confirmed by quantitative PCR (q-PCR) for the mRNA encoding tPA (+49% at 1 h, P<0.05; +254% at 24 h, P<0.05 and +39% at 72 h, P<0.05 when compared with the sham operated animals at the same timel; Figure 1e). Zymography assay for the detection of the proteolytic activity of the protein tPA showed a similar transient increase in the levels of active tPA, evident at 24 h (+87%) and persisting at 72 h (+61%), (P<0.05 when compared with the sham operated animals at the same time; Figures 1e and f).
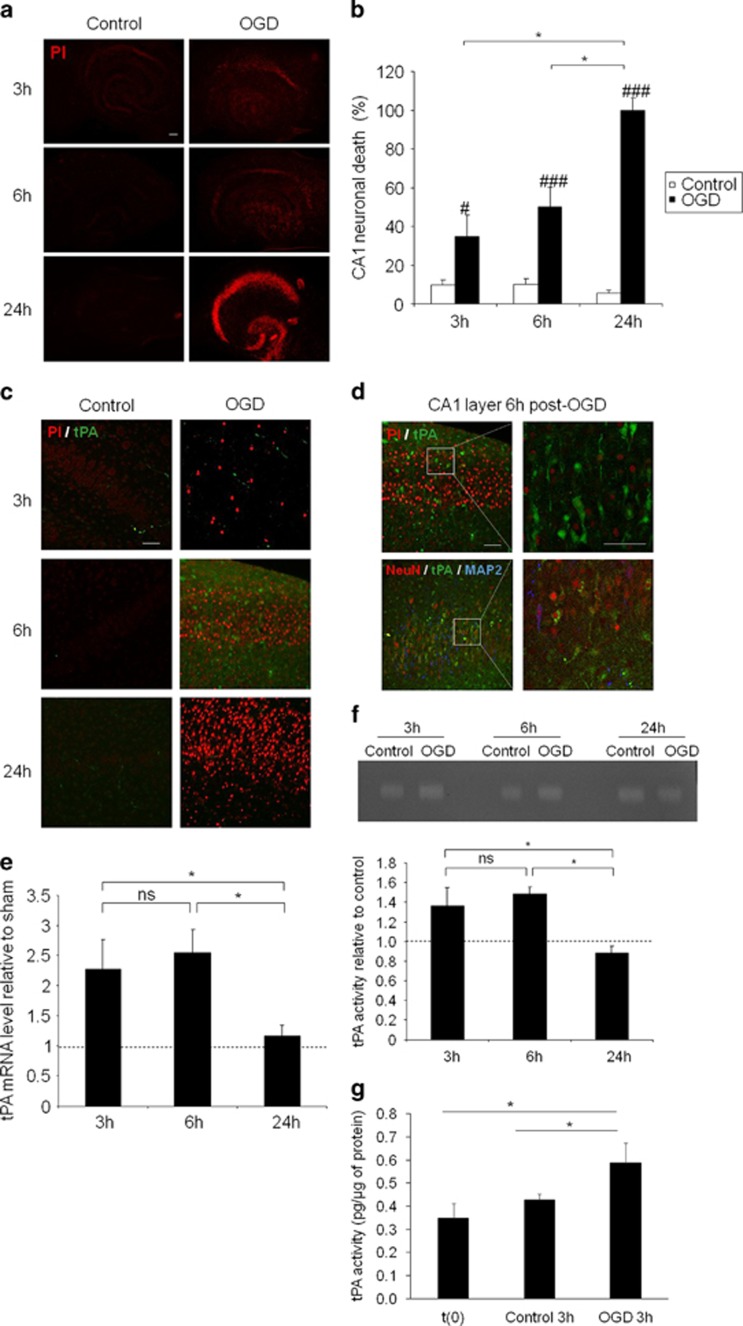
Similar experiments were performed in an in vitro model of rat hippocampal slices subjected to OGD (Figure 2). As previously reported,26 OGD (30 min) followed by different times of reoxygenation and glucose supply (3–24 h) led to a time-dependent increase in neuronal death measured by quantification of the level of propidium iodide (PI) staining, with a maximum at 24-h post-OGD (35% of neuronal death at 3 h, 50% at 6 h and 100% at 24 h when compared with control slices, #P<0.05; ###P<0.001; Figures 2a and b). Like after cardiac arrest in rats, OGD/reoxygenation led to an increased expression of tPA, mainly in neurons, beginning 3-h post stress and reaching a maximum at 6 h (Figures 2c and d).
Figure 2.
tPA increases in hippocampal slice after oxygen and glucose deprivation. (a) Representative propidium iodide staining of hippocampal slice subjected to oxygen/glucose deprivation during 30 min and reoxygenated during 3, 6 or 24 h (scale bar: 100 μm) and (b) quantification of neuronal death in CA1, expressed in percentage of OGD 24 h. (c) Representative immunostaining for tPA (FITC) and cell death (propidium iodide staining) in hippocampal slice subjected to OGD at 3, 6 and 24 h after reoxygenation (scale bar: 50 μm). (d) Representative immunostainings for tPA, neuron (NeuN and MAP-2) in hippocampal slice subjected to OGD and after 6 h of reoxygenation (scale bar: 50 μm). (e) tPA mRNA expression relative to control (*P<0.05; n=4) and (f) tPA activity relative to control in hippocampal slice subjected to OGD and after 3, 6 and 24 h of reoxygenation (*P<0.05; n=6). (g) tPA activity expressed in pg of tPA per μg of protein in slices after 7 DIV (t(0)) and in control slices and in slices subjected to OGD after 3 h of reoxygenation (*P<0.05; n=3)
These patterns of immunostainings were correlated with q-PCR (Figure 2e) and zymography assays (Figure 2f). tPA mRNA levels were increased of 127% at 3 h and of 154% at 6-h post-OGD, returning to basal levels at 24 h, when compared with control slices (Figure 2e). Similar observations were made from zymography assays with an increased proteolytic activity of tPA after OGD at 3 and 6 h returning to basal levels at 24 h (+36% at 3 h and +48% at 6-h post-OGD, Figure 2f). These data were also confirmed using a chromogenic assay for tPA activity (+27% at 3 h after OGD compared with control; Figure 2g). Altogether, these data demonstrate that ischemic brain injury is associated with an increased transcription and translation of tPA, leading to higher levels of endogenous active tPA in hippocampal neurons.
Endogenous tPA mediates neuroprotection by activating EGFRs
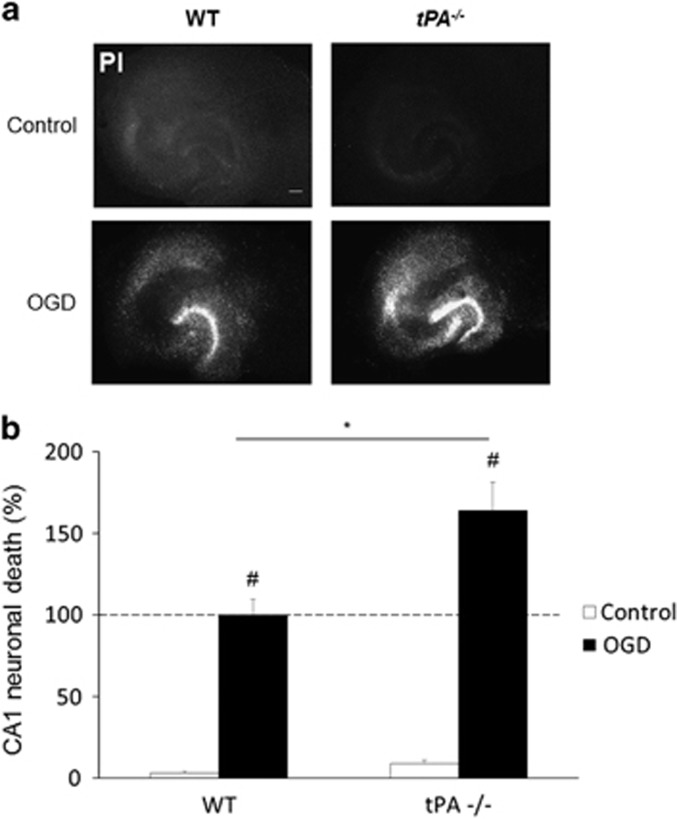
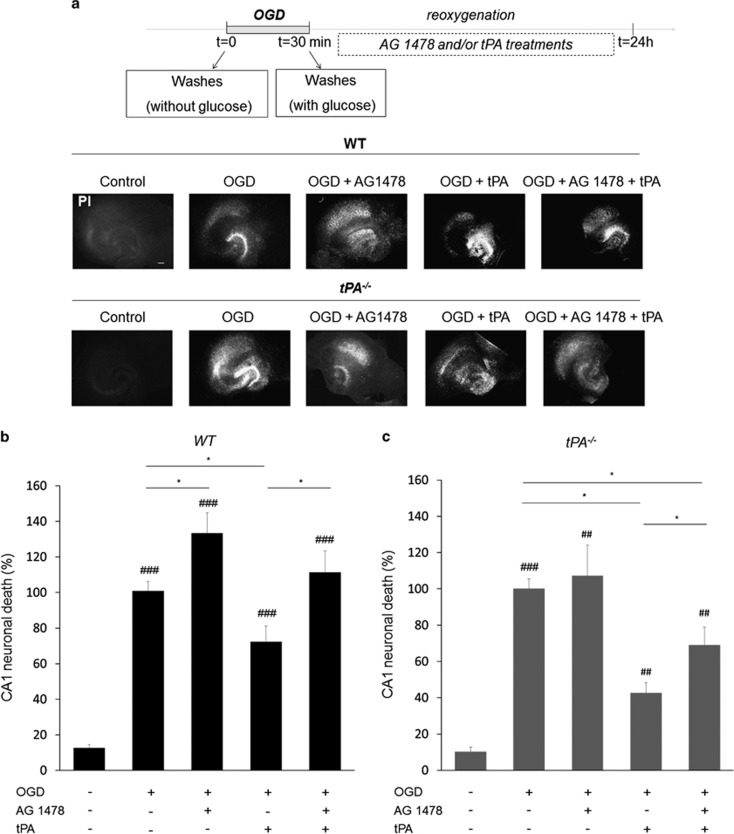
Then, we sought to investigate whether such an increase in endogenous tPA influences neuronal fate and if so, by which mechanism(s). Here, we used hippocampal slices collected from wild-type or tPA-deficient mice. OGD was induced as above and neuronal death was quantified by PI staining at 24 h after a 30-min period of OGD. As expected, OGD led to an important neuronal death in wild-type slices, an effect increased in tPA-deficient slices (+64%, *P<0.05 when compared to wild-type slices subjected to OGD; Figures 3a and b). These data show that endogenous tPA is neuroprotective following OGD. In order to determine the mechanism(s) that could mediate this neuroprotective effect of endogenous tPA, rat hippocampal slices were subjected to OGD in the presence or not of inhibitors of the main receptors of tPA: an antibody against the binding site of tPA on NMDA receptors (anti-ATD-GluN1),27 the chaperone protein receptor-related protein (RAP) known to prevent the interaction of tPA with LRP28 or AG1478 a molecule previously characterized for preventing the transphosphorylation of EGFRs29 (Figures 4a and b). OGD increased neuronal death by a fivefold (P<0.05 when compared with non-OGD treated slices). Blockage of the ability of tPA to activate either NMDARs or LRP did not influence neuronal death induced by OGD. The cotreatment with AG1478 (5 μM) worsened OGD-induced neuronal death (+67%, P<0.05 when compared with OGD alone), suggesting that endogenous tPA mediates its neuroprotective effect after OGD by a mechanism involving activation of EGFRs. In order to validate this hypothesis, similar experiments were performed using hippocampal slices from wild-type or tPA-deficient mice (Figures 5a, b and c). Neuronal death was measured, as previously, using PI staining. Blockage of the transphosphorylation of EGFR by AG1478 aggravated OGD-induced neuronal death in wild-type slices (+33%, P<0.05), but not in tPA-deficient slices. Recombinant tPA protects neurons from wild-type slices (−27%, P<0.05) and tPA-deficient slices (−57%, P<0.05) after OGD, an effect prevented in the presence of AG1478 (Figures 5b and c).
Figure 3.
Neuronal death increases in tPA-/- hippocampal slices. WT and tPA−/− hippocampal slices were exposed 30-min OGD experiment (a) and neuronal death was quantified by propidium iodide (PI) 24 h later (b), neuronal death was expressed in percentage of WT tPA OGD 24 h. (scale bar: 100 μm) (*P<0.05 WT versus tPA−/−; #P<0.05 compared with control (non-OGD); n=4; n=7–9)
Figure 4.
Inhibition of EGF receptor phosphorylation increases hippocampal neuronal death. Hippocampal slices were exposed to AG 1478 (5 μM) or anti-ATD-GluN1 (10 μg/ml) or RAP (0.5 nM) after 30 min OGD experiment (a) and neuronal death was quantified by propidium iodide 24 h later (b), neuronal death was expressed in percentage of OGD 24 h. (scale bar: 100 μm) (*P<0.05 treated vs untreated; #P<0.05 OGD versus control for each treatment; n=6–9; n-10–22)
Figure 5.
tPA prevents neuronal death in tPA-/- hippocampal slices. WT and tPA−/− hippocampal slices were exposed to AG 1478 (5 μM) or/and tPA (300 nM) after 30-min OGD experiment (a) and neuronal death was quantified by propidium iodide 24 h after OGD (b), neuronal death was expressed in percentage of WT tPA OGD 24 h and (c) neuronal death was expressed in percentage of tPA−/− OGD 24 h. (scale bar: 100 μm) (##P<0.01 OGD versus control; ###P<0.001 OGD versus control; *P<0.05; n=3–5; n=8–33)
DISCUSSION
In the brain parenchyma, prosurvival and proneurotoxic effects of tPA involve mechanisms that are beyond its ability to activate plasminogen into plasmin, including modulation of NMDAR-dependent signaling,11 LRP-mediated PSD95 activation,16 annexin-II-dependent processes.9 Here we demonstrate that in a model of cultured hippocampal slices subjected to OGD, in both rats and mice, tPA may display neuroprotection by a mechanism involving activation of EGFR. These data are in agreement with a previous demonstration that tPA may have EGFR-dependent oligotrophic activity.6 Activation of NMDAR has been suggested as a possible mechanism of tPA-dependent neuroprotection following OGD in cortical neurons,20 but we did not confirm these results in our present model. This may be explained by the use of different strategies to block tPA-dependent NMDAR signaling, that is, MK-801 as a broad antagonist of NMDAR on one hand and an antibody previously characterized to specifically prevent the tPA-dependent potentiation of NMDAR signaling without affecting their basal activity30 on the other hand. Although LRP was also reported to mediate some of the neuroprotective functions of tPA,31 we excluded its implication in our models using RAP, a well-characterized antagonist of the interaction of tPA with LRP.32
Another important point of discussion in the field is to know whether exogenous tPA and endogenous tPA have differential effects on neuronal death/survival. Our present data are in agreement with a recent literature demonstrating that endogenous tPA display neuroprotective activities.20 Nevertheless, using tPA-deficient mice, we showed that application of exogenous tPA may also protect hippocampal neurons subjected to OGD. These data are in agreement with previous demonstrations that exogenous tPA may have either proneurotoxic or prosurvival effects depending on the type of paradigm, on the type of neurons (hippocampal versus cortical) or on the model used (wild-type neurons, tPA-deficient neurons, tPA overexpressing neurons in vitro and in vivo).11, 13, 17, 19, 20, 21 Thus, as reported previously,19, 20 we can postulate that, when released by suffering neurons at low concentrations, the endogenous tPA displays neuroprotective effects.
Surprisingly, we observed that addition of tPA on wild-type slices after OGD increased the number of PI-positive cells in the dentate gyrus (DG). This deleterious effect of tPA could be explained either by a differential sensitivity of the DG to tPA under ischemic conditions or to a different mechanism of neuronal death when subjected to OGD (excitotoxicity versus apoptosis). Indeed, although tPA is protective in an apoptotic paradigm,17 it is deleterious in a context of excitotoxicity.11 Another possibility could be the previously reported lower level of EGF receptors in the DG (when compared to CA1),33 which could explain the lack of a protective effect of tPA in the DG.
The third important point of our present study is that hippocampal neurons can respond to ischemic conditions by overexpressing tPA and thus protect themselves. Previous data had reported early transcription of neuronal tPA when subjected to paradigms of long-term potentiation-induced synaptic plasticity34 or exposure to amphetamine.35 To our knowledge, our present data are the first to demonstrate such a type of early transcription of tPA by neurons subjected to ischemia.
In sham animals, we found evidence of tPA immunostaining only in endothelial cells, and a promoting effect of global ischemia on endothelial and neuronal cells. Previous studies have suggested that astrocytes and microglia also express tPA, which contrasts with our present obervations.2 However, most of these studies investigated tPA mRNA or activity, not tPA immunoreactivity. We do not exclude, however, that under our control and OGD paradigms, tPA expression occurs in nonendothelial cells, but at too low levels to be detected by immunohistochemistry in our hands.
As neurons and endothelial cells, respectively, release tPA in the brain parenchyma and in the circulation, we can propose that the tPA released by neurons following global ischemia or OGD may display either autocrine or paracrine protective effects.
To conclude, stressed neurons might overproduce endogenous tPA to protect them from ischemic death, by a mechanism involving an activation of EGFR. In cerebral ischemia, this pathway has also been shown to protect oligodendrocytes in the white matter.6 In addition, owing to its fibrinolytic activity, tPA is up to now the only treatment approved by the authorities at the acute phase of the disease.36 However, intravenous infusion of tPA was also reported to display neurotoxicity in animals29 or to promote seizures in humans,37 in addition to a risk of hemorrhagic transformations.38 We can postulate that the proportion of beneficial or deleterious actions of tPA may differentially occur in the ischemic core versus the penumbra, at the acute phase of stroke–trauma versus the recovery phases, when exogenous tPA is injected intravenously for thrombolysis versus not. Altogether, these pleiotropic effects of tPA possibly contribute to the limitations of the benefit of tPA treatment.
Here we demonstrate that tPA can also neuroprotective effects, especially when produced and released by stressed neurons an effect mediated by its ability to activate EGFR-dependent signaling pathways. We can thus postulate that strategies contributing to promote either endogenous production of tPA or its signaling, including EGFR pathway, may have beneficial effects following brain injury such as stroke.
Like in our present study, exogenous tPA was already reported to protect cultured hippocampal neurons from OGD-induced death by a mechanism independent of its proteolytic activity.19, 39 Accordingly, in a model of global ischemia and in agreement with our present study, a beneficial effect of both endogenous and exogenous tPA was reported by Yepes' group, an effect also confirmed in cultured hippocampal neurons subjected to OGD.19 These data are in agreement with another study from the same group, demonstrating a beneficial effect of endogenous tPA against ischemic paradigms, both in vitro and in vivo. The proposed mechanisms involve a decreased activation of the mTor–HIF-1 alpha-dependent signaling pathway40 and/or an activation of the AMPK-dependent signaling pathway,21 all these mechanisms being dependent on NMDA receptor activation. By contrast, endogenous tPA was considered deleterious in a model of global ischemia in rats.41 However, this conclusion was raised based on an indirect neuroprotective effect of aprotinin, a potent inhibitor of the tPA-plasmin cascade.
Thus, despite some controversies in the literature, we can propose that endogenous tPA is neuroprotective following brain ischemia by a mechanism independent of its proteolytic activity possibly involving, either interconnected or independently, EGF receptors-, AMPK-, mTor-HIF-1 alpha-dependent signaling pathways.20, 40 Possible roles of either NMDA receptors,19, 20, 40 or LRP-119 were also reported. Further investigations are needed to investigate how these pathways may interfere to mediate the neuroprotective effect of tPA.
MATERIALS AND METHODS
Rat model of global cerebral ischemia
Male Wistar rat (250–300 g; CURB, Caen, France) were housed in a temperature-controlled room on a 12-h light/12-h dark cycle with food and water ad libitum. Experiments were performed in accordance with French ethical laws (act no. 87–848; Ministère de l'Agriculture et de la Forêt) and European Communities Council Directives of 24 November 1986 (86/609/EEC) guidelines for the care and use of laboratory animals.
Global ischemia was induced in rats by a 3-min long circulatory arrest followed by resuscitation, as previously reported.24 Anesthetized and intubated animals were placed on the dorsal side. To record blood pressure and to make the study of various blood parameters (blood gases, pH), a catheter was inserted into the femoral artery. Furthermore, a catheter was placed in the femoral vein to allow administration of epinephrine during resuscitation. After 10 min of control period, 200 μl NaCl of heparin 20 UI/ml were injected prior to respiratory arrest. Asphyxia was induced by placing an empty syringe into the cannula to intubation. This causes asphyxia circulatory arrest defined as a fall in mean arterial pressure (BP) below 25 mm Hg. Resuscitation was started after 3 min of circulatory arrest. The resuscitation procedure involved injecting 0.2 mg/kg of adrenaline, ventilation was restored at 100% O2 and 120 breaths per min and a cardiac massage was performed. Return of spontaneous circulation was defined as the mean arterial pressure above 60 mm Hg. The analysis of blood parameters (10 min before circulatory arrest, 5, 20, 30 and 40 min after resuscitation) was carried out at fixed time. Forty minutes after return of spontaneous circulation arterial and venous catheters were removed and the incision was closed. When the animals recovered spontaneous breathing, mechanical ventilation was stopped and the animals were extubated.
Organotypic hippocampal slice cultures and oxygen and glucose deprivation
Seven days old rat or mouse pups of either sex were decapitated and their brain collected in PBS containing 5 mg of glucose. Hippocampal slices (400 μm) were prepared using a McIlwain tissue chopper (Brinkman Instruments, World Precision Instruments, Hitchin, UK) and placed on 0.4 μm Millicell culture inserts (Millipore, Saint Quentin en Yvelines, France), as previously described.42 Slices were maintained in a humidified incubator with 5% CO2 at 37 °C with 1 ml of MEM containing 20% horse serum, supplemented with 10 mm HEPES and insulin, pH 7.2–7.3.
OGD was performed in a hypoxic chamber (IN VIVO 500, Ruskinn, AWEL International, Blain, France) programmed at 1% O2, 5% CO2 and 37 °C. At 7–8 DIV, hippocampal slices were submitted to an OGD or not, for 30 min. In the chamber, hippocampal slices were switched to glucose-/serum-free deoxygenated DMEM. After 30 min, cells were removed from the hypoxic chamber and the medium was replaced with oxygenated medium without serum and then were treated during reoxygenation with AG 1478 (5 μM; inhibitor of EGFR transphosphorylation) or anti-ATD-GluN1 (10 μg/ml; antibody against tPA/NMDAR interaction) or RAP (0.5 nM; inhibitor of LRP1 binding) or rtPA (300 nM). Neuronal death was assessed 24 h later by propidium iodide fluorescence. Propidium iodide (50 μg/μl; Sigma, Lyon, France) was added in medium at 23.5 h, 30 min later were washed in phosphate-buffered saline solution (PBS) and were immersed in fixative during 1 h (4% of paraformaldehyde in 0.1 M PBS, pH 7.4). Images were digitally captured using a Leica DM6000 microscope-coupled (Leica Microsystems SAS, Nanterre, France) coolsnap camera and visualized with Metavue 5.0 software (Molecular Devices, Downington, PA, USA) and further quantified using ImageJ 1.45r software (National Institute of Health, Bethesda, MD, USA).
Immunohistochemistry
Animal studies
Deeply anesthetized rat were transcardially perfused with cold heparinized saline followed by 300 ml of fixative (PBS 0.1 M. pH 7.4 containing 2% paraformaldehyde and 0.2% picric acid). Brains were post-fixed (18 h; 4 °C) and cryoprotected (sucrose 20% in veronal buffer; 24 h; 4 °C) before freezing in Tissue-Tek (Miles Scientific, Naperville, IL, USA). Cryomicrotome-cut sections (10 μm) were collected on polylysine slides and stored at −80 °C before processing. Fluoro-jade C staining was performed for neuronal death assessment. For immunostaining, sections were coincubated overnight with rabbit anti-tPA (1 : 1500; kind gift from Dr. R. Lijnen), anti-NeuN (1 : 800; Millipore), anticollagen type IV (1 : 800, Southern Biotech, Clinisciences, Montrouge, France), anti-GFAP (1 : 800, Abcam, Cambridge, UK) or anti-Iba1 (1 : 800, Abcam) in veronal buffer (pH 7.4). Primary antibodies were revealed using Fab'2 fragments of Donkey anti-rabbit IgG linked to TRITC and Fab'2 fragments of Donkey anti-mouse IgG linked to FITC (1 : 500, Jackson ImmunoResearch, West Grove, PA, USA). Washed sections were coverslipped with antifade medium containing DAPI and images were digitally captured using a Leica DM6000 microscope-coupled coolsnap camera and visualized with Metavue 5.0 software (Molecular Devices) and further processed using ImageJ 1.45r software (NIH).
In vitro studies
After a brief washing in phosphate-buffered saline solution (PBS), slices were immersed in fixative during 1 h (4% of paraformaldehyde in 0.1 M PBS, pH 7.4). Slices were collected and washed in PBS. Slices were coincubated overnight with rabbit anti-tPA (1 : 1500; kind gift from Dr. R. Lijnen), anti-NeuN (1 : 800; Millipore), chicken MAP2 (1 : 6000; Abcam) in PBS. Primary antibodies were revealed using Fab'2 fragments of Donkey anti-rabbit IgG linked to FITC, Fab'2 fragments of Donkey anti-mouse IgG linked to TRITC, Fab'2 fragments of Donkey anti-rabbit IgG linked to dylight649 (1 : 500, Jackson ImmunoResearch). Confocal laser-scanning microscopy was performed using a Leica SP5 confocal microscope (Leica Microsystems SAS).
Zymography assays
Zymography was performed by adding plasminogen (4.5 mg/ml) and casein (5 mg) to a 12.6% SDS-PAGE. Electrophoresis was performed at 4 °C, as previously described.43 Gels were washed with Triton X-100 (2.5%) and incubated at 37 °C. Caseinolytic bands were visualized after Coomassie staining.
Assay for fibrin-bound tPA (SOFIA)
Solid-phase fibrin was prepared as previously described,44, 45 with minor modifications.46 In this assay, tPA coming from the hippocampal slices was first bound to solid-phase fibrin by incubating 25 μg of lysed samples (diluted in buffer 0.05 mol/l PO4, pH 6.8, 4% albumin, 0.08 mol/l NaCl) with the fibrin surface at 37 °C in microtitration plates.
After 1 h at 37 °C, the plate was washed thrice with Assay buffer (0.05 mol/l PO 4, pH 7.4, 2 mg/ml albumin, 0.08 mol/l NaCl, 0.01% Tween 20, 0.01% Thymerosal) to eliminate unbound proteins. Then, the amount of tPA bound to the fibrin surface was measured by the addition of 0.250 μM of glu-plasminogen (Enzyme research Laboratories) and 0.3 mM of s-2251 (Val-Leu-Lys-p-nitroaniline, Chromogenix, Werfen, Le pré Saint Gervais, France), a specific plasmin chromogenic substrate, as previously described.46 Under these conditions, the measured absorbance was proportional to the amount of cleaved substrate, the first derivation of the absorbance was proportional to the rate of substrate cleavage (e.g. plasmin activity) and the secondary derivation of the absorbance was proportional to the rate of plasmin formation (e.g. tPA activity).47
Absorbance (OD 405nm) was monitored using a multiwall plate reader (Biotek ELx808, BioTek France, Colmar, France) thermostatically controlled at 37 °C. Rates of plasmin production were calculated from the slopes of curves of OD 405 vs time and transformed into plasmin concentration. All samples were run in triplicate. Positive control was a range of known recombinant human t-PA concentrations (Actilyse, Boehringer Ingelheim France, Paris, France).
Quantitative real-time PCR
Total RNAs were extracted from tissue and hippocampal slice with TRI-reagent (Sigma) according to the manufacturer. Total RNA was quantified by spectrophotometry (Nanodrop Technologies; Wilmington, USA). We performed the reverse transcription from 1 μg of total RNA and the iScript kit (iScript Select cDNA Synthesis Kit, Biorad; Marnes-la-Coquette, France) in a total volume of 20 μl with the following cycle conditions: 42 °C (90 min); 85 °C (5 min). The cDNA products were then stored at −20 °C until their use. q-PCR were performed from 1 μl of 1:20 diluted cDNA in 15 μl total of a 1X solution of IQ SYBR Green Supermix (Biorad) containing 200 nM of each primer. Primers were designed for each gene using the Beacon Designer software (Bio-Rad). Primer alignments were performed with the BLAST database to ensure the specificity of primers). Assays were run in triplicate on the CFX96 Real-Time system c1000 thermal cycler (Biorad), with the following cycle conditions: 95 °C (3 min); [95 °C (2 s), 60 °C (20 s)]x39; 70 °C (30 sec). Cyclophilin A was used as housekeeping genes for samples. The levels of expression of gene of interest were computed as follows: relative mRNA expression=E−(Ct of gene of interest)/E−(Ct of housekeeping gene), where Ct is the threshold cycle value and E is efficiency.
Statistical analyses
Results are the mean±S.E.M. Statistical analyses were performed by the two-tail Kruskall–Wallis' test, followed by post hoc comparisons with the two-tailed Mann–Whitney's test, using the Statview software (Statview, Cary, NC, USA).
Acknowledgments
This work was supported by INSERM, the french ministry of higher education and research, the University of Caen Basse Normandie. We are grateful to Dr. Benoit Roussel for his helpful expertize with qPCR experiments, to Dr. Axel Montagne and Laurent Chazalviel for their help at the beginning of this work and to Dr. Sara Martinez de Lizarrondo for his helpful expertize with tPA activity assays.
Glossary
- EGFR
epidermal growth factor receptor
- LRP
low-density lipoprotein receptor-related protein
- NMDAR
N-methyl-d-aspartate receptor
- OGD
oxygen glucose deprivation
- RAP
receptor-related protein
- tPA
tissue-type plasminogen activator
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by JM Hardwick
Supplementary Material
References
- Rijken DC, Sakharov DV. Basic principles in thrombolysis: regulatory role of plasminogen. Thromb Res 2001; 103: S41–S49. [DOI] [PubMed] [Google Scholar]
- Docagne F, Parcq J, Lijnen R, AliC, Vivien D. Understanding the functions of endogenous and exogenous tissue-type plasminogen activator during stroke. Stroke 46: 314–320. [DOI] [PubMed] [Google Scholar]
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron 1998; 21: 813–825. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park JH, Hong SH, Koh JY. Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science 1999; 284: 647–650. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Rogove AD, Strickland S. Neuronal cell death and tPA. Nature 1996; 384: 123–124. [DOI] [PubMed] [Google Scholar]
- Correa F, Gauberti M, Parcq J, Macrez R, Hommet Y, Obiang P et al. Tissue plasminogen activator prevents white matter damage following stroke. J Exp Med 2011; 208: 1229–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson L, Ehnman M, Fieber C, Eriksson U. Structural requirements for activation of latent platelet-derived growth factor CC by tissue plasminogen activator. J Biol Chem 2005; 280: 26856–26862. [DOI] [PubMed] [Google Scholar]
- Samson AL, Nevin ST, Croucher D, Niego B, Daniel PB, Weiss TW et al. Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function. J Neurochem 2008; 107: 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci 2002; 22: 3352–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Tsirka SE. Truncation of monocyte chemoattractant protein 1 by plasmin promotes blood-brain barrier disruption. J Cell Sci 2011; 124: 1486–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med 2001; 7: 59–64. [DOI] [PubMed] [Google Scholar]
- Mantuano E, Lam MS, Gonias SL. LRP1 assembles unique co-receptor systems to initiate cell signaling in response to tissue-type plasminogen activator and myelin-associated glycoprotein. J Biol Chem 2013; 288: 34009–34018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med 1998; 4: 228–231. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Castel H, Boulouard M, Bluthé R, Fernandez-Monreal M, Roussel BD et al. Anti-NR1 N-terminal-domain vaccination unmasks the crucial action of tPA on NMDA-receptor-mediated toxicity and spatial memory. J Cell Sci 2007; 120: 578–585. [DOI] [PubMed] [Google Scholar]
- Lopez-Atalaya JP, Roussel BD, Levrat D, Parcq J, Nicole O, Hommet Y et al. Toward safer thrombolytic agents in stroke: molecular requirements for NMDA receptor-mediated neurotoxicity. J Cereb Blood Flow Metab 2008; 28: 1212–1221. [DOI] [PubMed] [Google Scholar]
- Martin AM, Kuhlmann C, Trossbach S, Jaeger S, Waldron E, Roebroek A et al. The functional role of the second NPXY motif of the LRP1 beta-chain in tissue-type plasminogen activator-mediated activation of N-methyl-D-aspartate receptors. J Biol Chem 2008; 283: 12004–12013. [DOI] [PubMed] [Google Scholar]
- Liot G, Roussel BD, Lebeurrier N, Benchenane K, López-Atalaya JP, Vivien D et al. Tissue-type plasminogen activator rescues neurones from serum deprivation-induced apoptosis through a mechanism independent of its proteolytic activity. J Neurochem 2006; 98: 1458–1464. [DOI] [PubMed] [Google Scholar]
- Lee HY, Hwang IY, Im H, Koh JY, Kim YH. Non-proteolytic neurotrophic effects of tissue plasminogen activator on cultured mouse cerebrocortical neurons. J Neurochem 2007; 101: 1236–1247. [DOI] [PubMed] [Google Scholar]
- Echeverry R, Wu J, Haile WB, Guzman J, Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J Clin Invest 2010; 120: 2194–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Nicholson AD, Haile WB, Torre E, An J, Chen C et al. Tissue-type plasminogen activator mediates neuronal detection and adaptation to metabolic stress. J Cereb Blood Flow Metab 2013; 33: 1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature 1995; 377: 340–344. [DOI] [PubMed] [Google Scholar]
- Wu F, Echeverry R, Wu J, An J, Haile WB, Cooper DS et al. Tissue-type plasminogen activator protects neurons from excitotoxin-induced cell death via activation of the ERK1/2-CREB-ATF3 signaling pathway. Mol Cell Neurosci 2013; 52: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Hébert M, Jullienne A, Lesept F, Le Béhot A, Louessard M et al. Memantine improves safety of thrombolysis for stroke. Stroke 2012; 43: 2774–2781. [DOI] [PubMed] [Google Scholar]
- Hendrickx HH, Rao GR, Safar P, Gisvold SE. Asphyxia, cardiac arrest and resuscitation in rats. I. Short term recovery. Resuscitation 1984; 12: 97–116. [DOI] [PubMed] [Google Scholar]
- Unal-Cevik I, Kilinç M, Gürsoy-Ozdemir Y, Gurer G, Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res 2004; 1015: 169–174. [DOI] [PubMed] [Google Scholar]
- Pringle AK, Iannotti F, Wilde GJ, Chad JE, Seeley PJ, Sundstrom LE. Neuroprotection by both NMDA and non-NMDA receptor antagonists in in vitro ischemia. Brain Res 1997; 755: 36–46. [DOI] [PubMed] [Google Scholar]
- Macrez R, Bezin L, Le Mauff B, Ali C, Vivien D. Functional occurrence of the interaction of tissue plasminogen activator with the NR1 Subunit of N-methyl-D-aspartate receptors during stroke. Stroke 2010; 41: 2950–2955. [DOI] [PubMed] [Google Scholar]
- Bu G, Schwartz AL. RAP, a novel type of ER chaperone. Trends Cell Biol 1998; 8: 272–276. [DOI] [PubMed] [Google Scholar]
- Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 1996; 379: 557–560. [DOI] [PubMed] [Google Scholar]
- Macrez R, Obiang P, Gauberti M, Roussel B, Baron A, Parcq J et al. Antibodies preventing the interaction of tissue-type plasminogen activator with N-methyl-D-aspartate receptors reduce stroke damages and extend the therapeutic window of thrombolysis. Stroke 2011; 42: 2315–2322. [DOI] [PubMed] [Google Scholar]
- An J, Zhang C, Polavarapu R, Zhang X, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain. Blood 2008; 112: 2787–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest 2003; 112: 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-García C, Prochnow S, Simeonova IK, Strelau J, Hölzl-Wenig G, Mandl C et al. Growth/differentiation factor 15 promotes EGFR signalling, and regulates proliferation and migration in the hippocampus of neonatal and young adult mice. Development 2014; 141: 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature 1993; 361: 453–457. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Kajii Y, Nishikawa T. Psychotomimetic-induction of tissue plasminogen activator mRNA in corticostriatal neurons in rat brain. Eur J Neurosci 1998; 10: 3387–3399. [DOI] [PubMed] [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- Alvarez V, Rossetti AO, Papavasileiou V, Michel P. Acute seizures in acute ischemic stroke: does thrombolysis have a role to play? J Neurol 2013; 260: 55–61. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012; 379: 2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin MP, Zhao G. Tissue plasminogen activator protects hippocampal neurons from oxygen-glucose deprivation injury. J Neurosci Res 2001; 63: 388–394. [DOI] [PubMed] [Google Scholar]
- Wu F, Wu J, Nicholson AD, Echeverry R, Haile WB, Catano M et al. Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. J Neurosci 2012; 32: 9848–9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Nagai N, Urano T. Role of tissue plasminogen activator/plasmin cascade in delayed neuronal death after transient forebrain ischemia. Neurosci Lett 2005; 381: 189–193. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 1991; 37: 173–182. [DOI] [PubMed] [Google Scholar]
- Roussel BD, Macrez R, Jullienne A, Agin V, Maubert E, Dauphinot L et al. Age and albumin D site-binding protein control tissue plasminogen activator levels: neurotoxic impact. Brain 2009; 132: 2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angles-Cano E. A spectrophotometric solid-phase fibrin-tissue plasminogen activator activity assay (SOFIA-tPA) for high-fibrin-affinity tissue plasminogen activators. Anal Biochem 1986; 153: 201–210. [DOI] [PubMed] [Google Scholar]
- Boutière B, Arnoux D, Benchimol P, Sampol J, Risède P, Anglés-Cano E. Solid-phase fibrin-tissue-plasminogen-activator activity assay (SOFIA-tPA): tPA but not UK is specifically detected. Thromb Res 1986; 43: 129–132. [DOI] [PubMed] [Google Scholar]
- Fleury V, Loyau S, Lijnen HR, Nieuwenhuizen W, Anglés-Cano E. Molecular assembly of plasminogen and tissue-type plasminogen activator on an evolving fibrin surface. Eur J Biochem 1993; 216: 549–556. [DOI] [PubMed] [Google Scholar]
- Longstaff C, Thelwell C, Williams SC, Silva MM, Szabó L, Kolev K. The interplay between tissue plasminogen activator domains and fibrin structures in the regulation of fibrinolysis: kinetic and microscopic studies. Blood 2011; 117: 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.