Abstract
Objectives. To describe salient epidemiological characteristics of Zika virus outbreaks across the world and to examine the clinical presentations, complications, and atypical manifestations related to their occurrence in recent history.
Methods. We conducted a systematic review of the literature by searching through MEDLINE, Embase, and Global Health Library, as well as the epidemiological bulletins and alerts from the World Health Organization, the Pan American Health Organization, and the European Centre for Disease Prevention and Control over the period 1954 to 2016.
Results. The search yielded 547 records. We retained 333 for further analysis, to which we added 11 epidemiological bulletins from various sources. Of these, we systematically reviewed 52 articles and reports, revealing some epidemiological features and patterns of spread of the Zika virus worldwide, as well as pathological outcomes suspected to be linked to Zika outbreaks. Neurologic disorders among zika patients were similar in Brazil and French Polynesia but a causal link is not established. Incidence of zika infection in pregnant women is not known. In Brazil, during the zika outbreak the incidence of microcephaly increased more than 20 times. Among 35 infants with microcephaly, born from women suspected to have Zika infection during pregnancy in northeast Brazil, 74% of the mothers reported rash during the first and second trimester.
Conclusions. On February 1, 2016, The World Health Organization declared the ongoing Zika crisis an emergency and that, although not yet scientifically proven, the link between the virus and growing numbers of microcephaly cases was “strongly suspected.” However, the causal relationship between zika and microcephaly is not universally accepted.
Public Health Implications. The current situation with regard to Zika is not encouraging, because there is no vaccine, no treatment, and no good serological test, and vector control remains a challenge.
Among diseases emerging in the 21st century, Zika is raising one of the greatest amounts of concern for public health globally. Zika virus (ZIKV) has presented as outbreaks since 2007; however, more recently, it has become the main suspected cause of an unusual and completely unexpected microcephaly epidemic, exposing the urgent needs for knowledge about this disease.
Zika fever is an exanthematous disease, related to dengue fever, West Nile, and yellow fever.1 This infection is characterized by symptoms that can last 1 week, with a clinical presentation similar to that of other arbovirus infections such as chikungunya and dengue, including mild fever, rash, arthralgia, arthritis, myalgia, headache, conjunctivitis, and edema. Severe cases involving hospitalization are uncommon, and deaths are rare.2 This disease is caused by a flavivirus, isolated for the first time in 1947 from the blood of a sentinel rhesus monkey (Macacamulatta) in the Zika forest near Entebbe, Uganda.3
Zika virus has been isolated from Aedes africanus,4 Aedes luteocephalus,5 Aedes aegypti,6 Aedes albopictus,7,8 Aedes furcifer,9 and Aedes vittatus5,9 mosquitos and, therefore, although A aegypti is the main vector in the Brazil epidemic, all of these Aedes species are probably involved in the transmission of ZIKV to humans. Zika was the predominant virus identified during the Yap Island outbreak, even though it was not isolated from Aedes hensilli. The evidence that this species was the most likely vector of dengue made this the suspected vector of ZIKV in Micronesia.10 Aedes species present special difficulty to vector control agencies, mainly because they can reproduce in extremely small amounts of water (e.g., the water in a bottle cap) and their eggs are extremely hardy (e.g., the eggs can survive drying for more than a year).
Recently, a large increase was observed in the circulation of ZIKV worldwide, which initially was endemic only in Africa and Asia. Cases have been reported in countries of Europe, Oceania, and the Americas, particularly in Latin America where it is rapidly spreading to new areas.11 From places with established autochthonous transmission, such as Brazil, viremic travelers have the capacity to introduce ZIKV into new countries, where Aedes mosquitoes would become infected and perpetuate local transmission cycles. In South America, Brazil had large concentration of cases of Zika, especially in the Northeast region, and serious complications occurred simultaneously with the outbreak of this arbovirus. Until this moment, only 1 review on Zika has been published,12 and that was before the virus’s introduction in the American region. Thus, we decided to produce another review with the newly available information, and to identify gaps in the available knowledge about this disease.
The aim of our systematic review was to describe the current knowledge on the epidemiological characteristics, frequency, spatial distribution, clinical presentation, and complications or atypical manifestations related to the occurrence of Zika outbreaks.
METHODS
This study is being reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The protocol was registered on PROSPERO, an international database of prospectively registered systematic reviews in health and social care. The registration number is CRD42016033168.
We searched MEDLINE, Embase, and Global Health Library to identify studies reporting epidemiological aspects of Zika disease worldwide (age, sex, prevalence, attack rates), clinical presentation, atypical manifestations, and negative fetal outcomes. We searched databases in January 2016 with the following approach: (1) Zika terms “Zika” and “Zika infections,” and (2) “epidemic,” “epidemiology,” “outbreak,” “seroprevalence,” “attack rates,” “clinical presentation,” “clinical manifestation,” “clinical symptoms,” “clinical features,” “atypical manifestations,” “neurological symptoms,” “cardiovascular symptoms,” “eye disease,” “kidney disease,” “ocular symptoms,” and “vertical transmission.” We supplemented database searches by screening bibliographies of the articles. We also included epidemiology bulletins from World Health Organization (WHO), Pan American Health Organization (PAHO), European Centre for Disease Prevention and Control (ECDC), and ministries of health from countries affected by Zika virus. We included studies published in English, Portuguese, French, and Spanish. We reviewed all titles and abstracts of publications identified in the course of the primary search for relevance and eligibility after we removed the duplicate articles. (Full electronic search strategy for MEDLINE can be found in Table A, available as a supplement to the online version of this article at http://www.ajph.org.)
Eligibility criteria were original studies that reported cases of Zika infections, and epidemiology bulletins from the WHO, PAHO, ECDC, and ministries of health from countries affected by Zika virus. Eligible study designs were case–control, cohort, cross-sectional case series, case reports, and ecological studies. We excluded reviews, in vitro studies, animal studies, studies of disease vectors, and studies examining Zika and other exanthematous diseases together or without the epidemiological aspects or clinical presentation.
Two independent reviewers (ESP, FB) screened article titles and abstracts to select articles for full-text screening. The reviewers assessed full texts independently; in case of disagreement, they consulted a third author (MNC), and agreed upon a decision by consensus.
We used a uniform tool to extract data from eligible articles and bulletins: study design, year of publication, study location, period of study, authors, and population characteristics such as age, sex, ethnicity, clinical symptoms, frequency of outcomes, and laboratory confirmation.
The epidemiological characteristics of the patients with Zika (mean age, sex) and clinical presentation are presented and the attack rate and proportion of complications calculated.
RESULTS
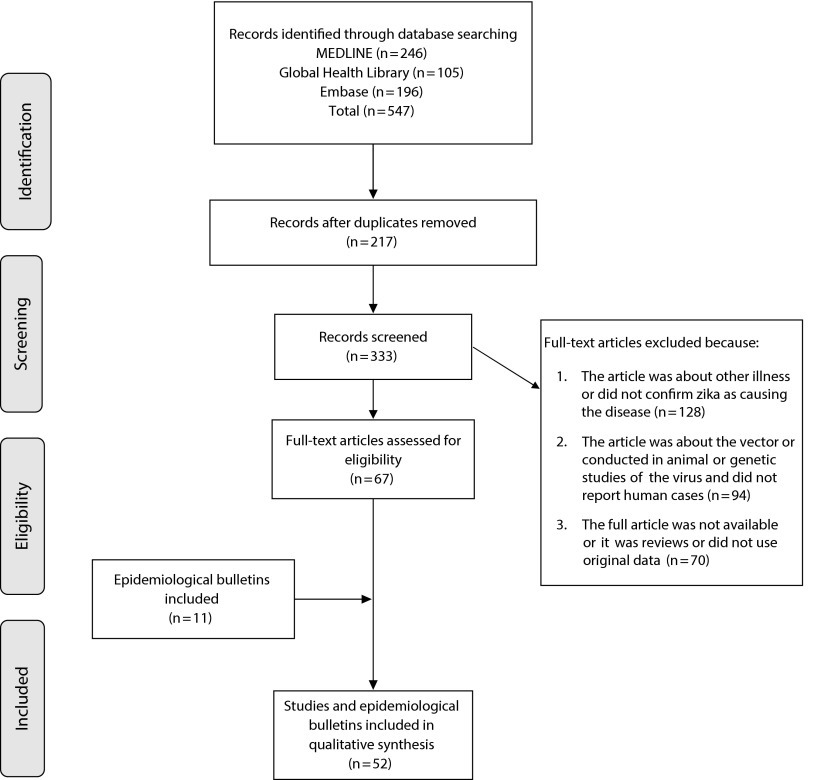
Results of the search are presented in Figure 1. The primary search identified 547 papers. We removed 217 duplicates. We screened 333 articles to assess eligibility, and excluded 292 that did not meet the inclusion criteria. We included 41 articles in the synthesis (30 case reports or case series and 11 surveillance or cross-sectional studies). We also included 11 epidemiological bulletins and alerts from WHO, PAHO, ECDC, and ministries of health from Brazil and French Polynesia.
FIGURE 1—
Process of Selection of the Studies for a Systematic Review on Epidemiology and Atypical Manifestations of Zika Virus Outbreaks
Study Characteristics
The case report, case series surveillance, and cross-sectional study characteristics are described in Table A. The studies were published from 1954 to 2016. The majority were case reports describing clinical symptoms of patients with laboratory-confirmed illness. These cases presented as a mild nonspecific disease; the most common symptoms reported were rash, fever, arthralgia, conjunctivitis, myalgia, and headache; 55% of the case reports described Zika in travelers.13–29 Coinfection occurred with dengue,30 chikungunya,31 and HIV,32 but the patients recovered without complications. Although Zika is a vector-borne disease, there is some evidence of sexual27,33 and perinatal34 transmission, and a theoretical possibility of transmission via blood transfusion.35 The virus was found in blood,35 semen,33 urine,14,36 and saliva samples,37 and 1 study proposed the bite of a monkey as a plausible route of transmission.15
Epidemiology
After the first evidence of human infection in 1952,38 sporadic cases and serological evidence of Zika were reported in surveys and case reports, showing that Zika was active in several countries in Africa and Asia39–42 before spreading to the Pacific region and more recently to the Americas. In 1954, a serological surveillance in French Equatorial Africa showed only 0.5% were positive for Zika antibodies.39 In Nigeria (1971–1975), 38% of the individuals had neutralizing antibodies to Zika in sera,38 and this disease was serologically confirmed in 3.1% of febrile patients in an hospital in Java, Indonesia (1977–1978).42
From 1983 until 2006, there were no publications on Zika, until the Yap Island outbreak when it was detected for the first time in Oceania. The overall attack rate (of Zika illness presenting to a health care facility) was 14.6 per 1000 inhabitants. It was higher among women and the mean age was 36 years.43 A household survey conducted in this area estimated a Zika infection rate (positive immunoglobulin M) of 73% (95% confidence interval = 68%, 77%), and clinical manifestations in approximately 1 in every 5 infected people. Males were more likely to have Zika infection than females; this result did not differ across age groups. No hospitalizations or deaths attributable to Zika were detected, but the population was small (fewer than 10 000 people).43
From 2008 to 2013, 4 studies were published on Zika—3 case reports and 1 surveillance study. Three cases of Zika occurred in Senegal and Colorado (possible non–vector-borne transmission: 2 scientists acquired Zika in Senegal and returned home to Colorado, and 1 transmitted Zika to his wife via semen),27 and 2 in Cambodia44,45; the surveillance study was conducted in Cameroon.46 No further transmission was reported in the Pacific region until 2013, when French Polynesia identified its first case. Statistics of the country estimated that 11.5% (32 000) of the population used the health facilities with Zika-like symptoms. A total of 383 cases were serologically confirmed; the mean age of those patients was 28 years.47 A serological surveillance conducted in 2013 to 2014 among blood donors found that 2.8% were reverse-transcription polymerase chain reaction (RT-PCR) positive for Zika. Out of the 42 positive donors, 26.2% reported Zika fever–like disease from 3 to 10 days after blood donation.35
During the Zika outbreak in French Polynesia, an unusual increase in the number of neurological and autoimmune complications was identified. Among patients that visited health care facilities with Zika-like symptoms, 2.3 per 1000 had neurological complications and 1.3 per 1000 (42 cases) had Guillain–Barré Syndrome (GBS). Among the GBS cases, 88% reported a viral syndrome up to 23 days before the onset of the neurological syndrome. Only 1 case was laboratory-confirmed during the infection by RT-PCR48 and several other cases were found to be immunoglobulin G–positive against Zika after the neurological signs; the average age was 46 years and 74% were men.47 Fifteen cases required intensive care, and 9 needed mechanical ventilation; however, no deaths were reported.49,50
After the report of the Brazilian epidemic of microcephaly in 2013, French Polynesia authorities looked back and reported central nervous system malformations in fetuses and newborns to women who were pregnant during the Zika outbreak on the island. They identified 17 cases of malformations.51 None of the pregnant women reported clinical signs of Zika; however, a serological test was performed in 4 women and they were immunoglobulin G–positive for flavivirus, suggesting a possible asymptomatic Zika infection.51 Perinatal Zika transmission was reported in 2 cases in French Polynesia in 2013 to 2014.34
The French Polynesian outbreak spread to other Pacific islands and autochthonous cases have been reported in New Caledonia (1400 confirmed cases),52 Cook Islands (932 suspected cases, 50 confirmed),52 Fiji, Samoa, and Solomon Island.53 Countries such as Philippines54 and Thailand55 have been reporting cases of Zika. Imported cases (without autochthonous transmission) were reported in Japan,14,18 Australia,15,16 Italy,24 Germany,26 Norway,25 Canada,20 United States,19,29,27 and United Kingdom28 in persons who visited countries with local transmission.
The autochthonous circulation of Zika in the Americas was first confirmed on Easter Island (Chile) in 2014. A total of 51 of 89 samples from cases suspected of Zika were positive by RT-PCR and the majority of the positive patients were women.56 In early 2015, Zika infection was laboratory-confirmed in Brazil and autochthonous transmission established.57,58 According to preliminary estimates from the Brazilian Ministry of Health, between 440 000 and 1 300 000 cases of Zika occurred in Brazil in 2015.59 In Bahia (northeast state of Brazil), the attack rate in 2015, detected among reported cases, was approximately 4.4 per 1000 inhabitants. In some cities of Bahia, such as Camaçari, Itabuna, Senhor do Bomfim, and Monte Santo, the attack rate was greater than 25 per 1000 inhabitants.60
As in French Polynesia, an unusual increase in the number of neurological manifestations and GBS occurred in Brazil. In Bahia, the proportion of neurological complications temporally associated with Zika was 2.3 per 1000 (proportion estimated with reported cases)60; GBS was diagnosed in 1 of every 1000 reported cases. Laboratory testing was performed in 224 samples of suspected Zika cases; this virus was confirmed in 10 patients, and 7 of those with viral confirmation had a neurological syndrome.61 In Brazil, 2 deaths of adults were attributed to Zika and 7 are under investigation by the Ministry of Health.59
At the end of 2015, Brazilian authorities reported possible links between Zika infection during pregnancy and microcephaly.62 Over the past 5 years, an annual average of 163 (5.6 per 100 000 live births) cases of microcephaly were routinely identified each year, according to routine birth reports. In 2015, there were 3530 (121.7 per 100 000 live births) suspected cases of microcephaly reported including 46 deaths, mainly in Pernambuco, a state that concentrated 35% of the total of suspected cases of microcephaly.63 ZIKV RNA was found in amniotic fluid samples from 2 pregnant women with fetal microcephaly.64 The 2 women had Zika-like symptoms at gestation weeks 18 and 19.
Currently, autochthonous Zika transmission has occurred in 27 counties in the Americas including Colombia (16 419 reported cases; 66.4% were female; 798 laboratory-confirmed cases); Guatemala (17 suspected cases); Mexico (confirmed local transmission); Panama (3 cases); Paraguay (6 laboratory-confirmed cases); Venezuela (4 laboratory-confirmed cases, 15 GBS cases); El Salvador (240 cases, 46 GBS cases, 54% of them male, and 2 deaths); Honduras, and Martinique. Bolivia, Guyana, Ecuador, Guadeloupe, Guatemala, Puerto Rico, Barbados, Saint Martin, and Haiti have reported sporadic transmission following recent introduction.65
DISCUSSION
We systematically reviewed 52 studies and epidemiology bulletins reporting epidemiological aspects of Zika disease worldwide (age, sex, seroprevalence, attack rates), clinical presentation, atypical manifestations, and negative fetal outcomes. On the basis of the data published in these documents, it is possible to know some epidemiological characteristics of Zika outbreaks and to have some idea where this illness has been circulating worldwide. In addition, we report some complications that have occurred during Zika outbreaks.
Epidemiological Patterns of Emergence and Spread
Zika infection is an acute exanthematous disease that for many years circulated silently in Africa and Asia. During this period, data about cases of Zika were restricted to case reports and serological surveys; the clinical presentation was similar to that of nonspecific viral illness. There were a few epidemiological characteristics of the patients reported, and atypical presentations were not reported.
The highest attack rate estimated among patients that presented to health care facilities was documented in French Polynesia. In Brazil, the true attack rate of the epidemic is not known; however, a study conducted in 1 city of Bahia showed that, from February to June 2015, an outbreak of indeterminate acute exanthematous disease occurred, in which 14 835 cases were reported. It was suggested that this outbreak was caused by ZIKV and the overall attack rate was 5.5 per 1000 inhabitants,66 almost 3 and 20 times lower than in Micronesia and French Polynesia, respectively. The overall attack rate, estimated by reported cases, greatly varied between the countries. We suggest 3 possible reasons for this: (1) the competence of mosquito vector (the predominant vector in Yap was A hensilli whereas in French Polynesia and Brazil it was A aegypti), (2) The multiplicity of analogous diseases circulating simultaneously (dengue and Zika in Yap and French Polynesia and Zika, dengue and chikungunya in Brazil) increase the probability of misdiagnosis and consequently undernotification, and (3) the recent introduction in Brazil which did not allow the outbreak to reach its peak.
In places where gender information was available (Yap, Brazil, Thailand, and Easter Island) among patients who had access to health care facilities, the disease was more frequently seen in women,43,55,56,66 possibly because women attended health services more often than did men. The mean age of the patients was slightly different, but all outbreaks reached all age groups, which is the classic pattern of an introduction of new disease in a susceptible population.
The estimated rate of asymptomatic Zika infection, as was described for other arboviruses such as West Nile virus67 and dengue,68 is high. If we use the ratio of 1 to 5 clinical cases observed in Micronesia to estimate the attack rate of Zika infection in French Polynesia and Brazil, we estimate that 55% of the French Polynesian and 2.5% of Brazilian inhabitants have been infected. Challenges for accurate estimates include a high rate of unapparent infections and the cross-reactivity of the serologic test of zika and dengue.
Potential Pathological Outcomes Linked to Zika Outbreaks
Hospitalizations for Zika were not reported until the French Polynesia outbreak, when neurological complications were identified temporally and spatially connected with Zika. In Brazil, Venezuela, and El Salvador, GBS cases correlating with Zika outbreaks were also reported. The proportions of neurological disorders including GBS among Zika patients were very similar in Brazil and French Polynesia. In mice, ZIKV has a brain tropism, suggesting that the virus can cross the blood–brain barrier and cause negative outcomes3; however, more investigations are required to prove that this complication was caused by ZIKV. The literature comprises cases of neurological disorders including GBS caused by other flaviviruses such as West Nile virus69 and dengue.70
Authorities of French Polynesia and Brazil reported possible links between Zika infection during pregnancy and microcephaly, and perinatal transmission of Zika was confirmed in 2 reported cases.34 The incidence of Zika infection in pregnant women is not known, and data on pregnant women infected with ZIKV are scarce. In Brazil, during the Zika outbreak, the incidence of microcephaly increased more than 20 times. Among 35 infants with microcephaly born from women suspected to have Zika infection during pregnancy in northeast Brazil, 74% of the mothers reported having a rash during the first and second trimester.71
The causal relationship between Zika and microcephaly, although sufficiently established for public health actions, is not universally accepted. Experts agree that the reported number is likely inflated because of the search for cases and because of misdiagnosis; so far, out of the cases of microcephaly investigated with neuroimaging, 270 cases were confirmed and 462 were rejected as false diagnoses. According to the Latin American Collaborative Study of Congenital Malformations, the number of suspected cases of microcephaly is too high to be plausible.72 Epidemiological research including case–control studies and prospective studies of pregnant women with rash will finally establish the causal association between Zika infection and the microcephaly outcomes.
Limitations of the Study
This literature review has some limitations. Zika is an emerging disease, and so there is a small number of studies that address this infection, the majority of which are case reports and a small number of serological surveys mainly conducted before the spreading of Zika to the American region. We also included in this review data provided by the passive surveillance systems, which can vary with the quality and coverage of the local surveillance system, and over time, and may underrepresent the real number of cases. Another limitation is the lack of epidemiological studies about this disease and the potential complications that occurred during the outbreaks. We did not carry out an assessment of quality of the studies and we did not exclude studies with potential weaknesses; as a consequence, any limitations of the original studies are pointed out in this review. The inclusion of publications in 4 languages reduced selection bias.
Perspectives
The rapid spread of Zika infection raises new challenges for the health authorities and researchers about the magnitude and possible complications in future outbreaks. Cases of Zika in travelers also raise concerns among unaffected countries since nonautochthonous cases have been diagnosed in Europe; ZIKV has the potential to rapidly spread across Latin America and the Caribbean.73 It has been suggested that global warming may have favored the reemergence, emergence, and rapid spread of arboviruses worldwide.74 With regard to Zika outbreaks, there are more questions than answers, and further studies are required to address questions about competence of the vector, proportion of asymptomatic and symptomatic cases, long-lasting natural immunity, and whether the relationship with Zika and microcephaly and neurologic disorders is causal.
The current situation with regard to Zika is not encouraging, because there is no vaccine, no treatment, and no good serological test, and vector control remains a challenge. There is no information about the burden caused by the cocirculation with other arboviruses such as dengue and chikungunya, both endemic diseases in countries where Zika recently has been introduced. In Brazil, for example, in 2015, more than 1 649 008 cases of dengue and more than 20 000 cases of chikungunya have been reported.75 Zika is transmitted by the same vectors as dengue; thus, the prospect of Zika spreading to more than a hundred countries where dengue is endemic–epidemic is concrete. However, if it is confirmed that Zika is causing complications such as GBS and congenital malformations, the future scenario presented will be much more worrisome than it was for dengue, and this illness can be considered one of the greatest challenges and problems of our time for global public health.
ACKNOWLEDGMENTS
This study was supported by the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico; process 205427/2014-6).
Note. The funders had no role in the study.
HUMAN PARTICIPANT PROTECTION
For this systematic review, we used data already published from other studies, so ethics approval was not required.
REFERENCES
- 1.Kuno G, Chang G, Tsuchiya K et al. Phylogeny of the genus Flavivirus. J Virol. 1998;72(1):73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Symptoms, diagnosis, & treatment. National Center for Emerging and Zoonotic Infectious Diseases. 2015. Available at: http://www.cdc.gov/zika/symptoms. Accessed January 28, 2016.
- 3.Dick GW, Kitchen S, Haddow A. Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 4.Haddow AJ, Williams M, Woodall J et al. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bull World Health Organ. 1964;31(1):57–69. [PMC free article] [PubMed] [Google Scholar]
- 5.Diagne CT, Diallo D, Faye O et al. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect Dis. 2015;15(1):492. doi: 10.1186/s12879-015-1231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18(3):411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- 7.Grard G, Caron M, Mombo IM et al. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8(2):e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong PS, Li M, Chong C et al. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis. 2013;7(8):e2348. doi: 10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diallo D, Sall A, Diagne C et al. Zika virus emergence in mosquitoes in Southeastern Senegal, 2011. PLoS One. 2014;9(10):e109442. doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledermann JP, Guillaumot L, Yug L et al. Aedes hensilli as a potential vector of chikungunya and zika viruses. PLoS Negl Trop Dis. 2014;8(10):e3188. doi: 10.1371/journal.pntd.0003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15(9):1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioos S, Mallet HP, Leparc Goffart I et al. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44(7):302–307. doi: 10.1016/j.medmal.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Korhonen EM, Huhtamo E, Smura T et al. Zika virus infection in a traveler returning from the Maldives, June 2015. Euro Surveill. 2016;21(2):30107. doi: 10.2807/1560-7917.ES.2016.21.2.30107. [DOI] [PubMed] [Google Scholar]
- 14.Shinohara K, Kutsuna S, Takasaki T et al. Zika fever imported from Thailand to Japan, and diagnosed by PCR in the urines. J Travel Med. 2016;23(1):tav011. doi: 10.1093/jtm/tav011. [DOI] [PubMed] [Google Scholar]
- 15.Leung GH, Baird RW, Druce J et al. Zika virus infection in Australia following a monkey bite in Indonesia. Southeast Asian J Trop Med Public Health. 2015;46(3):460–464. [PubMed] [Google Scholar]
- 16.Pyke AT, Daly M, Cameron J et al. Imported Zika virus infection from the Cook Islands into Australia, 2014. PLoS Curr. 2014:6. doi: 10.1371/currents.outbreaks.4635a54dbffba2156fb2fd76dc49f65e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwong JC, Druce J, Leder K. Zika virus infection acquired during brief travel to Indonesia. Am J Trop Med Hyg. 2013;89(3):516–517. doi: 10.4269/ajtmh.13-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutsuna S, Kato Y, Takasaki T et al. Two cases of Zika fever imported from French Polynesia to Japan, December 2013 to January 2014. Euro Surveill. 2014;19(4):20683. doi: 10.2807/1560-7917.es2014.19.4.20683. [DOI] [PubMed] [Google Scholar]
- 19.Summers DJ, Acosta RW, Acosta AM. Zika virus in an American recreational traveler. J Travel Med. 2015;22(5):338–340. doi: 10.1111/jtm.12208. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca K, Meatherall B, Zarra D et al. First case of Zika virus infection in a returning Canadian traveler. Am J Trop Med Hyg. 2014;91(5):1035–1038. doi: 10.4269/ajtmh.14-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tappe D, Nachtigall S, Kapaun A et al. Acute Zika virus infection after travel to Malaysian Borneo, September 2014. Emerg Infect Dis. 2015;21(5):911–913. doi: 10.3201/eid2105.141960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karen BB, Whitney SP, Robert CF. Trouble in paradise. IDCases. 2014;1:95–96. doi: 10.1016/j.idcr.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zammarchi L, Tappe D, Fortuna C et al. Zika virus infection in a traveler returning to Europe from Brazil, March 2015. Euro Surveill. 2015;20(23):21153. doi: 10.2807/1560-7917.es2015.20.23.21153. [DOI] [PubMed] [Google Scholar]
- 24.Zammarchi L, Stella G, Mantella A et al. Zika virus infections imported to Italy: clinical, immunological and virological findings, and public health implications. J Clin Virol. 2015;63:32–35. doi: 10.1016/j.jcv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Wæhre T, Maagard A, Tappe D et al. Zika virus infection after travel to Tahiti, December 2013. Emerg Infect Dis. 2014;20(8):1412–1414. doi: 10.3201/eid2008.140302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tappe D, Rissland J, Gabriel M et al. First case of laboratory-confirmed Zika virus infection imported into Europe, November 2013. Euro Surveill. 2014;19(4):20685. doi: 10.2807/1560-7917.es2014.19.4.20685. [DOI] [PubMed] [Google Scholar]
- 27.Foy BD, Kobylinski KC, Chilson Foy JL et al. Probable non–vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hearn P, Atkinson B, Hewson R et al. Identification of the first case of imported Zika fever to the UK: a novel sample type for diagnostic purposes and support for a potential non-vectorborne route of transmission. Am J Trop Med Hyg. 2014;91(5 suppl 1):62–63. [Google Scholar]
- 29.McCarthy M. First US case of Zika virus infection is identified in Texas. BMJ. 2016;352:i212. doi: 10.1136/bmj.i212. [DOI] [PubMed] [Google Scholar]
- 30.Dupont-Rouzeyrol M, O’Connor O, Calvez E et al. Co-infection with Zika and dengue viruses in 2 patients, New Caledonia, 2014 [letter] Emerg Infect Dis. 2015;21(2):381–382. doi: 10.3201/eid2102.141553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villamil-Gómez WE, González-Camargo O, Rodriguez-Ayubi J et al. Dengue, chikungunya and Zika co-infection in a patient from Colombia [letter] J Infect Public Health. 2016 doi: 10.1016/j.jiph.2015.12.002. Epub ahead of print January 2, 2016. [DOI] [PubMed] [Google Scholar]
- 32.Calvet GA, Filippis AM, Mendonça MC et al. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro. Brazil. J Clin Virol. 2016;74:1–3. doi: 10.1016/j.jcv.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Musso D, Roche C, Robin E et al. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21(2):359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Besnard M, Lastere S, Teissier A et al. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):20751. [PubMed] [Google Scholar]
- 35.Musso D, Nhan T, Robin E et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014;19(14):20761. doi: 10.2807/1560-7917.es2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- 36.Gourinat AC, O’Connor O, Calvez E et al. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21(1):84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musso D, Roche C, Nhan TX et al. Detection of Zika virus in saliva. J Clin Virol. 2015;68:53–55. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979;83(2):213–219. doi: 10.1017/s0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellissier A. Serological investigation on the incidence of neurotropic viruses in French Equatorial Africa. Bull Soc Pathol Exot Filiales. 1954;47(2):223–227. [PubMed] [Google Scholar]
- 40.Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg. 1964;58:335–338. [PubMed] [Google Scholar]
- 41.MacNamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48(2):139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 42.Olson JG, Ksiazek TG, Suhandiman et al. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75(3):389–393. doi: 10.1016/0035-9203(81)90100-0. [DOI] [PubMed] [Google Scholar]
- 43.Duffy MR, Chen TH, Hancock WT et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 44.Heang V, Yasuda CY, Sovann L et al. Zika virus infection, Cambodia, 2010 [letter] Emerg Infect Dis. 2012;18(2):349–351. doi: 10.3201/eid1802.111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heang V, Yasuda C, Ngan C et al. Zika virus from fever syndromic surveillance in Cambodia. Am J Trop Med Hyg. 2011;85(6 suppl 1):183. [Google Scholar]
- 46.Fokam EB, Levai LD, Guzman H et al. Silent circulation of arboviruses in Cameroon. East Afr Med J. 2010;87(6):262–268. doi: 10.4314/eamj.v87i6.63085. [DOI] [PubMed] [Google Scholar]
- 47.Malet H, Vial A, Musso D. Epidemiological and statistiques health information bulletin [in French]. Papeete, French Polynesia: Health Surveillance Office. Available at: http://www.hygiene-publique.gov.pf/IMG/pdf/no13_-_mai_2015_-_zika.pdf. Accessed January 25, 2016.
- 48.Oehler E, Watrin L, Larre P et al. Zika virus infection complicated by Guillain-Barré syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19(9):20720. doi: 10.2807/1560-7917.es2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 49.European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus infection outbreak, French Polynesia. 2014. Available at: http://ecdc.europa.eu/en/publications. Accessed January 19, 2016.
- 50.Pan American Health Organization, World Health Organization, Regional Office for the Americas. Epidemiological alert: Zika virus infection. 2015. Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=30075. Accessed January 20, 2016.
- 51.European Centre for Disease Prevention and Control. Microcephaly in Brazil potentially linked to the Zika virus epidemic. 2015. Available at: http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?ID=1332&List=8db7286c-fe2d-476c-9133-18ff4cb1b568&Source=http%3A%2F%2Fecdc.europa.eu%2Fen%2FPages%2Fhome.aspx. Accessed January 22, 2016.
- 52.Roth A, Mercier A, Lepers C et al. Concurrent outbreaks of dengue, chikungunya and Zika virus infections—an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Euro Surveill. 2014;19(41):20929. doi: 10.2807/1560-7917.es2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization, Regional Office for the Western Pacific Region. Pacific syndromic surveillance report Manila, 2015. Available at: http://www.wpro.who.int/southpacific/programmes/communicable_diseases/disease_surveillance_response/PSS-16-August-2015/en. Accessed January 23, 2016.
- 54.Alera MT, Hermann L, Tac-An IA et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis. 2015;21(4):722–724. doi: 10.3201/eid2104.141707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buathong R, Hermann L, Thaisomboonsuk B et al. Detection of Zika virus infection in Thailand, 2012–2014. Am J Trop Med Hyg. 2015;93(2):380–383. doi: 10.4269/ajtmh.15-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tognarelli J, Ulloa S, Villagra E et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol. 2015 doi: 10.1007/s00705-015-2695-5. Epub ahead of print November 26, 2015. [DOI] [PubMed] [Google Scholar]
- 57.Zanluca C, Melo V, Mosimann A et al. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110(4):569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campos GS, Bandeira A, Sardi S. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21(10):1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zika virus epidemic in the Americas. potential association with microcephaly and Guillain-Barré syndrome. Stockholm, Sweden: European Centre for Disease Prevention and Control; 2015. Available at: http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association-with-microcephaly-rapid-risk-assessment.pdf. Accessed January 23, 2016.
- 60.Ministry of Health of Brazil, Secretary of Health of the State of Bahia. Epidemiological situation of arboviruses. Bull Epidemiol. 2015:11. [Google Scholar]
- 61. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. Washington, DC: Pan American Health Organization, World Health Organization, Regional Office for the Americas; 2015.
- 62.European Centre for Disease Prevention and Control. Microcephaly in Brazil potentially linked to the Zika virus epidemic. 2015. Available at: http://ecdc.europa.eu/en/publications/Publications/zika-microcephaly-Brazil-rapid-risk-assessment-Nov-2015.pdf. Accessed January 26, 2016.
- 63.Pan American Health Organization, World Health Organization, Regional Office for the Americas. Epidemiological alert: neurological syndrome, congenital anomalies, and Zika virus infection. 2016. Available at: http://www.paho.org/hq/index.php?option=com_content&view=category&layout=blog&id=1218&Itemid=2291. Accessed January 26, 2016.
- 64.Ministry of Health of Brazil. The Ministry of Health announces epidemiological bulletin. Available at: http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/20805. Accessed January 28, 2016.
- 65.European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update), 21 January 2016. Available at: http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Accessed January 28, 2016.
- 66.Cardoso CW, Paploski A, Kikuti M et al. Outbreak of exanthematous illness associated with Zika, chikungunya, and dengue viruses, Salvador, Brazil. Emerg Infect Dis. 2015;21(12):2274–2276. doi: 10.3201/eid2112.151167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mostashari F, Bunning M, Kitsutani P . Lancet. Epidemic West Nile encephalitis; New York: 1999. results of a household-based seroepidemiological survey. 2001;358(9278):261–264. [DOI] [PubMed] [Google Scholar]
- 68.Grange L, Simon-Loriere E, Sakuntabhai A et al. Epidemiological risk factors associated with high global frequency of inapparent dengue virus infections. Front Immunol. 2014;5:280. doi: 10.3389/fimmu.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hart J, Tillman G, Kraut M et al. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis. 2014;14(1):248. doi: 10.1186/1471-2334-14-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solbrig MV, Perng G. Current neurological observations and complications of dengue virus infection. Curr Neurol Neurosci Rep. 2015;15(6):29. doi: 10.1007/s11910-015-0550-4. [DOI] [PubMed] [Google Scholar]
- 71.Schuler-Faccini L, Ribeiro EM, Feitosa IM et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- 72.Butler D. Zika virus: Brazil’s surge in small-headed babies questioned by report. Nature News. Available at: http://www.nature.com/news/zika-virus-brazil-s-surge-in-small-headed-babies-questioned-by-report-1.19259. Accessed January 28, 2016.
- 73.Bogoch I, Brady O, Kraemer M et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016 doi: 10.1016/S0140-6736(16)00080-5. Epub ahead of print January 15, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomas SM, Tjaden N, Van den Bos S, Beierkuhnlein C. Implementing cargo movement into climate based risk assessment of vector-borne diseases. Int J Environ Res Public Health. 2014;11(3):3360–3374. doi: 10.3390/ijerph110303360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ministry of Health of Brazil. Secretary of Surveillance in Health. A–Z. Epidemiological Bulletin. Monitoring of cases of dengue, chikungunya fever and fever by Zika virus through epidemiological week 52, 2015 [in Portuguese]. Available at: http://portalsaude.saude.gov.br/images/pdf/2016/janeiro/15/svs2016-be003-dengue-se52.pdf. Accessed January 26, 2016.