Abstract
We describe the epidemic of microcephaly in Brazil, its detection and attempts to control it, the suspected causal link with Zika virus infection during pregnancy, and possible scenarios for the future. In October 2015, in Pernambuco, Brazil, an increase in the number of newborns with microcephaly was reported. Mothers of the affected newborns reported rashes during pregnancy and no exposure to other potentially teratogenic agents. Women delivering in October would have been in the first trimester of pregnancy during the peak of a Zika epidemic in March. By the end of 2015, 4180 cases of suspected microcephaly had been reported. Zika spread to other American countries and, in February 2016, the World Health Organization declared the Zika epidemic a public health emergency of international concern. This unprecedented situation underscores the urgent need to establish the evidence of congenital infection risk by gestational week and accrue knowledge. There is an urgent call for a Zika vaccine, better diagnostic tests, effective treatment, and improved mosquito-control methods.
Less than a year after the first identification, in April 2015, of Zika virus (ZIKV) in Brazil,1 there was an outbreak of an exanthematous disease in its northeastern region tentatively attributed to ZIKV. ZIKV was later detected in 20 of the 27 states in Brazil2 and in 18 countries in America.3 A sharp increase in microcephaly is expected among the offspring of women who were pregnant and infected during the subsequent outbreaks give birth.
Microcephaly is an abnormally small head at birth because of defective brain development. It can have genetic or environmental causes. Environmental exposures include radiation, drugs, fetal alcohol syndrome, and infections. Well-known agents of congenital infections include toxoplasmosis, rubella, cytomegalovirus, herpesvirus, and syphilis (TORCHES).4,5 Until November 2015, ZIKV has never been considered to be a cause of congenital infections or microcephaly.6
ZIKV is an RNA arbovirus, Flaviviridae family (genus Flavivirus), transmitted by the Aedes mosquito (which is also the vector for dengue). Because dengue and Zika share a vector, Zika could establish itself in any country where dengue is present. ZIKV is genetically close to dengue, West Nile, yellow fever, and Japanese encephalitis viruses.7 One study suggests that most (80%) ZIKV infections appear to be asymptomatic.8 When clinical features are present, they are similar to those of dengue and chikungunya7—both arboviruses that are circulating in Brazil.8 The force of transmission of ZIKV can be very high, as 73% of the population was estimated to have been infected in the 2007 outbreak in Yap, Federated States of Micronesia8; the rate of clinical cases was estimated to be 12% in the 2013–2014 outbreak in French Polynesia.9
ZIKV was isolated in Uganda in 1947, and only sporadic cases and small outbreaks were reported in Africa and Asia during the 1960s until early in the 21st century.7,10 In 2007, an outbreak was detected on Yap Island8; in 2013, an outbreak was detected in French Polynesia.9 In April 2015, ZIKV was identified in Brazil, and assessed to be the etiological agent of outbreaks of an acute exanthematous illness, which started in late 2014 in many cities of the northeast region.1,11
DETECTING THE EPIDEMIC
In October 2015, in the state of Pernambuco, Brazil, an increase was reported in the number of newborns with microcephaly: 26 cases in 3 weeks (from the 1st to the 21st). When the numbers were examined, the spike was clear: there had been 6 cases of microcephaly in the 7 months from January to July, 6 in August, 11 in September, and 39 in October. The State Secretariat for Health of Pernambuco raised an alert and notified the national surveillance system. A task force was set up to do a rapid assessment, including public health at the state and national levels. Thirty-eight mothers of 38 newborns with microcephaly were interviewed and 24 reported rash during the pregnancy. There was no common exposure during the pregnancy to pesticides, severe alcohol abuse, drugs, radiation, or other possible teratogenic agents. Women delivering in October would have been in the first trimester of pregnancy at the peak of the outbreak of exanthematous disease later identified as Zika (not then a reportable disease), which lasted from January to March 2015 (a physician, Carlos Brito, spotted the link). Evidence started to point toward the cases of microcephaly being caused by congenital Zika virus infection.
In November 2015, cases of microcephaly started to be diagnosed in other states of the northeast region.12 In the first week of November (epidemiological week 44) another 54 cases were reported in Pernambuco, and cases were diagnosed in 5 states of the northeastern region. On November 11, 2015, the Minister of Health declared a public health emergency as the increasing number of cases suggested that it could become a major epidemic. The World Health Organization (WHO) was notified, and on the first of December, the Pan American Health Organization issued an Epidemiological Alert to member states; by then cases of Zika virus had been reported in 9 countries in the Americas 13—the number was to rise to 18 by January 17, 2016.3
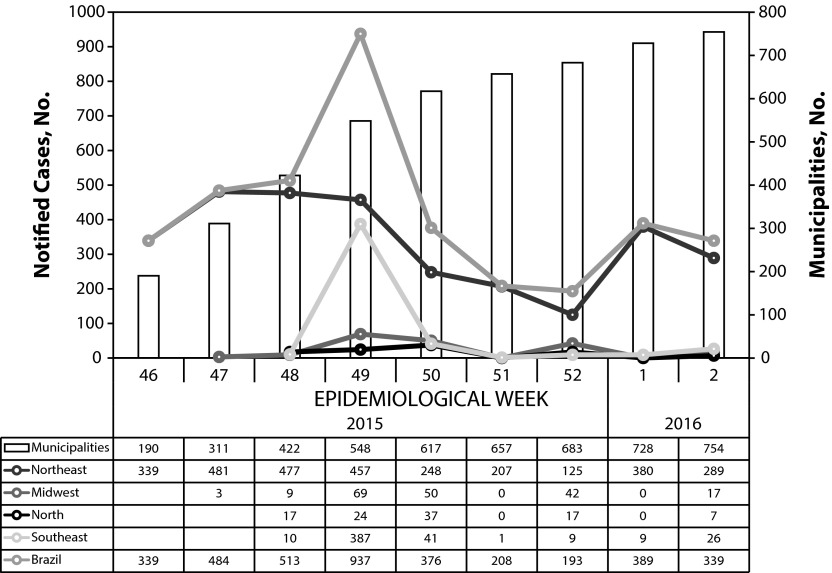
Epidemiological week 49 (Figure 1) saw the highest number of reports (900 cases in a week); by the end of the year, 4180 cases were reported in Brazil, in 21 of the 27 Brazilian states. Most (84.4%) of the municipalities with cases of microcephaly, and 90% of the cases reported until the second week of 2016 are in the northeastern region, which has fewer than 30% of the Brazilian population; 56 of the cases were stillbirths or spontaneous abortions.14 In the most-affected state, more than 1% of births in the period were reported with microcephaly. Microcephaly is not a very specific clinical diagnosis, and all reported cases are considered suspected until neurological damage is confirmed by magnetic resonance imaging. By the time of this writing, 500 reported cases had received magnetic resonance imaging, and 44% were confirmed as microcephaly.14 The remaining were considered normal indicating a degree of overreporting. If the proportion is maintained after all reported cases are examined, the epidemic up to January 2016 would consist of 1672 and not 4180 cases.
FIGURE 1—
Reported Cases of Microcephaly and Number of Municipalities, by Epidemiological Week, Brazil and Regions, 2015–2016
Note. Until epidemiological week 3.
Source. Ministry of Health of Brazil.
EVIDENCE FOR A LINK BETWEEN ZIKV AND MICROCEPHALY
The evidence for the epidemic of microcephaly resulting from congenital Zika is accumulating fast. The geographical distribution appears to reflect the areas of Zika outbreaks (Figures 1 and 2). Scientists from different disciplines reported that the abnormalities in the central nervous system appeared to have characteristics in common with other congenital infections (such as cytomegalovirus); arboviruses can cause congenital infections in animals15; cases were seronegative for TORCHES; and neurotropism of Zika was demonstrated in an animal model.16
FIGURE 2—
Distribution of Reported Cases of Microcephaly by Federated Unit, Brazil, 2015–2016
Note. Until epidemiological week 3.
Source. Ministry of Health of Brazil.
Most convincingly, a reexamination of the data revealed cases of microcephaly in women who were pregnant during the Zika outbreak in French Polynesia,17 increases in cases of Guillain-Barré syndrome were found after the outbreaks of Zika in French Polynesia and in Pernambuco,18,19 and Zika virus RNA was present in the amniotic fluid of 2 pregnant women whose fetuses had microcephaly, and in the tissue samples of a deceased case and in the placenta of a woman who reported rash and who had a miscarriage in the eighth week of pregnancy.20 Brazil declared that the cause of the epidemic was congenital Zika virus on November 28, 2015.21
CONTROL ACTIVITIES
In the absence of a vaccine (or antiviral drugs for infected pregnant women) control is restricted to monitoring the number of cases, advising women to consider avoiding pregnancy, advising pregnant women to avoid mosquito bites (long-sleeved clothes, repellent, screens on windows), and intensifying measures to combat Aedes. The agility, responsibility, and collective response by professionals, policymakers in Brazil (with support from the Pan American Health Organization), and scientists enabled a rapid recognition of this unpredictable event, proposal of a plausible hypothesis, production of evidence, and adoption of the actions for public health and for clinical support that were possible with the existing knowledge and that produced during the first few months of the epidemic.2
There is no doubt that WHO valued the information received from Brazil and other nations participating in the first meeting of the emergency committee convened by the Director-General under the International Health Regulations regarding clusters of microcephaly cases and other neurological disorders in some areas affected by Zika virus. This meeting was held by teleconference on February 1, 2016. Summarizing the report she received from the emergency committee, the Director-General of WHO declared “that the recent cluster of microcephaly cases and other neurological disorders reported in Brazil, following a similar cluster in French Polynesia in 2014, constitutes a Public Health Emergency of International Concern.”22 Yet, she added that “The experts agreed that a causal relationship between Zika infection during pregnancy and microcephaly is strongly suspected, though not yet scientifically proven. All agreed on the urgent need to coordinate international efforts to investigate and understand this relationship better.”22
Sharing information is important when there is a marked public concern. Therefore, the Brazil Ministry of Health released an accessible Web site that gives information and advice in a clear and friendly, but rigorous, way, and releases a weekly epidemiological bulletin on the ZIKV epidemic.23
In spite of all these measures, the risks for women to be infected and to transmit the infection to the fetus remain high. Pregnancy terminations are illegal in Brazil and in many other Latin American countries, independent of gestational age and presence of severe malformations. In light of the severity of the malformations being identified (not just neurological but also of hearing and sight),24,25 with likely extreme negative consequences for the families affected, it would be sensible to reopen the legalization of terminations debate to offer the women choice over the decision of continuing or interrupting such pregnancies with adequate medical care and legal protection.
CHALLENGES, RESEARCH PRIORITIES, AND PERSPECTIVES
Because Zika was only recently suspected to cause congenital disease, it was not a reportable disease in Brazil and there were no serological survey, and therefore, it is not possible to establish with certainty the proportion (or trends in the proportion) of the population who were infected by ZIKV since its introduction in different geographical areas. This information would be useful to predict the course of the microcephaly epidemic.
The main vector for Zika virus, Aedes aegypti, also the main vector for dengue, has high competence as a vector, is present in more than 100 countries, and has been resilient to existing control measures, even when implemented adequately: there have been successive, expanding dengue epidemics in Brazil and in the world.26,27 An expert entomologist at the Centers for Disease Control and Prevention, reported that 2 species of mosquitoes capable of transmitting the virus, A aegypti and Aedes albopictus, live in the southern, more tropical parts of the United States,28 so the emergence of ZIKV in the United States is a possibility, too.
Aedes is not known to reproduce in very cold climates, but there are also health concerns for countries where the vectors are not present, and the transmission by mosquitoes cannot occur: visitors to areas where Zika is circulating can be infected. Imported cases of Zika have been diagnosed in Europe29 and in the United States.30 Pregnant women who were infected in Zika-endemic countries might have microcephalic babies: the first US case was in Hawaii,31 and this led to the Centers for Disease Control and Prevention issuing an alert.32 The complete genome of Zika virus was recovered from the brain of a fetus with microcephaly who was born from a Slovenian mother who had lived in Brazil from December 2013 to 2015 and reported a Zika-like illness during the 13th week of gestation.33
Diagnosis of Zika is difficult. Because of the small number of laboratory-confirmed cases, and because the disease was not yet officially reportable case by case, it is not possible to make accurate predictions of the expected number of microcephaly cases in Brazil or in the rest of the Americas, but the perspective seems bleak.
We do not know if there is substantive, lasting immunity to ZIKV. If there is, and the attack rate is very high, it is possible that only the first outbreaks in each population will include adults, and that after the susceptibles are exhausted, ZIKV will become a disease predominantly of children, with much reduced risk of microcephaly. Researchers are working to produce robust evidence of the causal link, to estimate the risk of congenital infection in pregnant women by gestational week at infection, to establish the severity and clinical progression of affected newborns, and to investigate the biology and interaction between virus and host and the physiopathology. In addition, researchers are also working to develop better diagnostic testing and a vaccine. Such evidence is required to accrue knowledge and to develop effective and safe drugs for infected pregnant women and new technologies for vector control.
However, understanding and addressing such an unexpected, unpredicted, extreme, and rapidly expanding phenomenon requires a joint effort of the national and international scientific communities, public health policymakers, and funders. Otherwise, the world can only watch while this tragedy develops, compromising the cognitive and psychomotor development of a generation of children.
ACKNOWLEDGMENTS
We acknowledge the team of the Ministry of Health of Brazil.
HUMAN PARTICIPANT PROTECTION
Institutional review board approval was not required because no human participants were involved.
REFERENCES
- 1.Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21(10):1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde. Situação epidemiológica de ocorrência de microcefalias no Brasil, 2015. Bol Epidemiol. 2016;46(34):1. Available at: http://portalsaude.saude.gov.br/images/pdf/2016/janeiro/13/COES-Microcefalias—Informe-Epidemiol–gico-08—SE-01-2016—Valida—-o-12jan2016–-VALIDADO-PELO-CLAUDIO–e-com-os-estados-por-webconfer–n.pdf. Accessed January 16, 2016.
- 3.Pan American Health Organization, World Health Organization. Epidemiological update: neurological syndrome, congenital anomalies, and Zika virus infection. 17 January 2016. Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32879&lang=en. Accessed January 17, 2016.
- 4.Pan American Health Organization, World Health Organization. Congenital anomalies are the second-leading cause of death in children under 5 in the Americas. 2015. Available at: http://www2.paho.org/Hq/index.php?option=com_content&view=article&id=10487-2015-congenital-anomalies-second-leading-cause-of-death-in-children&catid=740&Itemid=1926&lang=en. Accessed January 16, 2016.
- 5.World Health Organization. Congenital anomalies. 2015. Available at: http://www.who.int/mediacentre/factsheets/fs370/en. Accessed January 16, 2016.
- 6.John CC, Carabin H, Montano SM et al. Global research priorities for infections that affect the nervous system. Nature. 2015;527(7578):S178–S186. doi: 10.1038/nature16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ioos S, Mallet HP, Leparc Goffart I et al. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44(7):302–307. doi: 10.1016/j.medmal.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Duffy MR, Chen TH, Hancock WT et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 9.Mallet HP, Vial AL, Nusso D. Bilan de l’epidemie a virus Zika en Polynesie Francaise, 2013–2014. Bull Inf Sanit Epidemiol Stat. 2015;13:1–5. [Google Scholar]
- 10.Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15(9):1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso CW, Paploski IAD, Kikuti M et al. Outbreak of exanthematous illness associated with Zika, chikungunya, and dengue viruses, Salvador, Brazil. Emerg Infect Dis. 2015;21(12):2274–2276. doi: 10.3201/eid2112.151167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde. Situação epidemiológica de ocorrência de microcefalias no Brasil, 2015. Bol Epidemiol. 2015;46(34):1–3. Available at: http://portalsaude.saude.gov.br/images/pdf/2015/novembro/19/Microcefalia-bol-final.pdf. Accessed January 16, 2016.
- 13.Pan American Health Organization. Epidemiological alert—neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas: 1 December 2015. Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed January 16, 2016. [Google Scholar]
- 14. Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde. Situação epidemiológica de ocorrência de microcefalias no Brasil, 2016. Bol Epidemiol. 2016. Available at: http://portalsaude.saude.gov.br/images/pdf/2016/janeiro/28/COES-Microcefalias—Informe-Epidemiol–gico-10—SE-03-2016—26jan2016—20h34.pdf. Accessed January 29, 2016.
- 15.Parsonson IM, Della-Porta AJ, Snowdon WA. Congenital abnormalities in newborn lambs after infection of pregnant sheep with Akabane virus. Infect Immun. 1977;15(1):254–262. doi: 10.1128/iai.15.1.254-262.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 17.Centre D’Hygiene et de Salubrite Publique. Note sur les investigations autour des malformations cérébrales congénitales ayant suivi l’épidémie de zika de 2013–2014. Available at: http://www.hygiene-publique.gov.pf/IMG/pdf/note_malformations_congenitales_cerebrales.pdf. Accessed December 1, 2015.
- 18.Oehler E, Watrin L, Larre P et al. Zika virus infection complicated by Guillain-Barré syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19(9):20720. doi: 10.2807/1560-7917.es2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 19. Fundação Oswaldo Cruz (Pernambuco). Nota Fiocruz PE esclarece detalhes relativos a pesquisas sobre zika na unidade. Available at: http://portal.fiocruz.br/pt-br/content/fiocruz-pernambuco-esclarece-duvidas-sobre-virus-zika. Accessed November 1, 2015.
- 20.Fundação Oswaldo Cruz (Paraná) Pesquisa da Fiocruz Paraná confirma transmissão intra-uterina do zika vírus. Available at: http://www.icc.fiocruz.br/pesquisa-da-fiocruz-parana-confirma-transmissao-intra-uterina-do-zika-virus. Accessed January 29, 2016.
- 21. Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde. Ministério da Saúde divulga novos dados de microcefalia. Available at: http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/svs/noticias-svs/21020-ministerio-da-saude-divulga-novos-dados-de-microcefalia. Accessed January 29, 2016.
- 22.World Health Organization. WHO director-general summarizes the outcome of the emergency committee regarding clusters of microcephaly and Guillain-Barré syndrome. Available at: http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en. Accessed February 2, 2016.
- 23. Brasil Ministério da Saúde. Prevenção e combate—dengue, chikungunya e Zika. 2015. Available at: http://combateaedes.saude.gov.br/situacao-epidemiologica. Accessed January 16, 2016.
- 24.Schuler-Faccini L, Ribeiro EM, Feitosa IM et al. Possible association between Zika vírus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- 25.Ventura CV, Maia M, Vasco Bravo-Filho ALG et al. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;387(10015):228. doi: 10.1016/S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- 26.Ooi E-E, Goh K-T, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12(6):887–893. doi: 10.3201/10.3201/eid1206.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teixeira MG, Costa M da CN, Barreto F et al. Dengue: twenty-five years since reemergence in Brazil. Cad Saude Publica. 2009;25(suppl 1):S7–S18. doi: 10.1590/s0102-311x2009001300002. [DOI] [PubMed] [Google Scholar]
- 28.Stein R. Big Zika virus outbreak unlikely in the US, officials say. Available at: http://www.npr.org/sections/health-shots/2016/01/26/464459350/big-zika-virus-outbreak-unlikely-in-the-u-s-officials-say. Accessed February 2, 2016.
- 29.Zammarchi L, Tappe D, Fortuna C et al. Zika virus infection in a traveller returning to Europe from Brazil, March 2015. Euro Surveill. 2015;20(23):21153. doi: 10.2807/1560-7917.es2015.20.23.21153. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy M. First US case of Zika virus infection is identified in Texas. BMJ. 2016;352 doi: 10.1136/bmj.i212. i212. [DOI] [PubMed] [Google Scholar]
- 31.Governor of the State of Hawaii. Hawaii Department of Health receives confirmation of Zika infection in baby born with microcephaly. Available at: http://governor.hawaii.gov/newsroom/doh-news-release-hawaii-department-of-health-receives-confirmation-of-zika-infection-in-baby-born-with-microcephaly. Accessed January 29, 2016.
- 32.Centers for Disease Control and Prevention. Travel health notices—alert level 2. 2016. Available at: http://wwwnc.cdc.gov/travel/notices. Accessed February 2, 2016.
- 33.Mlakar J, Korva M, Tul N et al. Zika virus associated with microcephaly. N Engl J Med. 2016 doi: 10.1056/NEJMoa1600651. Epub ahead of print February 10, 2016. [DOI] [PubMed] [Google Scholar]