Abstract
OBJECTIVES:
Soluble CD163 (sCD163), a marker of Kupffer cell activation detectable in serum, correlates with inflammation and fibrosis in chronic viral hepatitis, but its role in nonalcoholic fatty liver disease is unknown. We hypothesized that sCD163 would correlate with nonalcoholic fatty liver disease activity and fibrosis.
METHODS:
Liver biopsies and serum were obtained from 145 obese subjects undergoing gastric bypass surgery. Subjects were divided into four groups based on fibrosis stage and nonalcoholic fatty liver disease activity score (NAS); Group 1: F0, NAS=0; Group 2: F<2, 0<NAS<5; Group 3: NAS≥5, F<3; or Group 4: F≥3, any NAS. Serum sCD163 and the monocyte/macrophage marker sCD14 were measured by enzyme-linked immunosorbent assay. Relationships between sCD163, sCD14, fibrosis stage, and NAS were examined. Area under the receiver operating charateristic for the diagnosis of nonalcoholic steatohepatitis based on the Clinical Research Network definition was calculated.
RESULTS:
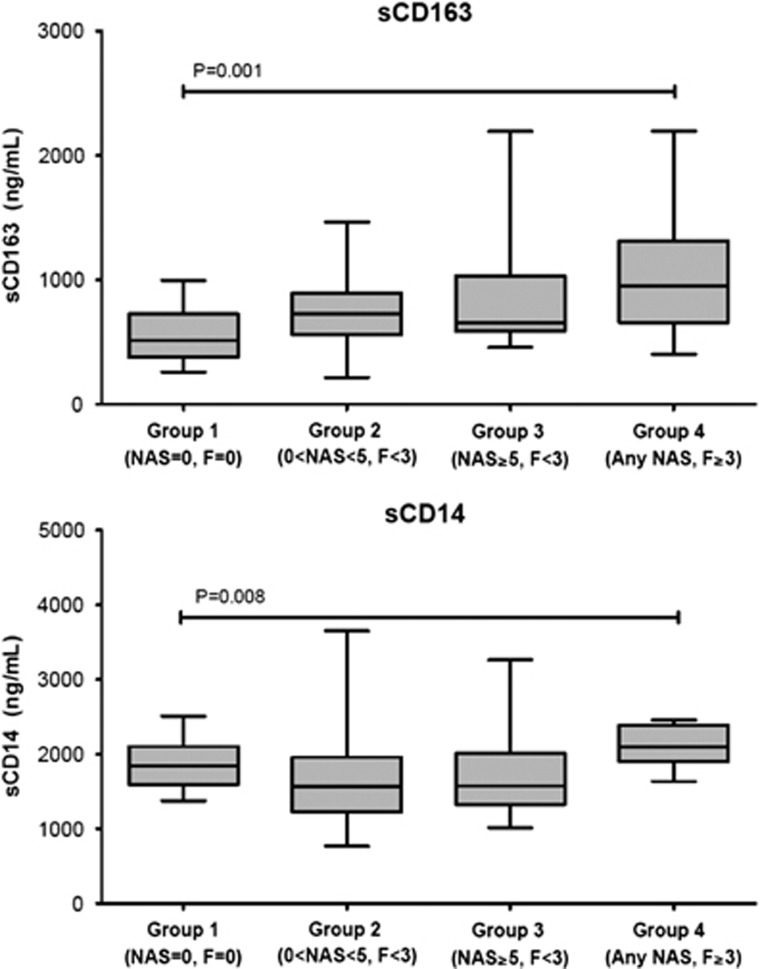
sCD163 increased with progressive liver histology, with lowest values in normal histology and highest levels in those with nonalcoholic steatohepatitis and advanced fibrosis (Group 1: 552 ng/ml, Group 2: 721 ng/ml, Group 3: 803 ng/ml, and Group 4:1,031; P=0.001). sCD14 also differed significantly across groups (Group 1: 1,877 ng/ml, Group 2: 1632 ng/ml, Group 3: 1,706 ng/ml, and Group 4: 2111; P=0.008, respectively). sCD163 correlated with steatosis grade (P<0.001), lobular inflammation (P=0.033), and hepatocyte ballooning (P<0.001). In a multivariable ordered logistic regression model, there was a significant association between every 100 ng/ml increase in sCD163 and higher fibrosis stage, with an odds ratio of 1.16 (95% confidence interval 1.02–1.31), P=0.020. The odds ratios of the association between every 100 ng/ml increase in sCD163 and higher NAS was 1.17 (95% confidence interval 1.04–1.32), P=0.010. A sCD163-based predictive score demonstrated an area under the receiver operating charateristic of 0.70 (95% confidence interval: 0.58–0.82) for the diagnosis of nonalcoholic steatohepatitis. Soluble CD14 did not correlate with fibrosis stage or NAS.
CONCLUSIONS:
In obese subjects, serum sCD163, but not sCD14, correlated with fibrosis stage and NAS. These data support a role for activated Kupffer cells in the pathogenesis of nonalcoholic steatohepatitis and fibrosis, and suggest potential clinical utility for assessment of sCD163 levels.
INTRODUCTION
In the United States, nonalcoholic fatty liver disease (NAFLD) afflicts ~30% of the general population and is the leading cause of chronic liver disease.1 NAFLD encompasses a broad spectrum of disorders, ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), the progressive form of NAFLD that can lead to cirrhosis,2 hepatocellular carcinoma, and the need for transplantation.3, 4, 5 Distinguishing between simple steatosis and NASH, as well as the staging of NASH fibrosis requires histologic diagnosis via liver biopsy. Biopsies are invasive, expensive, and can result in serious complications.6 Furthermore, they are subject to sampling error and interobserver variability. Therefore, there exists a need for accurate, non-invasive, and cost-effective testing to assist in risk stratification of NASH.
Although hepatic steatosis is a hallmark of NAFLD, hepatic inflammation has a key role in the pathophysiology of NASH and fibrosis.7 The cause of this inflammation has long been debated, and may be directly due to free fatty acid or cholesterol accumulation within hepatocytes,8 microbial translocation from the gastrointestinal tract leading to intrahepatic immune cell activation,9 or alterations in gut microbiota.10 The ability to detect hepatic inflammation and distinguish steatosis from NASH non-invasively would allow for rapid diagnosis of NASH and monitoring of disease progression and response to therapy without the need for serial liver biopsies. Circulating monocyte-macrophage associated proteins might serve as biomarkers for NASH. CD163 is a hemoglobin scavenger receptor that is expressed by cells of the monocyte-macrophage lineage.11 During cellular activation and inflammation, its soluble form (sCD163) is shed via proteolytic cleavage at the cell surface12 and can be detected within serum or plasma. Kupffer cells, hepatic macrophages that have a key role in liver inflammation and fibrosis, express high levels of CD163 and represent over 80% of tissue macrophages,13 raising the possibility that serum levels of sCD163 disproportionately reflect chronic hepatic inflammation. Indeed sCD163 has been examined in various liver conditions, including hepatitis C virus (HCV) and hepatitis B virus (HBV) infection. Recent work has shown sCD163 levels are elevated in patients with HCV-related cirrhosis compared with those with minimal or no fibrosis.14 Soluble CD163 has also been shown to correlate with inflammation, specifically hepatic activity index, and fibrosis among patients with chronic hepatitis B and C.15 In addition, in patients with cirrhosis of varying etiologies, elevated sCD163 levels are significantly associated with portal hypertension, Child-Pugh score, and model for end-stage liver disease score.16, 17 There is a paucity of data on sCD163 in NAFLD. One study of liver biopsies from children with NAFLD found an association between CD163 staining and the severity of steatosis and fibrosis.18
Soluble CD14 (sCD14) is another more general marker of monocyte activation, lipopolysaccharide, and bacterial translocation. CD14 is expressed on Kupffer cells and hepatocytes,19, 20 and serum sCD14 is elevated in cirrhotic subjects with ascites and evidence of bacterial translocation.21 In a recent study of 113 subjects with NAFLD sCD14 correlated with histologic evidence of inflammation and fibrosis.22
To our knowledge, the roles of sCD163 and sCD14 have not been compared in liver disease, and more specifically have not been explored in NASH. We hypothesized that both sCD163 and sCD14 would correlate with hepatic inflammation and fibrosis, but sCD163 would have a stronger correlation with fibrosis and inflammation because of its relative specificity for the activation of Kupffer cells, which have a key role in liver injury and inflammation.
METHODS
Cohort Selection
We performed a cross-sectional analysis of serum sCD163, sCD14, and hepatic histology. Liver biopsies and serum samples were obtained from patients undergoing gastric bypass surgery at Bon Secours Health System in Richmond, VA. This study was approved by the Bon Secours Health System and Partners Institutional Review Boards, and all patients gave written consent before participation. Standard of care core liver biopsies and serum samples were obtained at time of gastrbypass surgery. Inclusion criteria included age ≥18 years of age, liver biopsy at the time of surgery and ability to provide informed consent. Exclusion criteria included infection with HBV, HCV or HIV, or other causes of chronic liver disease and significant alcohol consumption (defined by >1 drink/day in women or 2 drinks/day in men).
Liver biopsies were reviewed by a single blinded pathologist at Massachusetts General Hospital and scored for presence of fibrosis (modified Brunt stage, F0–F4) and assigned scores for grade of steatosis (grade 0=<5% steatosis; 1=5–33% 2=34–66% 3=>66%), hepatocyte ballooning (0=no ballooning; 1=few; 2=many), and lobular inflammation per 200 × (0=no foci; 1=<2 foci; 2=2–4 foci; 3=>4 foci).23 Biopsies considered insufficient (<15 mm in length or <10 portal tracts) were excluded. NAFLD activity score (NAS) ranges from 0 to 8 and is a sum of the scores for steatosis grade, lobular inflammation, and hepatocyte ballooning. Baseline demographics, including age, gender, and race, as well as baseline clinical and laboratory values were collected on all patients.
Subjects were divided into four groups: Group 1, normal histology with NAS=0 and F=0, Group 2 (0<NAS<5, F<3); Group 3, (NAS≥5, F<3); and Group 4, advanced fibrosis (F≥3, any NAS).
NASH was defined based on the Clinical Research Network recommendations as having a score of 1 or greater in each of the three components of the NAS score: steatosis, hepatocyte ballooning, and lobular inflammation.24 This definition provides similar categorization as the recently described FLIP algorithm,25 but the decision tree was not used by pathologists during specimen analysis. Subjects were divided into three groups based on NASH: Group 1, normal, obese subjects (steatosis, lobular inflammation and hepatocyte ballooning all =0); Group 2, simple steatosis (steatosis score ≥1 with ballooning and/or lobular inflammation score <1) and group ; Group 3, NASH (steatosis score ≥1, hepatocyte ballooning ≥1, and lobular inflammation ≥1).
Biochemical analysis
For all but eight patients, blood draws occurred on the day of gastric bypass surgery when liver tissue was obtained; seven patients had blood drawn within six months before surgery and one patient had blood drawn within one year before surgery. Blood from one 10-ml EDTA-coated tube was separated by centrifugation and serum was stored at −80 °C. Samples were thawed and serum sCD163 and sCD14 levels were batch analyzed in duplicate using the Quantikine enzyme-linked immunosorbent assay system (R&D Systems, Minneapolis, MN). The mean assay coefficient of variance was 3.3% and 4.8% for sCD163 and sCD14, respectively.
Statistical analysis
Data are distributed normally and are therefore presented as mean ± s.d. Differences between multiple groups were compared using one-way analysis of variance (or χ2 test, and differences between two specific groups were compared using unpaired Student's t-tests. Spearman's rank correlation was used to assess the relationship between sCD163 or sCD14 levels and histological fibrosis stage or NAS, whereas Pearson's correlation was used to assess the relationship between continuous variables.
Univariate and multivariate ordered logistic regression analyses were performed with NAS or fibrosis stage as dependent variables and sCD163 or sCD14 as explanatory variables. These models generated odds ratios for a given fibrosis stage or NAS corresponding to specific increases in sCD163 or sCD14. These models included age, gender, race, diabetes, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin as covariates.
We evaluated the prediction power of sCD163 plus ALT by calculating the receiver-operator characteristics (ROC) curves. We randomly partitioned the sample into two half-samples, each containing 50% of the cases (Group 3) and 50% of controls (Group 2). The first-half sample was used as the training set on which we fitted a logistic regression model and estimated the coefficients on sCD163 and ALT.26 We used the second half-sample as the validation set and calculated the ROC curve of the score based on the coefficients estimated from the training set. We calculated the area under the ROC curve (AUROC) for both the training and validation sets. The software SAS, version 9.3 (SAS Institute Inc., Cary, NC) and SPSS version 17.0 (SPSS Inc., Chicago, IL) were used for the analyses. P values <0.05 were considered significant.
RESULTS
In this cohort, 145 patients who underwent gastric bypass surgery had samples available and were included in the analyses. There were 20 patients with normal liver histology (Group 1), 78 patients with NAS <5, F<3 (Group 2), 37 patients with NAS ≥5, F<3 (Group 3), and 10 patients with advanced fibrosis or cirrhosis (Group 4). Subjects had NAS ranging from 0 to 7 and fibrosis stages ranging from 0 to 4.
The characteristics of the 145 patients and their respective groups are shown in Table 1. Patients with advanced fibrosis (Group 4) were more likely to be male, older, white, diabetic, and had significantly elevated ALT, AST, and total bilirubin compared with those with normal histology (Group 1). Differences in sCD163 and sCD14 levels between two groups are shown in Supplementary Table 1 online.
Table 1. Baseline characteristics of subjects.
| Group 1 NAS=0, F=0 (n=20) | Group 2 0<NAS<5, F<3 (n=78) | Group 3 NAS≥5, F<3 (n=37) | Group 4 Any NAS, F≥3 (n=10) | P value | |
|---|---|---|---|---|---|
| Diabetes (n, %) | 1 (5.0) | 29 (37.2) | 17 (45.9) | 8 (80.0) | <0.001 |
| Male (n, %) | 1 (5.0) | 14 (17.9) | 9 (24.3) | 5 (50.0) | 0.028 |
| Smoker (n, %) | 2 (10.0) | 7 (9.0) | 3 (8.1) | 2 (20.0) | 0.089 |
| White (n, %) | 7 (35.0) | 57 (75.0)a | 30 (81.1) | 10 (100.0) | 0.001 |
| Age (years) | 39.6 (9.0) | 46.3 (11.4) | 43.6 (11.6) | 55.7 (9.1) | 0.002 |
| BMI (kg/m2) | 46.3 (5.7) | 45.8 (6.8) | 49.1 (7.9) | 47.0 (4.2) | 0.117 |
| ALT (IU/l) | 40 (29) | 41 (17) | 65 (31) | 97 (86) | <0.001 |
| AST (IU/l) | 17 (10) | 23 (14) | 38 (26) | 66 (57) | <0.001 |
| Total billirubin (mg/dl) | 0.44 (0.25) | 0.48 (0.23) | 0.49 (0.24) | 0.65 (0.28) | 0.139 |
| sCD163 ng/ml | 552 (206) | 721 (261) | 803 (350) | 1031 (529) | 0.001 |
| sCD14 ng/ml | 1877 (336) | 1632 (497) | 1706 (491) | 2111 (262) | 0.008 |
AST, aspartate aminotransferase; BMI, body mass index; F, modified brunt fibrosis score; NAS, Nonalcoholic fatty liver disease activity score. Data are mean (s.d.).
Two subjects missing race information.
In a univariate ordered logistic regression model, age, male gender, white race, diabetes status, ALT, AST, sCD163 levels, and sCD14 levels all predicted increasing fibrosis stage (P<0.05 for all analyses; results not shown). Similarly, diabetes status, male gender, white race, ALT, AST, and sCD163 levels predicted increasing NAS (P<0.05 for all analyses; results not shown).
sCD163
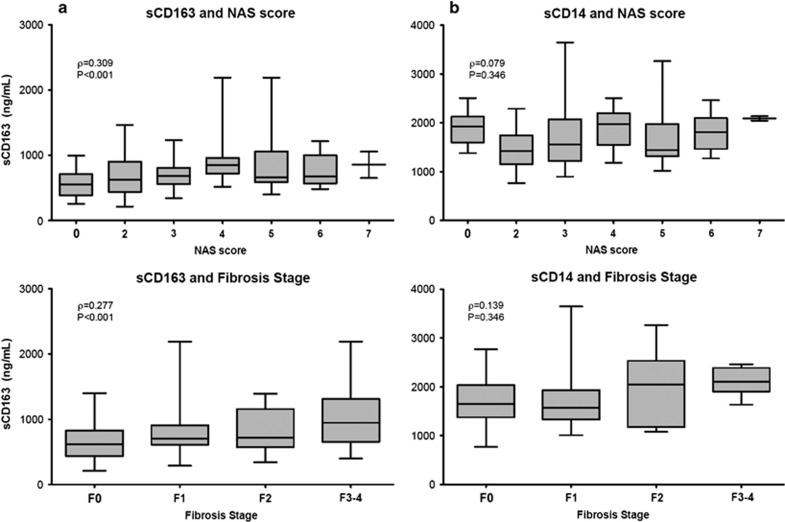
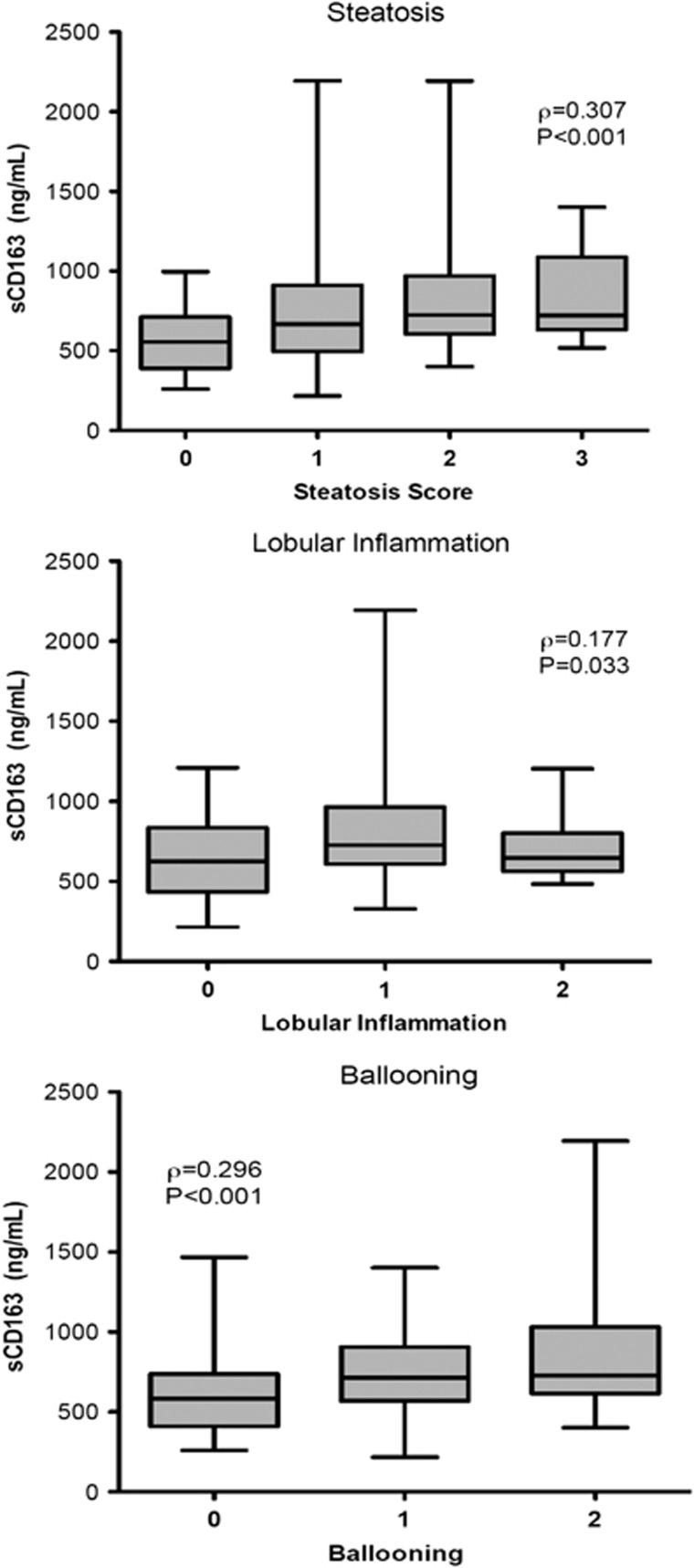
Mean levels of sCD163 in Group 2 (Figure 1; 721 g/ml [261]), Group 3, (803 ng/ml [350]), and Group 4 (1031ng/ml [529]) differed significantly from controls (Table 2; Group 1, 552 ng/ml [206] P=0.009, P=0.005, and P=0.001, respectively) and across all groups (P=0.001). In addition, mean sCD163 levels were significantly elevated in the advanced fibrosis group vs. the steatosis group (P=0.003). In the univariate analysis, sCD163 correlated with both fibrosis stage and NAS (Figure 2a; ρ=0.277, P=0.001 and ρ=0.309, P<0.001, respectively) and also correlated with each of the components of NAS, including steatosis (ρ=0.307, P<0.001), lobular inflammation (ρ=0.177, P=0.033), and hepatocyte ballooning (ρ=0.296, P<0.001, Figure 3). sCD163 also correlated with traditional biochemical markers of inflammation such as AST and ALT (r=0.295 and 0.356, respectively, P<0.0001).
Figure 1.
Differences in soluble CD163 (sCD163) and sCD14 (sCD14) across all four groups. Although both sCD163 and sCD14 were significantly different across groups, only sCD163 increased across Group 1 to Group 4 and was significantly higher in all groups compared with controls. P values are for ANOVA. ANOVA, analysis of variance.
Table 2. Baseline characteristics of subjects.
| Group 5 Normal (n=21) | Group 6 NAFLD (n=49) | Group 7 NASH (n=75) | P value | |
|---|---|---|---|---|
| Diabetes (n, %) | 2 (9.5) | 14 (28.6) | 39 (52.0) | <0.001 |
| Male (n, %) | 1 (4.8) | 8 (16.3) | 20 (26.7) | 0.063 |
| Smoker (n, %) | 3 (14.3) | 4 (8.2) | 7 (9.3) | 0.772 |
| White (n, %) | 8 (38.1) | 38 (77.6)* | 58 (77.3) | 0.003 |
| Age (years) | 40.9 (10.7) | 46.9 (12.0) | 45.5 (11.1) | 0.130 |
| BMI (kg/m2) | 46.4 (5.6) | 45.0 (6.6) | 49.1 (7.9) | 0.048 |
| ALT (IU/l) | 39 (29) | 42 (26) | 60 (40) | 0.006 |
| AST (IU/l) | 18 (10) | 24 (20) | 35 (29) | 0.006 |
| Total billirubin (mg/dl) | 0.46 (0.26) | 0.47 (0.20) | 0.50 (0.26) | 0.675 |
| sCD163 ng/ml | 555 (201) | 691 (270) | 824 (350) | 0.001 |
| sCD14 ng/ml | 1901 (347) | 1554 (411) | 1773 (524) | 0.007 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index. Data are mean (s.d.). P values are for χ2 test or unpaired t-test.
Figure 2.
(a) Soluble CD163 (sCD163) and (b) soluble CD14 (sCD14) compared with fibrosis stage and NAFLD Activity Score (NAS) score. Serum sCD163 correlated with both fibrosis stage and NAS score, whereas sCD14 did not. P values are for Spearman's rank correlation. NAFLD, nonalcoholic fatty liver disease.
Figure 3.
Association of soluble CD163 (sCD163) with each component of the nonalcoholic fatty liver disease Activity Score (NAS). Serum sCD163 correlated with all three individual components of the NAS—steatosis, lobular inflammation and ballooning. sCD14 did not (data not shown). P values are for Spearman's rank correlation.
In a multivariable ordered logistic regression model of fibrosis that included age, gender, race, diabetes status, body mass index, ALT, AST, bilirubin, and sCD163, for every 100 ng/ml increase in sCD163 the odds ratiofor increasing fibrosis score was 1.16 (95% confidence interval (CI) 1.03–1.32, P=0.018). In this model, race and diabetes status also independently predicted increasing fibrosis (Supplementary Table 2). Similarly, for every 100ng/ml increase in sCD163 the odds ratio for increasing NAS was 1.17 (1.04–1.32), P=0.010, and race, diabetes status, and body mass index also predicted increasing NAFLD activity (Supplementary Table 3).
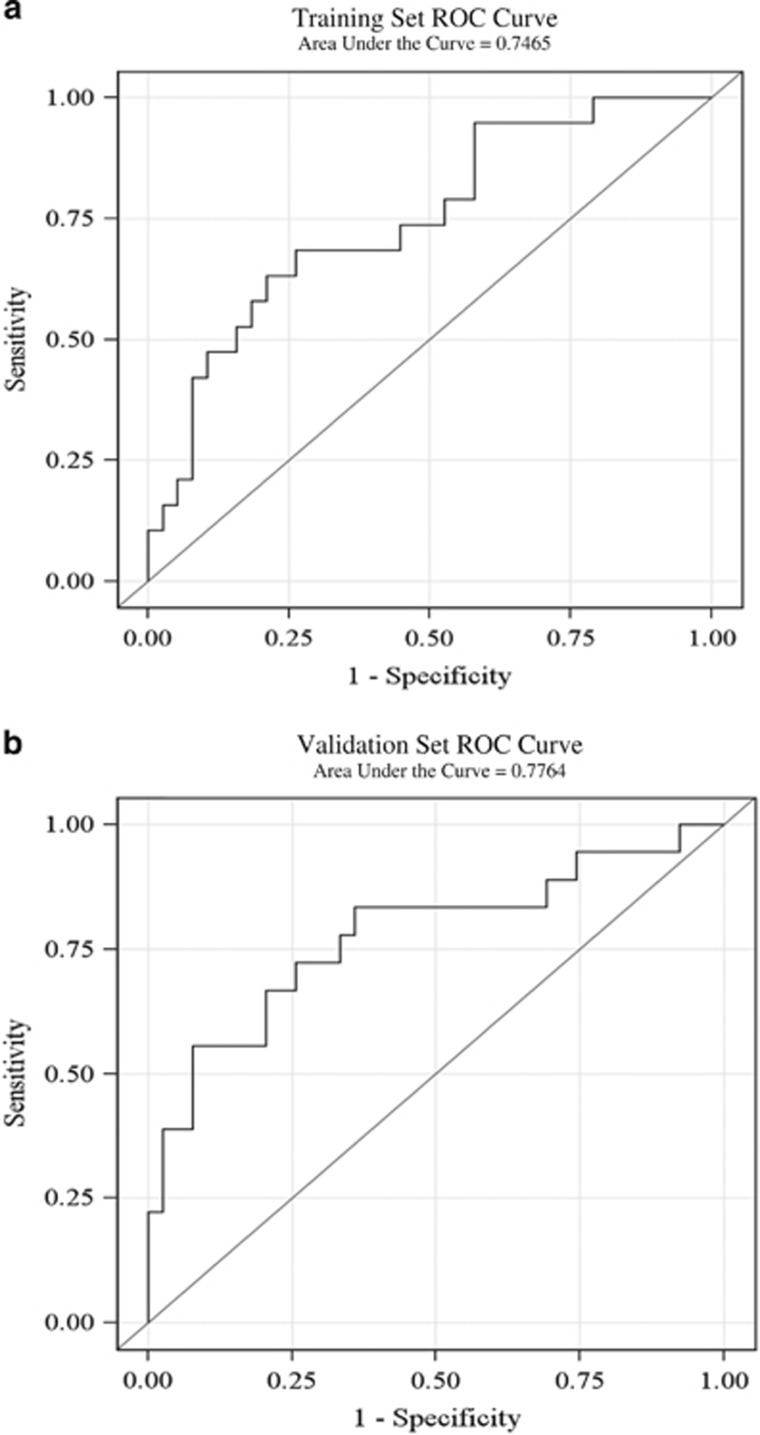
Moreover, sCD163 was an independent predictor of NAS ≥5 among patients without advanced fibrosis, as our sCD163-based predictive score (which included sCD163 and ALT levels) demonstrated an area under the receiver operating characteristic curve (AUROC) of 0.75 (95% CI: 0.61–0.88). The area under the ROC curve (AUROC) in the validation set was not different from the AUROC in the training set. Figure 4a and b show the ROC curves in the training set and the validation set, respectively. Only two variables, sCD163 and ALT were included in the model due to a limited sample size. When those with advanced fibrosis were included in the analysis, our sCD163-based predictive score demonstrated an AUROC of 0.76 (95% CI: 0.68–0.85) for NAS ≥5.
Figure 4.
Training and validation receiver-operator characteristic (ROC) curves for model using sCD163 and alanine aminotransferase (ALT) as a predictor for nonalcoholic steatohepatitis (NASH). Receiver-operator characteristic (ROC) curves were obtained using sCD163 and ALT as a predictor for the presence of NASH in both a training (4A) and validation (4B) selection of patients from the cohort.sCD163, soluble CD163.
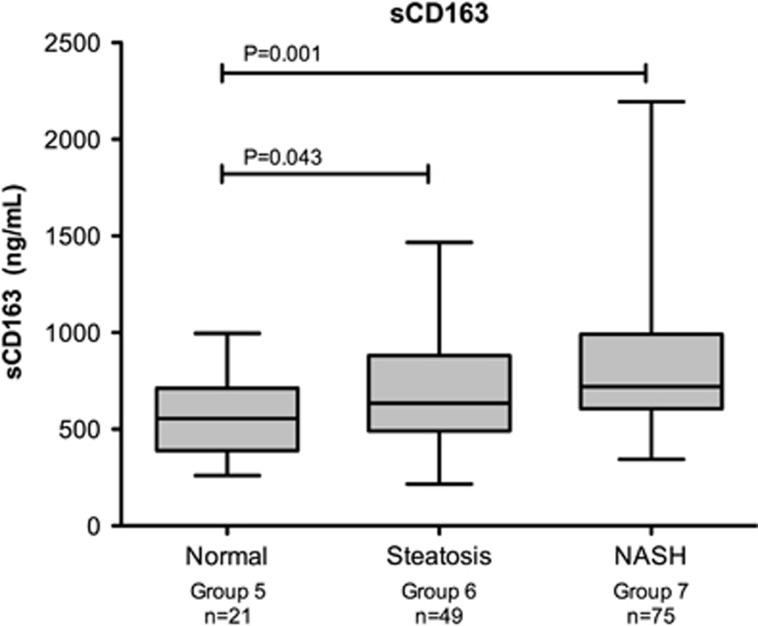
In a second analysis, there were 21 patients with normal liver histology (Group 5), 49 patients with steatosis (Group 6), and 75 patients with NASH (Group 7). The characteristics of the 145 patients and their respective groups are shown in Table 2. Diabetes status, gender, age, race, ALT, AST, sCD163, and sCD14 levels were significantly different between groups. Levels of sCD163 were significantly different between each group and are shown in Figure 5. Soluble CD163 levels were lower in normal individuals (mean 555 [201] ng/ml) compared with those with NAFLD (mean 691 [270] ng/ml, P=0.043) and NASH (mean 824 [350] ng/ml, P=0.001). Furthermore, sCD163 levels were significantly different between NASH subjects and those with steatosis (P=0.026). Our sCD163-based predictive score (which included sCD163 and ALT levels) demonstrated an area under the receiver operating characteristic curve (AUROC) of 0.70 (95% CI: 0.58–0.82) for the diagnosis of NASH (NASH vs. non NASH) and an AUROC of 0.66 (95% CI: 0.52–0.80) in a NASH vs. steatosis analysis. Furthermore, when the eight patients that did not have their blood drawn on day of gastric bypass were excluded, the NASH vs. non-NASH and NASH vs. steatosis AUROC improved to 0.73 (95% CI: 0.61–0.85) and 0.73 (95% CI: 0.60–0.86), respectively.
Figure 5.
Mean sCD163 levels across normal, steatosis, and NASH groups. P=0.001. NASH, nonalcoholic steatohepatitis.
sCD14
Mean sCD14 levels were elevated in Group 4 (Figure 1; 2111 ng/ml [562]) when compared with Groups 1 (1877 ng/ml [336]), 2 (1632 ng/ml [497]), and 3 (1706 ng/ml [491) (Supplementary Table 1; P=0.065, P=0.004, and P=0.016, respectively). sCD14 levels differed significantly between the steatosis group and controls (P=0.040). In univariate analysis sCD14 differed significantly across groups (Figure 1, P=0.008, respectively).
In contrast to sCD163, sCD14 levels did not correlate with fibrosis stage or NAS (Figure 2b; ρ=0.139, P=0.095 and ρ=0.079, P=0.346, respectively) and did not predict increasing fibrosis stage or NAS in the ordered logistic regression model (results not shown).
DISCUSSION
In this study, we found a significant association between sCD163, NASH, and fibrosis. This association remained significant after adjustment for age, gender, race, presence of diabetes, and aminotransferase levels; indeed, in a multivariate logistic regression model only sCD163, white race, and presence of diabetes were significantly associated with both NAS and fibrosis stage. In contrast another widely used circulating marker of macrophage activation, sCD14, was not associated with NAS or fibrosis stage, consistent with its reflection of overall macrophage status rather than liver specific status.
These data have significance for the understanding of the pathophysiology of NAFLD and fibrosis. First, the cohort of patients with NAS <5 (Group 2) had significantly higher sCD163 than controls (Group 1), suggesting that early hepatic lipid accumulation may be associated with Kupffer cell activation and inflammation, even potentially before it is visible histologically. Second, the level of sCD163 was even higher in those with NAS ≥5 and higher yet in those with advanced fibrosis, suggesting ongoing and increasing Kupffer cell/macrophage activation throughout all stages of NAFLD. This activation persists even in those with advanced fibrosis. This confirms the recent study by Kazankov et al.27who also found sCD163 to be elevated in NAS ≥5 compared with those with NAS<5 as well as in patients with bridging fibrosis compared with lower fibrosis stages. Therefore, preventing or inhibiting Kupffer cell activation may be a rational therapeutic strategy for all stages of NAFLD.
The specific relationship between sCD163, fibrosis and NAS as compared with sCD14 is striking. Both are markers of macrophage activation, and both have been associated with tissue inflammation (e.g., carotid artery disease in HIV-HCV coinfection).28 Although sCD14 has been associated with disease progression in HBV/HCV,29 only sCD163 has been associated with fibrosis in HBV, HCV and HIV/HCV coinfection14, 15, 30 as well as acute liver failure31 and portal hypertension.32 CD14 is a co-receptor for lipopolysaccharide along with toll-like receptor 4, and is expressed primarily on macrophages, but also neutrophils and dendritic cells. Its shed form, sCD14, appears to be a general marker of inflammation. In contrast, CD163 is highly expressed on Kupffer cells, and sCD163 is significantly higher in the hepatic veins compared with the portal veins.17 Taken together these findings suggest that sCD163 is a marker of macrophage activation with a higher sensitivity for liver injury compared with other soluble markers. Soluble CD163 is not uniquely specific for the liver, however; elevated sCD163 is also associated with many conditions of immune activation: coronary plaque burden in HIV infection,33 rheumatoid arthritis activity,34 and, most interestingly in obesity.35, 36 The fact that in our study of obese patients sCD163 was further increased in those with significant liver disease demonstrates the potential utility of sCD163 in this population, and underlines the role of inflammation in the pathogenesis of NAFLD.
Although sCD163 alone cannot be used as a definitive diagnostic test for NASH, as part of a panel with other predictors of steatohepatitis, it may serve as a useful screening tool. Currently the American Association for the Study of Liver Disease practice guidelines suggest considering biopsy for NAFLD patients at the highest risk of developing NASH, based on the presence of metabolic syndrome and the NAFLD fibrosis score.37 Another biomarker, circulating cytokeratin 18, has been suggested in one study to be markedly increased in NASH patients when compared with patients with simple steatosis (AUROC=0.82, 95% CI: 0.78–0.88),4 but other studies have not corroborated its predictive value.38
There are some limitations to this study. The data are cross-sectional, and therefore exploratory, as individuals were not followed after gastric bypass surgery. Further longitudinal data are required to determine whether sCD163 levels change with disease regression or progression. Our cohort was limited to those with obesity warranting weight loss surgery and further exploration of the value of sCD163 in non-obese individuals with NAFLD as well as in the general NAFLD population is needed. Nonetheless, levels of serum sCD163 in those with NAS=0 (Group 5) are similar to those reported in other “healthy control” groups,39 supporting the role of sCD163 as a specific marker of hepatic inflammation. The use of obese controls in this study and including body mass index as a covariate aims to eliminate obesity as a potential confounder. Finally, only 10 subjects had advanced fibrosis, and 5 of these had NAS scores consistent with NASH, suggesting that the association of sCD163 and fibrosis scores may be confounded by concomitant steatohepatitis. However, previous studies14, 15 have reported a correlation between sCD163 and advanced fibrosis or cirrhosis.
Furthermore, although random division of the original cohort into a training and validation set for the AUROC analysis is a popular strategy when a true validation cohort is unavailable,26 this method does not replace an independent validation cohort. Unfortunately, a true validation cohort was unavailable, so this strategy is our best attempt to reduce the bias associated with a single study cohort.
CONCLUSIONS
In summary, serum sCD163, but not sCD14, correlated with overall NAS score, histologic elements of steatohepatitis, and fibrosis stage in patients with NAFLD. The assay for sCD163 is rapid, inexpensive, and highly reproducible. Measurement of sCD163 may have a role, together with other assessments, in the diagnosis and staging of NAFLD/NASH. Further studies addressing its prognostic value in other patient populations as well as the effect of therapeutic interventions (such as weight loss) are needed.
Study Highlights
Acknowledgments
We would like to thank the patients and staff at the Bons Secours Health System in Ricmond, VA.
Guarantor of the article: Raymond T. Chung, MD.
Specific author contributions: J.L.M. performed the experiments, analyzed the data and wrote the manuscript. E.R.F. performed the experiments, analyzed the data and wrote the manuscript. H.Z. analyzed the data and wrote the manuscript. J.M. performed the experiments and analyzed the data. A.K. performed the experiments and analyzed the data. N.A. performed the experiments and analyzed the data. L.Y.K. performed the experiments and analyzed the data. L.G. performed the experiments. K.E.C. planned the study, analyzed the data and wrote the manuscript. R.T.C. planned the study, analyzed the data and wrote the manuscript.
Financial support: The following authors would like to acknowledge NIH grants; these grants were not connected specifically to this project but contributed to the work. Raymond T. Chung NIH K24 DK078772. Kathleen E Corey: NIH K23DK099422. Jessica L. Mueller: NIH T32 DK007191. Eoin R. Feeney: NIH/NIAID 5P30AI060354–09. Lindsay Y. King: NIH T32 DK007191.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis 2008; 28: 339–350. [DOI] [PubMed] [Google Scholar]
- Matteoni CA, Younossi ZM, Gramlich T et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999; 116: 1413–1419. [DOI] [PubMed] [Google Scholar]
- Ekstedt M, Franzen LE, Mathiesen UL et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44: 865–873. [DOI] [PubMed] [Google Scholar]
- Fassio E, Alvarez E, Dominguez N et al. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 2004; 40: 820–826. [DOI] [PubMed] [Google Scholar]
- Adams LA, Lymp JF St, Sauver J et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005; 129: 113–121. [DOI] [PubMed] [Google Scholar]
- Van Thiel DH, Gavaler JS, Wright H et al. Liver biopsy. Its safety and complications as seen at a liver transplant center. Transplantation 1993; 55: 1087–1090. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010; 52: 1836–1846. [DOI] [PubMed] [Google Scholar]
- Farrell GC, van Rooyen D, Gan L et al. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 2012; 6: 149–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz AG, Casafont F, Crespo J et al. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg 2007; 17: 1374–1380. [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012; 482: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller HJ, Peterslund NA, Graversen JH et al. Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood 2002; 99: 378–380. [DOI] [PubMed] [Google Scholar]
- Hintz KA, Rassias AJ, Wardwell K et al. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol 2002; 72: 711–717. [PubMed] [Google Scholar]
- Klein A, Zhadkewich M, Margolick J et al. Quantitative discrimination of hepatic reticuloendothelial clearance and phagocytic killing. J Leukoc Biol 1994; 55: 248–252. [DOI] [PubMed] [Google Scholar]
- Andersen ES, Rodgaard-Hansen S, Moessner B et al. Macrophage-related serum biomarkers soluble CD163 (sCD163) and soluble mannose receptor (sMR) to differentiate mild liver fibrosis from cirrhosis in patients with chronic hepatitis C: a pilot study. Eur J Clin Microbiol Infect Dis 2014; 33: 117–122. [DOI] [PubMed] [Google Scholar]
- Kazankov K, Barrera F, Moller HJ et al. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology 2014; 60: 521–530. [DOI] [PubMed] [Google Scholar]
- Rode A, Nicoll A, Moller HJ et al. Hepatic macrophage activation predicts clinical decompensation in chronic liver disease. Gut 2013; 62: 1231–1232. [DOI] [PubMed] [Google Scholar]
- Holland-Fischer P, Gronbaek H, Sandahl TD et al. Kupffer cells are activated in cirrhotic portal hypertension and not normalised by TIPS. Gut 2011; 60: 1389–1393. [DOI] [PubMed] [Google Scholar]
- De Vito R, Alisi A, Masotti A et al. Markers of activated inflammatory cells correlate with severity of liver damage in children with nonalcoholic fatty liver disease. Int J Mol Med 2012; 30: 49–56. [DOI] [PubMed] [Google Scholar]
- Imajo K, Fujita K, Yoneda M et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab 2012; 16: 44–54. [DOI] [PubMed] [Google Scholar]
- Pan Z, Zhou L, Hetherington CJ et al. Hepatocytes contribute to soluble CD14 production, and CD14 expression is differentially regulated in hepatocytes and monocytes. J Biol Chem 2000; 275: 36430–36435. [DOI] [PubMed] [Google Scholar]
- Albillos A, de la Hera A, Gonzalez M et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology 2003; 37: 208–217. [DOI] [PubMed] [Google Scholar]
- Quon BS, Ngan DA, Wilcox PG et al. Plasma sCD14 as a biomarker to predict pulmonary exacerbations in cystic fibrosis. PLoS One 2014; 9: e89341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Brunt EM, Kleiner DE et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011; 54: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedossa P, Poitou C, Veyrie N et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012; 56: 1751–1759. [DOI] [PubMed] [Google Scholar]
- Arlot S, Celisse A. A survey of cross-validation procedures for model selection. Statistics surveys 2010; 4: 40–79. [Google Scholar]
- Kazankov K, Tordjman J, Moller HJ et al. The macrophage activation marker sCD163 is independently associated with NAFLD severity in morbid obesity and reduced by bariatric surgery. J Gastroenterol Hepatol 2015; 30: 1293–1300. [DOI] [PubMed] [Google Scholar]
- Shaked I, Hanna DB, Gleissner C et al. Macrophage inflammatory markers are associated with subclinical carotid artery disease in women with human immunodeficiency virus or hepatitis C virus infection. Arterioscler Thromb Vasc Biol 2014; 34: 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Koh C, Roque A et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011; 141: 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniholm MH, Hanna DB, Landay AL et al. sCD163 is associated with non-invasive measures of liver fibrosis in HCV- and HCV/HIV-infected women. Hepatology 2014; 61: 734–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka A, Horiike N, Akbar SM et al. Soluble CD163 in patients with liver diseases: very high levels of soluble CD163 in patients with fulminant hepatic failure. J Gastroenterol 2005; 40: 52–56. [DOI] [PubMed] [Google Scholar]
- Gronbaek H, Sandahl TD, Mortensen C et al. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Aliment Pharmacol Ther 2012; 36: 173–180. [DOI] [PubMed] [Google Scholar]
- Burdo TH, Lo J, Abbara S et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204: 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisen SR, Moller HJ, Stengaard-Pedersen K et al. Soluble macrophage-derived CD163 is a marker of disease activity and progression in early rheumatoid arthritis 2011Clin Exp Rheumatol 29: 689–692. [PubMed] [Google Scholar]
- Zanni MV, Burdo TH, Makimura H et al. Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clin Endocrinol (Oxf) 2012; 77: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeldborg K, Christiansen T, Bennetzen M et al. The macrophage-specific serum marker, soluble CD163, is increased in obesity and reduced after dietary-induced weight loss. Obesity (Silver Spring) 2013; 21: 2437–2443. [DOI] [PubMed] [Google Scholar]
- Wieckowska A, Zein NN, Yerian LM et al. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 2006; 44: 27–33. [DOI] [PubMed] [Google Scholar]
- Cusi K, Chang Z, Harrison S et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol 2014; 60: 167–174. [DOI] [PubMed] [Google Scholar]
- Beltran LM, Munoz Hernandez R, de Pablo Bernal RS et al. Reduced sTWEAK and increased sCD163 levels in HIV-infected patients: modulation by antiretroviral treatment, HIV replication and HCV co-infection. PLoS One 2014; 9: e90541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.