Abstract
Background
The combination of chemotherapy with a tyrosine kinase inhibitor (TKI) is effective in the treatment of Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Ponatinib is a more potent BCR-ABL1 inhibitor and selectively suppresses the resistant T315I clones. We examined the efficacy and safety of combining chemotherapy with ponatinib for patients with Ph+ ALL in this ongoing Phase II prospective trial.
Methods
Adult patients with newly diagnosed Ph+ ALL and good performance and organ status received 8 cycles of hyper-CVAD alternating with high dose methotrexate/cytarabine every 21 days. Ponatinib was given at 45 mg daily for the first 14 days of cycle 1 then continuously for the subsequent cycles. Patients in complete remission (CR) received maintenance with ponatinib 45 mg daily with vincristine/prednisone monthly for 2 years followed by ponatinib indefinitely. The primary endpoint for this study was the improvement of median event-free survival from 24 to 36 months. The trial was registered on clinicaltrials.gov with the identifier NCT01424982.
Results
Thirty-seven patients with a median age of 51 years were treated. The overall complete cytogenetic response, major molecular response, and complete molecular response rates were 32/32 (100%), 35/37 (95%), and 29/37 (78%), respectively. By multiparameter flow cytometry, 35 patients (95%) had no detectable minimal residual disease after a median of 3 weeks of therapy. Grade ≥ 3 toxicity included infections during induction (20 patients), increased liver functional tests (14 patients), thrombotic events (3 patients), myocardial infarction (3 patients), hypertension (6 patients), skin rash (8 patients), and pancreatitis (6 patients). Two potentially related deaths from myocardial infarction were observed. Nine patients underwent allogeneic stem cell transplantation. With a median follow up of 26 months, 29 patients (78%) remain alive and in CR. The 2-year event-free and overall survival rates are 81% and 80%, respectively.
Conclusion
The first results of this ongoing trial indicate that the combination of chemotherapy with ponatinib is highly effective in achieving early sustained remissions in patients with newly diagnosed Ph+ ALL. New strategies, including dosing titration of ponatinib and optimized control of vascular risk factors may further improve outcomes. ARIAD Pharmaceuticals Inc. provided free drug and financial support from the ARIAD Investigator Sponsored Trial program.
Keywords: Philadelphia-positive ALL, ponatinib, outcome
Introduction
The incorporation of BCR-ABL1 tyrosine kinase inhibitors (TKIs) with chemotherapy has significantly improved the outcome of patients with Philadelphia chromosome (Ph)-positive acute lymphoblastic leukemia (ALL).1–3 The combination of cytotoxic chemotherapy with TKIs is now the standard of care for patients with Ph-positive ALL. It is established that TKIs should be started immediately upon recognition of Ph-positive disease and that continuous exposure to TKIs is superior to pulsed or intermittent administration.4–9
Despite the high efficacy of this combination, the 3-year event-free survival (EFS) and overall survival (OS) rates of Ph-positive adult ALL are roughly only 40% and 60%, respectively.1–2 These relatively low survival rates can mostly be attributed to TKI resistance. Both acquired and intrinsic resistance to TKIs have been described.10–11 Acquired resistance may be due to BCR-ABL–dependent mechanisms such as BCR-ABL overexpression or mutations in the kinase domains (KDs); many patients with Ph-positive ALL relapse with a T315I clone, which is resistant to imatinib and second-generation TKIs.12 The rates of T315I mutation depend on the regimen used but range from 33% to 70% in patients who relapse after being treated with dasatinib-based regimens.2,13,14 These high mutation rates and the relative intractability of T315I-mutant clones indicate an urgent need for new TKIs that can target T315I-mutant Ph-positive ALL.
Ponatinib is a more potent BCR-ABL1 inhibitor with activity observed in leukemias with both wild-type and mutated BCR-ABL1, including T315I.15 Clinical trials of ponatinib have demonstrated its high activity in Ph-positive chronic myeloid leukemia (CML) and Ph-positive ALL; the complete cytogenetic response (CCyR) rate is 50% to 70% in patients failing to respond to 2–3 TKIs and in those harboring a T315I mutation.16–17 We hypothesized that the combination of chemotherapy and ponatinib may be associated with better response rates, lower resistance rates, and a higher likelihood of eradication of minimal residual disease (MRD) than that reported with a combination of other TKIs and chemotherapy. Here we report the first results of an ongoing phase II study to evaluate the efficacy and safety of this combination.
Methods
Study design and participants
Adult patients with previously untreated Ph-positive ALL, determined by the identification of either t(9;22) karyotype or BCR-ABL1 fusion transcript, were eligible. Patients who had received < 2 courses of prior chemotherapy with or without TKIs were eligible as well. Patients had to be 18 years of age or older, have an Eastern Cooperative Oncology Group performance status of 2 or less, have normal cardiac function (defined by ejection fraction above 50%), and have adequate organ function (serum bilirubin ≤ 3.0 mg/dL and serum creatinine ≤ 3.0 mg/dL, unless higher levels were believed to be due to tumor). Patients were excluded if they had an active infection not controlled by antibiotics, clinical evidence of grade 3 to 4 heart failure as defined by the New York Heart Association criteria, active second malignancy, or prior history of treatment with ponatinib.
All patients were enrolled consecutively and signed a consent form in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. The trial was registered on clinicaltrials.gov with the identifier NCT01424982. There were no competing trials at our institution.
Procedures
The details of the hyper-CVAD regimen have been previously published.18–19 Ponatinib was given at 45 mg orally daily for the first 14 days of cycle 1 then continuously for the next 7 cycles of consolidation chemotherapy. Rituximab was also administered during the first 4 cycles in patients with CD20 expression ≥ 20%.20 For central nervous system (CNS) prophylaxis, intrathecal therapy with methotrexate and cytarabine was given alternately on days 2 and 7 of each course for a total of 12 doses. For patients presenting with active CNS disease, confirmed by cytologic examination of the cerebrospinal fluid (CSF), the intrathecal regimen was repeated twice weekly until the CSF became clear of leukemic cells and the CSF cell count normalized. After normalization, these patients received intrathecal therapy once per week for 4 weeks or until initiation of the next cycle of chemotherapy, when the regimen was resumed. Cranial irradiation was not administered for prophylaxis, but patients presenting with or developing cranial nerve palsies received radiation to the base of the skull in addition to intrathecal therapy.
Maintenance therapy was given for 2 years with monthly courses of intravenous vincristine on day 1 and oral prednisone 200 mg daily on days 1–5. Initiation of maintenance due to treatment-related toxicity prior to completion of 8 cycles was implemented, if appropriate. Daily oral ponatinib at a dosage of 45 mg was administered throughout the planned 2-year maintenance period and was continued indefinitely thereafter. Months 6 and 13 of maintenance were designed as intensification courses of hyper-CVAD and ponatinib. Patients with no evidence of MRD who were poor candidates for such intensification continued maintenance therapy uninterrupted. Appropriate dose reductions for the cytotoxic agents according to the type and degree of side effects or toxicity were permitted and followed previously published guidelines.18–19 Ponatinib dose reductions to 30 mg or 15 mg orally daily were allowed for significant drug-related toxicity during both initial therapy and the maintenance period. Beginning on August 1, 2014, the protocol was amended; ponatinib would be given at 45 mg daily for 14 days during induction therapy and then at 30 mg daily continuously starting with the second cycle, which could be further reduced to 15 mg daily continuously once a complete molecular response (CMR) was achieved. At any time during the intensive or maintenance therapy phases, patients with an available matched donor had the option to proceed to an allogeneic stem cell transplant (ASCT). The decision to proceed with ASCT was based on the discretion of the treating physician and previous experience at our institution.21
Supportive care
Supportive care measures were implemented according to standard guidelines. Tumor lysis prophylaxis with allopurinol, or alternatives such as rasburicase, and appropriate intravenous hydration were administered in the first course to all patients. Prophylactic antimicrobial therapy, including oral levofloxacin or trimethoprim/sulfamethoxazole, antiviral prophylaxis, and antifungal prophylaxis with azoles or echinocandins, was administered to all patients during periods of neutropenia beginning in induction. Transfusions of blood, platelets, or other blood products were given according to established guidelines to support periods of cytopenia or coagulopathy.
Complete remission (CR) was defined as the presence of fewer than 5% blasts in the bone marrow, with more than 1 × 109/L neutrophils and more than 100 × 109/L platelets in the peripheral blood and no extramedullary disease. Relapse was defined by recurrence of more than 5% lymphoblasts in a bone marrow aspirate unrelated to recovery or by the presence of extramedullary disease, and all patients underwent BCR-ABL1 mutation testing at time of relapse. CR duration was calculated from the time of CR until relapse. Event-free survival (EFS) was calculated from the beginning of treatment until an event (relapse, death during induction, or death during CR) occurred. Overall survival (OS) was calculated from the time of treatment initiation until death.
Follow-up assessments
All patients underwent baseline evaluation, which included history and physical examination; complete blood count with differential; full chemistry panel (including renal and hepatic panel); bone marrow aspiration for histology, flow cytometry, cytogenetics, fluorescent in situ hybridization, and reverse-transcription quantitative polymerase chain reaction (RT-qPCR) for BCR-ABL1 transcripts; and DNA PCR for immunoglobulin heavy-chain (IGH) gene rearrangements. BCR-ABL1 KD mutational testing was not performed at baseline.
Bone marrow evaluations were repeated on approximately days 14 and 21 of the first cycle of treatment. Complete blood counts, electrolytes, and renal and hepatic indices were obtained at least weekly during the intensive cycles of chemotherapy. Bone marrow aspiration material was assessed by morphology, cytogenetics, flow cytometry, and BCR-ABL1 RT-qPCR every 2 to 3 cycles.
CSF assessment was performed on day 2 of induction chemotherapy at the time of administration of the first intrathecal chemotherapy. Baseline cardiac function was evaluated with a multigated radionuclide ventriculography (MUGA) scan or transthoracic echocardiogram, and this assessment was repeated if clinically indicated.
MRD monitoring techniques
Molecular monitoring
BCR-ABL1 RT-qPCR was performed on total RNA extracted from leukocytes after red blood cell lysis. Reverse transcription was performed with random hexamers, and PCR was performed with TaqMan primers/probes for the e1a2, e1a3, e13a2 (b2a2), and e14a2 (b3a2) BCR-ABL1 transcripts in a single tube with normalization to total ABL transcripts. Post-PCR capillary electrophoresis was used to determine the type of fusion transcripts, with the method having a sensitivity of approximately 1 in 10 000 BCR-ABL1–expressing cells, as established by periodic dilution studies.22 Major molecular response (MMR) was defined as a BCR-ABL1/ABL1 ratio of less than 0.1% (IS). CMR was defined as undetectable disease at or below the 10−5 level. BCR-ABL1 KD mutation analysis that covered codons 221 to 500 was performed on cDNA with a nested PCR strategy at the time of treatment failure (defined by lack or loss of response).22 For patients with a T315I mutation, quantitation of mutation levels was performed with a pyrosequencing-based strategy with a detection rate of 1%.23
IGH clonality studies were performed on extracted genomic DNA with separate FR1, FR2, and FR3 PCR reactions with a consensus J primer. The sensitivity of detection of this method in a sample with low numbers of polyclonal B cells (such as post-treatment bone marrow and CSF) is approximately 0.2% to 1%.
Multiparameter flow cytometry
MRD assessment by flow cytometry was performed on whole bone marrow specimens by use of a standard stain-lyse-wash procedure using six-color combinations of CD34, CD10, and CD19, as previously described.23 A distinct cluster of at least 20 cells that showed altered antigen expression was regarded as an aberrant population, which yielded a sensitivity of 1 in 10 000 cells (for adequate specimens in which 2 × 105 cells could be collected).
Outcomes
Improvement of the 3-year event-free survival constituted the primary outcome of this trial. The secondary outcomes included overall response rates, survival, and safety.
Statistical analysis
This report represents a first analysis of an ongoing study originally designed to accrue 60 patients and powered to assess improvement in EFS as the primary endpoint. The initial study design was based on our previous experience with hyper-CVAD plus imatinib or dasatinib, for which the reported median EFS was 24 months. The current study has 94% power to prove if the combination of hyper-CVAD and ponatnib can achieve an improvement in median EFS to 36 months. The trial was continuously monitored, with an early stopping rule in place if it was ever likely that the trial’s EFS was less than that of previous similar trials. No stopping rules were met. All other statistical analyses were descriptive.
Survival curves were plotted by the Kaplan-Meier method and compared with the log-rank test. Differences in subgroups by different covariates were evaluated with the χ2 test for nominal values and the Mann-Whitney U and Fisher exact tests for continuous variables. Significance was defined as a p-value < 0.05.
Results
Patient characteristics and treatment
From November 2011 to September 2013, the first 37 patients were consecutively enrolled and treated in this ongoing study. Thirty-four patients (92%) had untreated Ph-positive ALL and 3 (8%) had been previously treated; 2 patients had active disease status after one prior cycle of chemotherapy without TKI before Ph-positive/BCR-ABL1 status was identified, and 1 patient had complete cytogenetic response after 2 cycles of chemotherapy and dasatinib. Eleven patients (30%) were CD20-positive and received rituximab during the first 4 cycles. Eighteen patients (49%) had no baseline cardiovascular risk factors. The patients’ characteristics are summarized in Table 1. Overall, patients received a median of 6 cycles (range, 2–8 cycles) of therapy. To date, 13 patients are receiving maintenance therapy in CR. Nine patients underwent an ASCT after a median of 4 cycles.
Table 1.
Patient characteristics (N=37)
| Parameter | N (%); Median [range] |
|---|---|
| Age (years) | |
| Median | 51 [27–75] |
| ≥50 | 20 (54%) |
| ≥60 | 12 (32%) |
| Performance status | |
| 0–1 | 31 (84) |
| 2 | 6 (16) |
| WBC (× 109/L) | 8 [1–630] |
| CNS disease | 3 (8) |
| CD20 positivity | 11 (30) |
| BCR-ABL1 transcript | |
| p190 | 27 (73) |
| p210 | 10 (27) |
| Cytogenetics | |
| Diploid | 5 (14) |
| Ph-positive | 32 (86) |
| Baseline cardiovascular risk factors | |
| Hypertension | 18 (49) |
| Dyslipidemia | 4 (11) |
| Coronary artery disease | 4 (11) |
| Peripheral arterial disease | 1 (3) |
Response
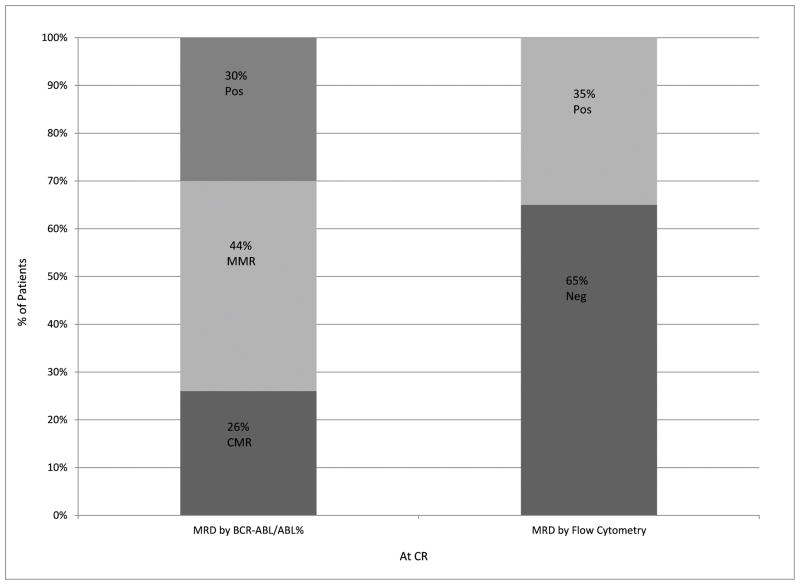
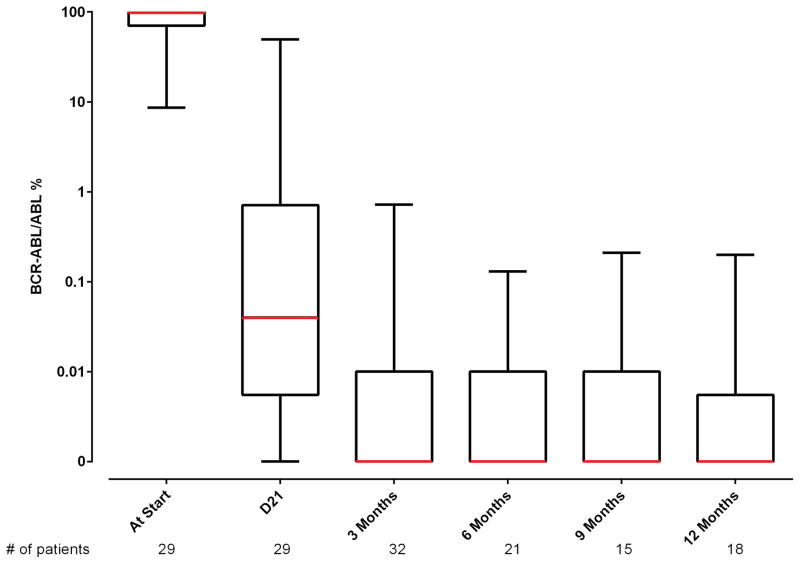
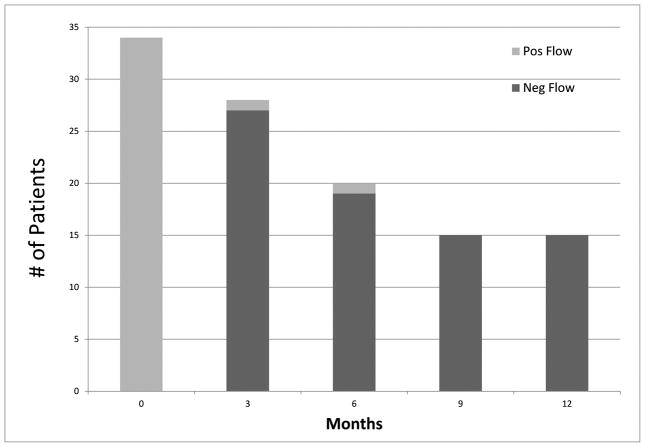
All patients were evaluable for response (Table 2). Minimal residual disease negativity assessed by 6-color multiparameter flow cytometry was observed in all but one patient (97%). Early mortality (death within 4 weeks of treatment start) did not occur. Of the 32 patients with Ph-positive metaphases at the start of therapy, CCyR, as assessed by conventional cytogenetics, was achieved in 30 (94%) after one course of induction therapy. One patient with a minor cytogenetic response (95% Ph-positive metaphases) after induction therapy converted to complete response after a second course. Furthermore, molecular response was achieved in the majority of patients: MMR was achieved in 35 of 37 (95%) of the patients overall and in 24 of those 35 patients (69%) after only induction therapy. The median time to MMR and CMR were 3 weeks (range, 2–14) and 11 (range, 2– 96) weeks, respectively. The median time to MRD negativity was 3 weeks (range, 3–14). Figure 1 shows the levels of residual disease after 1 cycle of therapy in CR for the entire cohort as measured by BCR-ABL1/ABL1 RT-qPCR or by flow cytometry. Figure 2 shows MRD status by PCR and by flow cytometry with follow-up.
Table 2.
Best overall response
| Parameter | N (%) |
|---|---|
| CR* | 36/36 (100) |
| CCyR** | 32/32 (100) |
| MMR | 35/37 (95) |
| CMR | 29/37 (78) |
| Flow negativity*** | 35/36 (97) |
| Early death | 0 (0) |
CR=complete response; CCyR=complete cytogenetic response; MMR=major molecular response; CMR=complete molecular response
1 patient in CR at start
5 patients were diploid by conventional cytogenetics at start
1 patient had no sample sent to flow cytometry
Figure 1.
Levels of residual disease after 1 cycle of protocol therapy in CR. MRD after 1 cycle at CR by BCR-ABL1/ABL1 percentage and flow cytometry.
Figure 2.
MRD status by PCR and by flow cytometry with follow-up. (A) MRD by time from therapy according to BCR-ABL1/ABL1 percentage.* The line connects the median values of the patients at the stated time points. Several patients at different time intervals had overlapping values. In 1 patient, BCR-ABL1 was undetectable at presentation by RT-qPCR and was detected by fluorescence in situ hybridization. (B) MRD by time from therapy according to multiparameter flow cytometry.
*The number of patients at start is only 29 because 3 had prior therapy and 5 had a BCR/ABL1 not performed at start or it was not quantified
Outcome
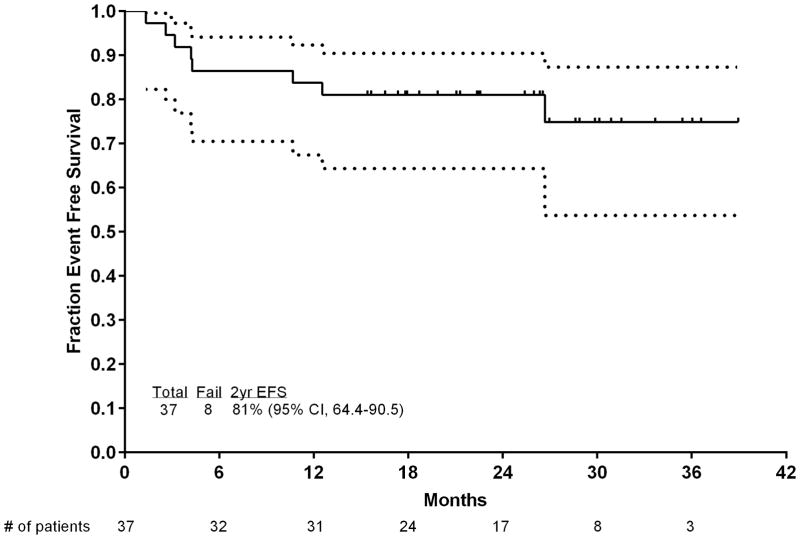
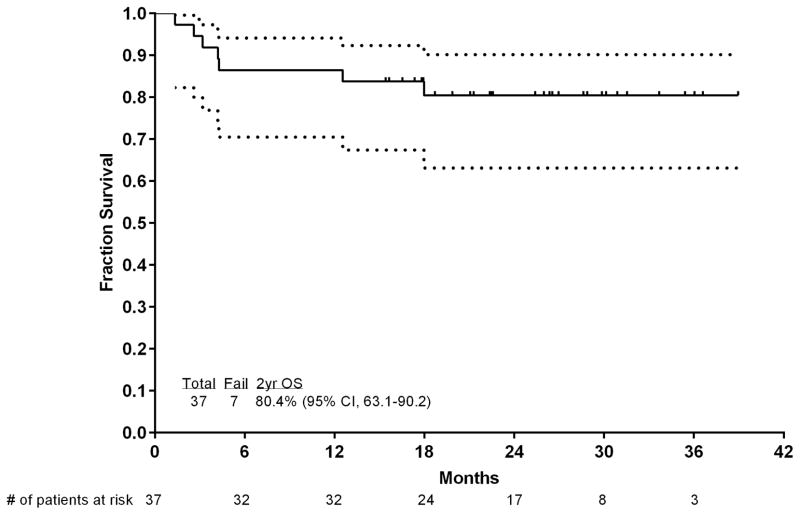
With a median follow-up of 26 months (range, 15–39 months), 29 (78%) patients were in CR, with 9 patients (24%) receiving ASCT, for an estimated 2-year survival rate of 80% (95% confidence interval [CI], 63%–90%), CR duration rate of 97% (95% CI, 80%–99.6%), and an EFS rate of 81% (95% CI, 64%–90%). Figure 3 shows the OS and EFS of the patients.
Figure 3.
Event-free survival and OS of the patients. (A) EFS and (B) OS. Numbers of patients at risk are indicated on the horizontal axis and in the table below the chart. Dotted lines are 95% CIs.
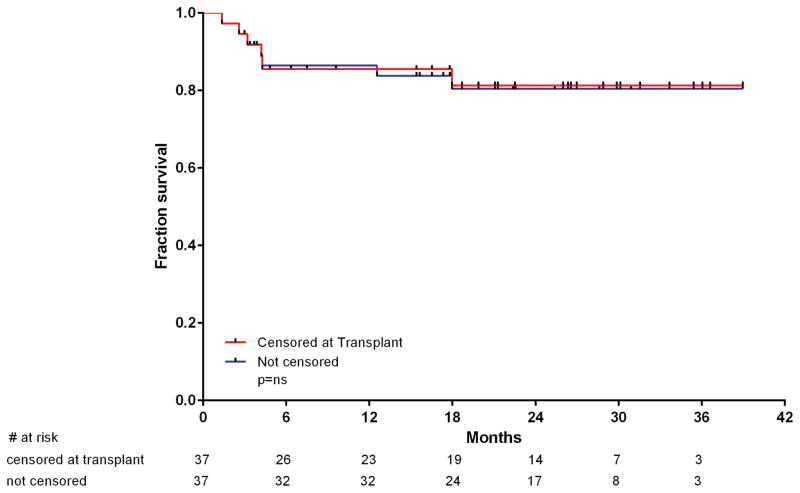
Six patients died in CR: the first from an unrelated cardiac event 4 months after electing to discontinue therapy due to deconditioning and logistical reasons and being placed on imatinib; the second from multiple organ failure after sepsis in neutropenia after the second cycle (C2D13); the third from a head injury sustained after a fall after cycle 4 (C4D13); and the fourth from sepsis and multiple organ failure after ASCT. The two other patients had no risk factors and died of arterial vascular events. The first was 37 years old, was receiving ponatinib 45 mg daily, and had a non-ST elevation myocardial infarction (NSTEMI) after cycle 2 (C2D41). The second was a 54-year-old woman, was receiving ponatinib 30 mg daily and had unexplained chest pain at C4D42 that preceded her death. Two patients (5%) relapsed after a median of 18 months. The first patient had achieved CMR and then decided to switch to dasatinib because of concerns about the vascular events encountered in ponatinib trials; she relapsed 6 months later. No kinase domain mutation was found. She failed to respond to 2 salvage regimens and died. The second patient achieved a CMR for 23 months and was on maintenance therapy with ponatinib at 15 mg daily. She relapsed with no kinase domain mutation identified and achieved a second CR after blinatumomab and dasatinib therapy. Overall, 9 patients underwent ASCT while in first CR (7 with major molecular response and 2 with complete molecular response before transplantation). All but one are alive and disease-free after transplantation. There was no difference in OS when patients were censored or not at the time of ASCT (Figure 4). After transplantation and engraftment, TKI therapy was resumed in all but one patient (2 imatinib, 3 dasatinib, 1 nilotinib, and 2 ponatinib).
Figure 4.
Outcome with and without censoring for ASCT.
Safety
Median time to platelet and neutrophil recovery for cycle 1 was 22 and 18 days, respectively, and 22 and 16 days for subsequent cycles. Infections occurred during induction in 20 patients, 14 had Grade 3–4 increased liver function tests, 6 had Grade 3 pancreatitis, and 8 had Grade 3 skin rashes. Compared to our previous experience with hyper-CVAD in combination with other TKIs, toxicities were similar except for higher rates of pancreatitis and vascular events, including hypertension. In contrast, there was less pleural effusion and renal dysfunction and a trend for less infection when compared with the toxicities encountered with hyper-CVAD and dasatinib (Supplemental Table 2).
Venous thrombotic events were observed in 3 patients, with 1 renal vein thrombosis and 2 pulmonary emboli. These events occurred early on during the induction phase, did not recur, and did not further impact ponatinib therapy. Three patients had myocardial infarction, of which 2 were unexplained and 1 was in the context of sepsis. Thirteen patients had hypertension; 6 of the episodes were Grade 3 and occurred during either induction and the first consolidation cycles (Table 3). Eighteen (49%) patients had their dose reduced to 30 mg after a median of 13 weeks due to skin rash (n=7), transaminitis (n=4), deconditioning (n=3), prolonged thrombocytopenia (n=2), pancreatitis (n=1), or pleural/pericardial effusion (n=1). Two patients had further decreases to 15 mg daily after a median of 9 weeks because of persistent transaminatis and atrial fibrillation with rapid ventricular response (1 each). Two additional patients were switched to dasatinib after the 6th cycle owing to severe bullous skin lesions (1 patient) or imatinib after the second cycle owing to generalized deconditioning and exacerbation of comorbid medical conditions (1 patient).
Table 3.
Treatment-related toxicities encountered during induction and consolidation chemotherapy cycles regardless of causality
| N (%) | |||||
|---|---|---|---|---|---|
|
| |||||
| Parameter | Any Grade | Grade1–2 | Grade 3 | Grade 4 | Grade 5 |
| Infections overall | 32 (86) | 0 (0) | 25 (68) | 6 (16) | 1 (3) |
| Infections during induction | 20 (54) | 0 (0) | 19 (51) | 1 (3) | 0 (0) |
| Increased SGPT/SGOT | 36 (97) | 22 (59) | 12 (32) | 2 (5) | 0 (0) |
| Increased bilirubin | 34 (92) | 25 (68) | 7 (19) | 2 (5) | 0 (0) |
| Skin rash | 31 (84) | 23 (62) | 8 (22) | 0 (0) | 0 (0) |
| Increased Amylase/lipase | 12 (32) | 5 (14) | 5 (14) | 2 (5) | 0 (0) |
| Hypertension | 13 (35) | 7 (19) | 6 (16) | 0 (0) | 0 (0) |
| Pancreatitis | 9 (24) | 3 (8) | 6 (16) | 0 (0) | 0 (0) |
| Bleeding | 14 (38) | 9 (24) | 4 (11) | 0 (0) | 1(3) |
| Mucositis | 16 (43) | 12 (32) | 4 (11) | 0 (0) | 0 (0) |
| Abdominal pain | 18 (49) | 15 (41) | 3 (8) | 0 (0) | 0 (0) |
| Nausea | 23 (62) | 20 (54) | 3 (8) | 0 (0) | 0 (0) |
| Myocardial infarction* | 3 (8) | 0 (0) | 0 (0) | 0 (0) | 3 (8) |
| Thrombotic events** | 7 (19) | 4 (11) | 3 (8) | 0 (0) | 0 (0) |
| Diarrhea | 23 (62) | 21 (57) | 2 (5) | 0 (0) | 0 (0) |
| Vomiting | 16 (43) | 14 (38) | 2 (5) | 0 (0) | 0 (0) |
| Renal injury | 4 (11) | 2 (5) | 2 (5) | 0 (0) | 0 (0) |
| Anorexia | 16 (43) | 15 (41) | 1 (3) | (0) | 0 (0) |
| Pericardial effusion | 6 (16) | 5 (14) | 1 (3) | (0) | 0 (0) |
| Constipation | 21 (57) | 21 (57) | 0 (0) | 0 (0) | 0 (0) |
| Dry skin | 10 (27) | 10 (27) | 0 (0) | 0 (0) | 0 (0) |
| Pleural effusion | 9 (24) | 9 (24) | 0 (0) | 0 (0) | 0 (0) |
| Visual changes | 9 (24) | 9 (24) | 0 (0) | 0 (0) | 0 (0) |
All three patients had myocardial infarction Grade 5, of which 2 were unexplained and 1 was in the context of sepsis.
One renal vein thrombosis and two pulmonary emboli
After the increased incidence of vascular toxicities was recognized during the pivotal ponatinib trials in 2013 and based on our own experience with 2 possible related deaths on-study, we elected to modify our strategy for safety management. We offered our patients the option to switch TKIs, and for those who elected to stay on ponatinib, we reduced the ponatinib dose to 30 mg and further decreased it to 15 mg in patients in CMR. Thirteen patients remained on ponatinib at a dose of 15 mg daily in 12 and 30 mg daily in 1 (whose BCR-ABL transcript levels were 0.04%). Furthermore, beginning on August 1, 2014, the protocol was amended; ponatinib would be given at 45 mg daily for 14 days during induction therapy, then at 30 mg daily continuously starting with second cycle, and then further reduced to 15 mg daily continuously once a CMR was achieved. No further vascular events or any other serious adverse events were observed in patients receiving lower doses of ponatinib, and none after the amendment of the study protocol.
Overall, eleven patients switched TKIs to dasatinib (n=8), imatinib (n=2), or nilotinib n=1). Two of them switched owing to side effects encountered on ponatinib therapy, and 9 decided to electively switch TKIs. At the time of switching TKIs, 6 were receiving consolidation and 5 were receiving maintenance therapy. Their best response at the time of switch was MMR in all 11 patients, with 8 in CMR (73%).
Furthermore, we retrospectively compared characteristics and outcome between patients who solely stayed on ponatinib and those who switched TKIs for the reasons mentioned above. There was no difference in patient characteristics or in outcome (CR duration, EFS, and OS) between patients who remained on ponatinib throughout their treatment and those who were switched to other TKIs (Supplemental Table 1 and Figure 1).
Discussion
In this ongoing Phase II study, we show that the combination of hyper-CVAD with ponatinib is highly effective, with MMR and CMR rates of 37/37 (100%) and 29/37 (78%), respectively. Patients with Ph-positive ALL traditionally have a very poor outcome with anti-ALL chemotherapy, particularly if they did not undergo ASCT in first CR.24 Combinations of BCR-ABL1 TKIs with chemotherapy have significantly improved outcomes, especially when the TKIs are incorporated early and given daily and continuously with chemotherapy.1–8 Despite the high remission rates obtained with the combination of chemotherapy and first- and second-generations TKIs, the long-term survival rates remain 40–50%, with most relapsing patients acquiring the T315I mutation (30% to 70%).2,4,13–14
After a follow-up time of over 2 years, only two patients have relapsed, and no T315I mutations have been detected, a significant improvement compared to the experience with first- and second-generation TKIs.25 With a median follow-up of 26 months, the 2-year CR, EFS, and OS rates were 97%, 81%, and 80%, respectively. These results compare favorably with previous experiences in similar patients treated with hyper-CVAD and imatinib or dasatinib. The reported 2-year CR and OS rates for imatinib were 58% and 67%, respectively, and 70% and 64% for dasatinib.2,4 Furthermore, our results compare favorably with the recent results of the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) in 268 patients treated in a two-arm study comparing hyper-CVAD with imatinib to reduced-intensity chemotherapy with imatinib. The reported 2-year EFS and OS rates in that trial were 55% and 60%, respectively. Overall, the regimen was well tolerated. Grade 3 and 4 side effects were consistent with the known intensity of hyper-CVAD toxicity, including episodes of neutropenic fever, infections, and liver dysfunction. An increased incidence of hypertension and vascular side effects were seen. The major safety concern of ponatinib is the development of vascular occlusive events, which were reported at a cumulative rate of 27% in the PACE study.27 These events have been observed with all TKIs, although with different frequencies.28 Because ponatinib is a multikinase inhibitor, it is possible that inhibition of certain kinases such as VEGF, FGFR, or PDGFR can promote endothelial dysfunction, which predisposes patients to thromboembolic events.29, 30 However, other mechanisms may play a role as well. Patients with preexisting risk factors for atherogenesis or thromboembolic risk are particularly prone to vascular events with ponatinib and warrant close monitoring or alternate TKI use. Pooled data from 683 patients who received ponatinib in phase 1, 2, or frontline trials suggested that dose intensity might be associated with the frequency of adverse events, including cardiovascular events.31 Therefore, we elected to modify our strategy and recommended that patients either switch TKI, or in those who elected to stay on ponatinib, reduce ponatinib dose to 30 mg and further decrease it to 15 mg upon achievement of CMR.
In patients who elected to stay on ponatinib at the lower dose, all but one (92%) maintained their response with no additional vascular events observed, confirming the retrospective observation of the relationship between dose intensity and vascular events. Similarly, switching to a less potent TKI when an optimal response (CMR) was achieved was feasible and safe; only one patient has lost response 6 months after the switch with no resistant mutation detected. There was no difference in outcome between patients who remained on ponatinib and those who were switched to other TKIs. Therefore, an induction strategy with the most potent TKI and further maintenance at lower doses or with other TKIs is conceivable. We are currently clinically testing this strategy.
Besides the particularly increased rates of vascular events encountered, a post-hoc analysis comparing the adverse events encountered with hyper-CVAD and ponatinib to similar trials using hyper-CVAD in combination with other TKIs showed similar rates of other Grade ≥3 adverse events.
Although ASCT has improved the outcome of patients with Ph-positive ALL,1,32 this study importantly questions whether responding patients should be referred to ASCT in first CR. Ravandi and colleagues found that the achievement and maintenance of a MRD-negative status was associated with improved survival in patients with Ph-positive ALL treated with combination chemotherapy and TKIs but without ASCT.21 Therefore, MRD monitoring may identify patients in first CR in whom further consolidation with ASCT may not be needed.
It should be noted that our study population is somewhat different than other similar published cohorts. Our patient population had a mean WBC level that was lower than expected based on similar trials, but the range for this value was very wide in our population. Similarly, the age of our patients is somewhat higher than that seen in other comparable studies. This higher age may be indicative of the nature of the cases referred to our institution. However this median is similar to our previous experience with the combination of Hyper-CVAD and other TKI, where the median of patients treated was 53 years (range, 21–79 years).2
In summary, we have demonstrated the feasibility of combining chemotherapy with ponatinib in patients with Ph-positive ALL. The regimen is effective in achieving early sustained responses, with a median follow-up of 26 months. In responding patients, long-term disease free survival was not affected by ASCT. A longer follow-up is needed to confirm these findings. New strategies, including dose titration of ponatinib, optimal control of vascular risk factors, and the addition of new monoclonal antibodies, may help to further improve outcomes.
Supplementary Material
Research in Context.
Evidence before this study
Tyrosine kinase inhibitors (TKIs), in combination with chemotherapy, are one of the primary therapies for Philadelphia chromosome (Ph)-positive acute lymphoblastic leukemia. Unfortunately, many malignancies acquire resistance to most TKIs through the T315I mutation in the BCR-ABL1 fusion protein. We sought to address this major clinical issue by combining chemotherapy with a new TKI, ponatinib, that can effectively target both wildtype and T315I BCR-ABL1.
Added value of this study
We show that the combination of ponatinib with the hyperCVAD chemotherapy regimen results in durable responses in Ph-positive ALL.
Implications of all the available evidence
Ponatinib in combination with hyperCVAD represents an effective treatment for Ph-positive ALL, with high response rates and durable responses. Further refinement of the combination may result in new standards of care in these patients.
Footnotes
Authorship
Conflict-of-interest disclosure: The authors received research grants from ARIAD. ARIAD Pharmaceuticals Inc. provided free drug and financial support from the ARIAD Investigator Sponsored Trial program. The authors declare no other conflict of interest.
References
- 1.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123(6):843–850. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravandi F, O’Brien S, Thomas D, et al. First report of phase II study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27(31):5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas DA, O’Brien SM, Faderl S, et al. Long-term outcome after hyper-CVA and imatinib (IM) for de novo or minimally treated Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph-ALL) J Clin Oncol. 2010;28(15s):Abstract 6506. [Google Scholar]
- 5.Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. Long-term follow up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant. 2013;19:150–155. doi: 10.1016/j.bbmt.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28:3644–3652. doi: 10.1200/JCO.2010.28.1287. [DOI] [PubMed] [Google Scholar]
- 7.Pfeifer H, Goekbuget N, Volp C, et al. Long-term outcome of 335 patients receiving different schedules of imatinib and chemotherapy as front-line treatment for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood (ASH Annual Meeting Abstracts) 2010;116:Abstract 173. [Google Scholar]
- 8.Wassmann B, Pfeifer H, Goekbuget N, et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2006;108(5):1469–1477. doi: 10.1182/blood-2005-11-4386. [DOI] [PubMed] [Google Scholar]
- 9.Dombret H, Gabert J, Boiron JM, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia---results of the prospective multicenter LALA-94 trial. Blood. 2002;100:2357–66. doi: 10.1182/blood-2002-03-0704. [DOI] [PubMed] [Google Scholar]
- 10.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293(5531):876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 11.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2(2):117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 12.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305(5682):399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 13.Rousselot P, Coude MM, Huguet F, et al. Dasatinib and low intensity chemotherapy for first-line treatment in patients with de novo Philadelphia positive ALL aged 55 and over: final results of the EWALL-Ph-01 study. Blood. 2012;120(Suppl 1):Abstract 666. [Google Scholar]
- 14.Foa R, Vitale A, Vignetti M, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118:6521–6528. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 15.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–12. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–88. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–96. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 20.Thomas DA, O’Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3880–3889. doi: 10.1200/JCO.2009.26.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122:1214–21. doi: 10.1182/blood-2012-11-466482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luthra R, Sanchez-Vega B, Medeiros LJ. TaqMan RT-PCR assay coupled with capillary electrophoresis for quantification and identification of bcr-abl transcript type. Mod Pathol. 2004;17(1):96–103. doi: 10.1038/modpathol.3800026. [DOI] [PubMed] [Google Scholar]
- 23.Jones D, Thomas D, Yin CC, et al. Kinase domain point mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia emerge after therapy with BCR-ABL kinase inhibitors. Cancer. 2008;113(5):985–994. doi: 10.1002/cncr.23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weir EG, Cowan K, LeBeau P, Borowitz MJ. A limited antibody panel can distinguish B-precursor acute lymphoblastic leukemia from normal B precursors with four color flow cytometry: implications for residual disease detection. Leukemia. 1999;13(4):558–567. doi: 10.1038/sj.leu.2401364. [DOI] [PubMed] [Google Scholar]
- 25.Soverini S, De Benedittis C, Papayannidis C, et al. Drug resistance and BCR-ABL kinase domain mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia from the imatinib to the second-generation tyrosine kinase inhibitor era: The main changes are in the type of mutations, but not in the frequency of mutation involvement. Cancer. 2014;120(7):1002–1009. doi: 10.1002/cncr.28522. [DOI] [PubMed] [Google Scholar]
- 26.Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711–3719. doi: 10.1182/blood-2015-02-627935. [DOI] [PubMed] [Google Scholar]
- 27.Cortes JE, Ibarz JP, Coutre PL, et al. Long-Term Follow-up of Ponatinib Efficacy and Safety in the Phase 2 PACE Trial. Blood. 2014;124:Abstract 3135. [Google Scholar]
- 28.Valent P, Hadzijusufovic E, Schernthaner G, et al. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2014 doi: 10.1182/blood-2014-09-594432. [DOI] [PubMed] [Google Scholar]
- 29.Rivera VMPJ, Gonzalvez F, Baker F, Gozgit JM, Hodgson G. Comparative TKI Profiling Analyses to Explore Potential Mechanisms of Ponatinib-Associated Arterial Thrombotic Events. Blood. 2014;124:Abstract 1783. [Google Scholar]
- 30.Hadzijusufovic EA-SK, Huber K. Nilotinib exerts direct pro-atherogenic and anti-angiogenic effects on vascular endothelial cells: a potential explanation for drug-iduced vasculopathy in CML. Blood. 2013;122:Abstract 257. [Google Scholar]
- 31.Knickerbocker RDD, Haluska FG, Baccarani M, Cortes J, Hochhaus A, Talpaz M. Impact of Dose Intensity of Ponatinib on Selected Adverse Events: Multivariate Analyses from a Pooled Population of Clinical Trial Patients. Blood. 2014;124:Abstract 4546. doi: 10.1016/j.leukres.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Fielding AK. Philadelphia-positive acute lymphoblastic leukemia--is bone marrow transplant still necessary? Biol Blood Marrow Transplant. 2011;17:S84–8. doi: 10.1016/j.bbmt.2010.11.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.