Abstract
Objectives. To determine the association between total breastfeeding duration and serum 25-hydroxyvitamin D (25-OHD) and to explore whether vitamin D supplementation influences this association.
Methods. We conducted a cross-sectional study of healthy children between September 2011 and August 2013 through the TARGet Kids! primary health care research network. Of the 4533 eligible children, we included only the 2508 who had 25-OHD measured. We assessed adjusted associations of total breastfeeding duration (in months) with serum 25-OHD and in supplemented versus nonsupplemented children, with the odds of 25-OHD less than 20 nanograms per milliliter.
Results. Each 1-month increase in total breastfeeding duration was associated with a 0.12 nanograms per milliliter lower median serum 25-OHD (95% confidence interval [CI] = –0.21 ng/mL, –0.02 ng/mL) among children who were not supplemented. The odds of serum 25-OHD less than 20 nanograms per milliliter increased by 6% (odds ratio [OR] = 1.06; 95% CI = 1.03, 1.10) for every 1-month increase in total breastfeeding duration among nonsupplemented children. The interaction between vitamin D supplementation, duration of breastfeeding, and median serum 25-OHD was statistically significant (P = .04).
Conclusions. Breastfed children who were not supplemented, particularly those breastfed more than 1 year, appear to have lower vitamin D status. Vitamin D supplementation may mitigate this risk. These findings support recommendations for supplementation during breastfeeding of any duration.
Vitamin D is a fat-soluble steroid that has numerous biological actions that affect health.1–4 In infants and young children, very low vitamin D intake can lead to rickets.5–8 Exclusively breastfed infants who are not supplemented with vitamin D are at increased risk for developing rickets because of the limited transfer of vitamin D in breast milk.9,10 Although previous research supports the association between exclusive breastfeeding and low serum vitamin D levels in children,11,12 little is known about the effect of total duration of breastfeeding, including the period of nonexclusive breastfeeding, on vitamin D status.
Guidelines for vitamin D supplementation during breastfeeding beyond the first year of life vary throughout the world. The American Academy of Pediatrics recommends, “Any breastfeeding infant, regardless of whether he or she is being supplemented with formula, should be supplemented with 400 IU of vitamin D.”10(p1146) The Committee on Nutrition of the French Society of Pediatrics also suggests continuous vitamin D supplementation beyond the first year of life,13 irrespective of breastfeeding status, and the Canadian Pediatric Society recommends, “400 IU/day for all infants during the first year.”14 An improved understanding of the relationship between breastfeeding beyond the first year of life, vitamin D supplementation, and vitamin D stores may assist with reducing variation in professional recommendations, current practice, and optimizing vitamin D status during breastfeeding beyond the first year.
We examined whether total duration of breastfeeding is associated with infant serum 25-hydroxyvitamin D (25-OHD) concentration in a cohort of healthy urban North American children. Our secondary objective was to explore how vitamin D supplementation influences the relationship between total duration of breastfeeding and 25-OHD.
METHODS
This cross-sectional study involved healthy urban children aged 1 to 5 years receiving primary health care at a TARGet Kids! participating family medicine or pediatrician’s office between September 2011 and August 2013. TARGet Kids! is a practice-based primary care research network and collaboration between child health outcomes researchers and primary care physicians from the Department of Pediatrics and the Department of Family and Community Medicine at the University of Toronto (http://www.targetkids.ca).15
We use the term “total duration of breastfeeding” to indicate the duration of breastfeeding of any kind, including exclusive and nonexclusive.
Study Population
Trained research personnel embedded in 7 TARGet Kids! participating pediatric and family medicine practices recruited children. Sociodemographic, lifestyle, and nutritional information were collected during a scheduled primary health care physician visit through a standardized parent-completed survey instrument derived from the Canadian Community Health Survey.16
We excluded children with health conditions affecting growth (e.g., cystic fibrosis), chronic conditions (except for asthma), or severe developmental delay from the study.
Outcome and Predictor Variables
The primary outcome variable was serum 25-OHD (ng/mL) level. We measured this from serum samples using a competitive 2-step chemiluminescence assay (DiaSorin LIAISON 25-OHD TOTAL, Saluggia, Italy)17 at the Mount Sinai Services Laboratory in Toronto. Extensive testing and validation of this machine has been performed and has demonstrated an intraassay imprecision of 7.2% at a concentration of 85 nanograms per milliliter (to convert to nmol/L, multiply by 2.496) and an interassay imprecision of 4.9% at 13 nanograms per milliliter, 8.9% at 31 nanograms per milliliter, and 17.4% at 85 nanograms per milliliter—values that are well within acceptable limits for biochemical measurements.18,19
The primary predictor variable was total duration of breastfeeding, which we determined from the response to the question “For how long has your child been breastfed?” Maternal recall has been found to be a valid and reliable estimate of breastfeeding duration.20 We classified children who had never been breastfed as having a total duration of breastfeeding of 0 months. We classified those currently breastfeeding as having a total duration of breastfeeding equal to the child’s current age.
We included covariates if they were associated with 25-OHD in 1 or more published studies and included age, gender, body mass index (BMI) z-score, use of vitamin D supplementation, skin pigmentation, median after-tax neighborhood household income, season of blood sampling (May–September vs October–April), daily outdoor play time, and total daily cups of cow’s milk and formula. Weight was measured using a precision digital scale (±0.025%; SECA, Chino, CA), and standing height was measured using a stadiometer (SECA). We calculated BMI z-scores using World Health Organization growth standards.21 We determined the use of vitamin D supplementation from the response to the question “Does your child take a vitamin D–containing supplement regularly?” In Canada, commercially available vitamin D supplements for children contain 400 international units of vitamin D. A trained research assistant measured skin pigmentation by using the Fitzpatrick scale, a widely used 6-category skin pigmentation classification system.22,23 We used 6-digit postal codes to obtain the median after-tax neighborhood household income for each participant (using the Statistics Canada Postal Code Conversion File and data from the 2006 Canadian census). Environmental measures of socioeconomic status have been used previously as proxies for individual-level measures of socioeconomic status.24–26
Statistical Analysis
We generated descriptive statistics for the main outcome and covariates. For our primary analysis, we developed a multiple linear regression model to examine the relationship between total duration of breastfeeding and serum 25-OHD level. We included all covariates in the final model regardless of their associated P values to avoid potential bias of the R2 and SEs.27 We handled missing data by multiple imputation using predictive mean matching.27 The maximum frequency of missing data for any variable was 13%. We performed log transformation of serum 25-OHD level to meet the normality assumption.
In our secondary analysis, we explored how vitamin D supplementation may influence the relationship between total duration of breastfeeding and serum 25-OHD level. To accomplish this, we repeated the primary analysis with the inclusion of an interaction term between total duration of breastfeeding and vitamin D supplement use. We also developed a multiple logistic regression model to assess the odds of serum 25-OHD level less than 20 nanograms per milliliter with vitamin D supplement use as an interaction term. We derived the predicted probability of 25-OHD less than 20 nanograms per milliliter by transforming the predicted log-odds from the logistic regression model. The Institute of Medicine considers 20 nanograms per milliliter of serum 25-OHD to be adequate for most children.28
All statistical tests were 2-tailed and considered significant at the .05 level. We performed statistical analyses using SAS version 9.3 (SAS Institute, Cary, NC) and R version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Of the 4533 children aged 1 to 5 years who participated in TARGet Kids! between September 2011 and August 2013, 2510 (55%) had serum 25-OHD level available. Two of the children had abnormally high serum 25-OHD levels (> 140 ng/mL), and we excluded them as outliers (data available as a supplement to the online version of this article at http://www.ajph.org). The final analysis included 2508 children. The median age of included children was 24.5 months (interquartile range [IQR] = 15.1-40.8 months), and 1329 (53%) children were male. The median serum 25-OHD level was 32 nanograms per milliliter (IQR = 26 ng/mL, 40 ng/mL), and 130 (5%) children had 25-OHD less than 20 nanograms per milliliter. The median total duration of breastfeeding was 10.5 months (IQR = 6–14 months); 53% of children received vitamin D supplementation. Population characteristics are presented in Table 1. Children who had 25-OHD testing were clinically similar to those who did not have 25-OHD level obtained (Table 1).
TABLE 1—
Baseline Characteristics of Participants: Toronto, Canada, 2011–2013
| Characteristic | Children With 25-OHD Testing, n = 2508, No. (%) or Median (IQR) | Children Without 25-OHD testing, n = 2025, No. (%) or Median (IQR) | Pa |
| Total duration of breastfeeding, mo | 10.50 (6.00, 14.30) | 9.50 (5.00, 14.00) | ≤ .001 |
| ≤ 1 y | 1809 (72) | ||
| > 1 y | 699 (28) | ||
| 25-OHD, nmol/L | 32.10 (26.40, 39.60) | NA | NA |
| 25-OHD < 20 ng/mL | 130 (5) | NA | NA |
| Vitamin D supplementation | ≤ .001b | ||
| Yes | 1336 (53) | 838 (41) | |
| No | 1140 (45) | 1165 (58) | |
| Age, mo | 24.50 (15.10, 40.80) | 22.01 (12.35, 37.36) | ≤ .001 |
| Gender | .13b | ||
| Male | 1329 (53) | ||
| Female | 1179 (47) | ||
| BMI z-scorec | 0.14 (–0.55, 0.82) | 0.15 (–0.61, 0.84) | .91 |
| Skin pigmentationd | ≤ .001e | ||
| Type I | 314 (13) | 178 (8) | |
| Type II | 956 (38) | 579 (29) | |
| Type III | 709 (28) | 771 (38) | |
| Type IV | 295 (12) | 232 (11) | |
| Type V | 96 (4) | 55 (3) | |
| Type VI | 58 (2) | 43 (2) | |
| Season of blood sampling | |||
| May–September | 1219 (49) | NA | |
| October–April | 1289 (51) | NA | |
| Median household income, $ | 55 105.00 (42 935.00, 68 951.00) | 54 768.00 (42 481.00, 71 580.00) | .23 |
| Milk (cow’s milk and formula) volume, cups | 2.00 (1.00, 3.00) | 2.00 (0.50, 3.00) | ≤ .001 |
| Total min outdoor activities | 60.00 (30.00, 90.00) | 30.00 (0.00, 60.00) | ≤ .001 |
Note. BMI = body mass index; IQR = interquartile range; NA = not applicable; 25-OHD = 25-hydroxyvitamin D. Participants were children aged 1–5 years.
Obtained using the t test unless otherwise specified.
Obtained using the χ2 test.
BMI z-scores were determined by using World Health Organization growth standards.21
Fitzpatrick scale: type I is lightest; type VI is darkest.
Obtained using the Cochran-Armitage trend test.
Univariate analysis revealed that total duration of breastfeeding was not significantly associated with serum 25-OHD. This association remained nonsignificant after adjusting for clinically relevant covariates (Table 2). However, the interaction between vitamin D supplementation and total duration of breastfeeding was statistically significant (P = .04), suggesting that vitamin D supplementation was an effect modifier of the association between total duration of breastfeeding and serum 25-OHD (Table 2). This is shown graphically in Figure 1. Among children who did not receive vitamin D supplementation, every 1-month increase in total duration of breastfeeding was associated with a 0.12 nanograms per milliliter lower median serum 25-OHD level (95% CI = −0.21 ng/mL, −0.02 ng/mL; P = .015). Among children who did receive vitamin D supplementation, total duration of breastfeeding was not significantly associated with 25-OHD (change in median serum 25-OHD = 0.0128; 95% CI = −0.26, 0.27; P = .76). Age, total daily milk (cow’s milk and formula) consumption, skin type, and season of blood sampling were statistically significant covariates (Table 2).
TABLE 2—
Adjusted Serum 25-OHD Differences by Total Duration of Breastfeeding, Vitamin D Supplementation, and Other Potential Confounders: Toronto, Canada, 2011–2013
| Variable | Unadjusted Change in Median Serum 25-OHD, ng/mLa (95% CI) | Adjusted Change in Median Serum 25-OHD, ng/mLa (95% CI) | Adjusted Among Nonsupplemented Children, Including Interaction Term, Change in Median Serum 25-OHD, ng/mLa (95% CI) |
| Total duration of breastfeeding | −0.06 (−0.12, 0.01) | −0.04 (−0.11, 0.02) | −0.12 (−0.21, −0.02) |
| BMI z-score | 0.03 (−0.39, 0.45) | −0.07 (−0.48, 0.35) | −0.07 (−0.48, 0.35) |
| Age, mo | 0.02 (−0.01, 0.05) | 0.03 (0.00, 0.06) | 0.04 (0.00, 0.06) |
| Total milk (cow’s milk and formula), intake/d | 1.01 (0.70, 1.32) | 1.10 (0.79, 1.41) | 1.11 (0.80, 1.42) |
| Skin typeb | |||
| Type I–III (Ref) | 1 | 1 | 1 |
| Type IV–VI | −3.05 (−4.08, −1.99) | −3.12 (−4.15, −2.06) | −3.13 (−4.15, −2.07) |
| Season of blood sampling | |||
| May–September (Ref) | 1 | 1 | 1 |
| October–April | −1.44 (−2.27, −0.58) | −1.55 (−2.38, −0.69) | −1.52 (−2.35, −0.70) |
| Gender | |||
| Male (Ref) | 1 | 1 | 1 |
| Female | −0.22 (−1.09, 0.68) | −0.16 (−1.01, 0.71) | −0.13 (−0.98, 0.75) |
| Vitamin D supplementation | |||
| No (Ref) | 1 | 1 | 1 |
| Yes | 2.65 (1.71, 3.62) | 2.85 (1.91, 3.82) | 1.36 (−0.28, 3.08) |
| Total duration of breastfeeding and supplement | 0.13 (0.00, 0.25) | 0.13 (0.01, 0.26) |
Note. 25-OHD = 25-hydroxyvitamin D; BMI = body mass index; CI = confidence interval. Participants were children aged 1–5 years. Linear regression models were also adjusted for median after-tax household income and total min outdoor activity/d.
Linear regression models. Negative values indicate a decrease in serum level; positive values indicate an increase in serum level.
Fitzpatrick scale: type I is lightest; type VI is darkest.
FIGURE 1—
Serum 25-OHD as a Function of Total Duration of Breastfeeding Among Children Aged 1–5 Years: Toronto, Canada, 2011–2013
Note. 25-OHD = 25-hydroxyvitamin D.
We also found a statistically significant association between total duration of breastfeeding and the odds of 25-OHD less than 20 nanograms per milliliter among children who were not supplemented. For every 1-month increase in total duration of breastfeeding, the odds of 25-OHD less than 20 nanograms per milliliter increased by 6% (adjusted odds ratio [AOR] = 1.06 per month; 95% CI = 1.03, 1.10) among those who did not receive supplementation. The odds of 25-OHD less than 20 nanograms per milliliter was not significantly associated with total duration of breastfeeding among children who received vitamin D supplementation (AOR = 0.98; 95% CI = 0.95, 1.02).
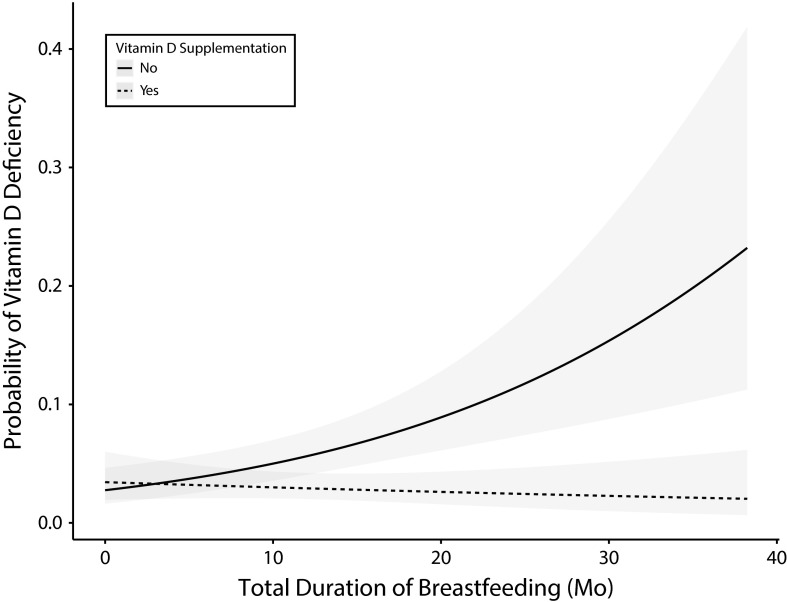
The adjusted predicted probability of vitamin D less than 20 nanograms per milliliter is shown in Figure 2 for both supplemented and nonsupplemented children. The predicted probability of vitamin D less than 20 nanograms per milliliter among children who did not receive supplementation was 16% with a total breastfeeding duration of 24 months and 29% with a total breastfeeding duration of 36 months.
FIGURE 2—
Predicted Probability of Serum 25-OHD < 20 ng/mL as a Function of Total Duration of Breastfeeding Among Children Aged 1–5 Years: Toronto, Canada, 2011–2013
Note. 25-OHD = 25-hydroxyvitamin D.
DISCUSSION
We identified an association between the total duration of breastfeeding and 25-OHD that was modified by vitamin D supplementation. Among children who did not receive vitamin D supplementation, each additional month of total breastfeeding duration was associated with a 0.12 nanograms per milliliter lower 25-OHD. Furthermore, the odds of 25-OHD less than 20 nanograms per milliliter was 6% higher for every 1-month increase in total duration of breastfeeding; thus, the predicted probability of 25-OHD less than 20 nanograms per milliliter was 16% by age 2 years and 29% by age 3 years among children who did not receive vitamin D supplementation. Among children who did receive vitamin D supplementation, we did not identify a relationship between total duration of breastfeeding and serum 25-OHD level, suggesting that the decline in serum 25-OHD during breastfeeding of any duration may be mitigated by supplementation with vitamin D.
According to the World Health Organization, breastfeeding is recommended for children up to age 2 years and beyond, as mutually desired by mother and child.29 Although breast milk contains many of the required nutrients for supporting growth, it is widely accepted that breast milk does not provide adequate amounts of vitamin D because of minimal transfer of vitamin D in breast milk.30–32 Exclusive breastfeeding in the first year of life without vitamin D supplementation is a known risk factor for rickets.11 Less is known about the relationship between total duration of breastfeeding, particularly beyond the first year of life and vitamin D status.
Although the American Academy of Pediatrics and the Committee on Nutrition of the French Society of Pediatrics recommend vitamin D supplementation during breastfeeding within the first few days of life and continuing throughout childhood,10,13 the Canadian Pediatric Society recommends vitamin D supplementation while breastfeeding up to aged 1 year.14 The Canadian Pediatric Society’s recommendation has been attributed to a lack of evidence for 400 iternational units per day of vitamin D supplementation in optimizing vitamin D status in older children.33 Our findings suggest that among children who did not receive vitamin D supplementation, longer total duration of breastfeeding was associated with lower 25-OHD and higher odds of 25-OHD less than 20 nanograms per milliliter, the level the Institute of Medicine considers deficient.28,34,35 Furthermore, the decline in serum 25-OHD with longer duration of breastfeeding may be minimized with vitamin D supplementation, suggesting that vitamin D supplementation may reduce the risk of low vitamin D stores while maximizing the benefits from a longer duration of breastfeeding.36 These observations support the American Academy of Pediatrics’ and French Society of Pediatrics’ recommendations for vitamin D supplementation beyond the first year of life while breastfeeding.
Strengths and Limitations
To the best of our knowledge, this is the first study to assess the effect of total duration of breastfeeding, including breastfeeding beyond age 1 year on serum 25-OHD. Strengths of our study include a relatively large sample size with comprehensive clinical data, which allowed the adjustment of multiple social, demographic, biological, and nutritional variables. Furthermore, we used a broad definition of breastfeeding duration, which included the period after the introduction of complementary foods. This is important both clinically and for current health policy objectives supporting breastfeeding through the first 2 years of life and beyond.29 Additionally, our primary care venue allowed us to measure serum 25-OHD among children who routinely receive primary health care, which is a setting well suited for the promotion of vitamin D supplementation during breastfeeding of any duration.
Limitations of this study include parent-completed questionnaire data, which may be subject to recall bias. The cross-sectional nature of this study did not allow us to address temporality. Confounders that were not accounted for in our questionnaire include maternal vitamin D stores, use of maternal vitamin D supplementation, and children’s dietary sources of vitamin D other than cow’s milk and vitamin D supplementation. Additionally, we did not quantify the dosage of vitamin D supplementation. However, vitamin D supplements for children in Canada contain 400 international units per dose. Lastly, we performed this study in Toronto, Canada, and the relationship between breastfeeding duration and vitamin D status may be different in areas with more or less ultraviolet radiation exposure.
Conclusions
We identified an association between longer total duration of breastfeeding and lower serum 25-OHD, which appeared to be modified by vitamin D supplementation. Vitamin D supplementation during breastfeeding beyond age 1 year may minimize the risk of serum 25-OHD levels less than 20 nanograms per milliliter. Our findings support the American Academy of Pediatrics and French Society of Pediatrics recommendations for vitamin D supplementation during breastfeeding of any duration.
ACKNOWLEDGMENTS
This study was supported by the Canadian Institutes of Health Research (MOP grant 106532) and the St Michael’s Foundation. The Pediatrics Outcomes Research Team is supported by the Hospital for Sick Children Foundation.
The authors thank all participating families for their time and involvement in TARGet Kids! and are grateful to all practitioners who are currently involved in the TARGet Kids! research network.
The steering committee comprised Tony Barozzino, Brian Chisamore, Mark Feldman, and Moshe Ipp. The research team comprised Kathleen Abreo, Tarandeep Malhi, Antonietta Pugliese, Megan Smith, and Laurie Thompson. At the Applied Health Research Centre were Gerald Lebovic, Magda Melo, and Patricia Nguyen. At the Mount Sinai Services Laboratory was Azar Azad. The TARGet Kids! Collaboration comprised the following—the scientific committee: Kawsari Abdullah, Laura N. Anderson, Catherine S. Birken, Cornelia M. Borkhoff, Sarah Carsley, Yang Chen, Mikael Katz-Lavigne, Kanthi Kavikondala, Christine Koroshegyi, Grace Jieun Lee, Jonathon L. Maguire, Dalah Mason, Jessica Omand, Patricia C. Parkin, Navindra Persaud, Meta van den Heuvel, and Weeda Zabih; site investigators: Jillian Baker, Tony Barozzino, Joey Bonifacio, Douglas Campbell, Sohail Cheema, Brian Chisamore, Karoon Danayan, Paul Das, Mary Beth Derocher, Anh Do, Michael Dorey, Sloane Freeman, Keewai Fung, Charlie Guiang, Curtis Handford, Hailey Hatch, Sheila Jacobson, Tara Kiran, Holly Knowles, Bruce Kwok, Sheila Lakhoo, Margarita Lam-Antoniades, Eddy Lau, Fok-Han Leung, Jennifer Loo, Sarah Mahmoud, Rosemary Moodie, Julia Morinis, Sharon Naymark, Patricia Neelands, James Owen, Michael Peer, Marty Perlmutar, Navindra Persaud, Andrew Pinto, Michelle Porepa, Nasreen Ramji, Noor Ramji, Alana Rosenthal, Janet Saunderson, Rahul Saxena, Michael Sgro, Susan Shepherd, Barbara Smiltnieks, Carolyn Taylor, Thea Weisdors, Sheila Wijayasinghe, Peter Wong, Ethel Ying, and Elizabeth Young.
Note. The Canadian Institutes of Health Research, the St Michael’s Foundation, and the Hospital for Sick Children Foundation were not involved in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the article.
HUMAN PARTICIPANT PROTECTION
Research ethics approval was granted through the research ethics board of the Hospital for Sick Children and of St. Michael’s Hospital. All parents of participating children provided written consent to participation in the study.
REFERENCES
- 1.Hayes CE, Cantorna MT, DeLuca HF. Vitamin D and multiple sclerosis. Proc Soc Exp Biol Med. 1997;216(1):21–27. doi: 10.3181/00379727-216-44153a. [DOI] [PubMed] [Google Scholar]
- 2.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 3.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39(2):365–379. doi: 10.1016/j.ecl.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9(12):941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 5.Özkan B. Nutritional rickets. J Clin Res Pediatr Endocrinol. 2010;2(4):137–143. doi: 10.4274/jcrpe.v2i4.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unuvar T, Buyukgebiz A. Nutritional rickets and vitamin D deficiency in infants, children and adolescents. Pediatr Endocrinol Rev. 2010;7(3):283–291. [PubMed] [Google Scholar]
- 7.Pettifor JM. Nutritional rickets: pathogenesis and prevention. Pediatr Endocrinol Rev. 2013;10(suppl 2):347–353. [PubMed] [Google Scholar]
- 8.Prentice A. Nutritional rickets around the world. J Steroid Biochem Mol Biol. 2013;136:201–206. doi: 10.1016/j.jsbmb.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. CMAJ. 2007;177(2):161–166. doi: 10.1503/cmaj.061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner CL, Greer FR American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 11.Choi YJ, Kim MK, Jeong SJ. Vitamin D deficiency in infants aged 1 to 6 months. Korean J Pediatr. 2013;56(5):205–210. doi: 10.3345/kjp.2013.56.5.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wall CR, Grant CC, Jones I. Vitamin D status of exclusively breastfed infants aged 2–3 months. Arch Dis Child. 2013;98(3):176–179. doi: 10.1136/archdischild-2012-302351. [DOI] [PubMed] [Google Scholar]
- 13.Vidailhet M, Mallet E, Bocquet A et al. Vitamin D: still a topical matter in children and adolescents. A position paper by the Committee on Nutrition of the French Society of Paediatrics. Arch Pediatr. 2012;19(3):316–328. doi: 10.1016/j.arcped.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Canadian Paediatric Society. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12(7):583–598. [PMC free article] [PubMed] [Google Scholar]
- 15.Carsley S, Borkhoff CM, Maguire JL et al. Cohort profile: the Applied Research Group for Kids (TARGet Kids!) Int J Epidemiol. 2015;44(3):776–788. doi: 10.1093/ije/dyu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Statistics Canada. Canadian community health survey 2000–2001. Available at: http://www23.statcan.gc.ca/imdb-bmdi/pub/instrument/3226_Q1_V1-eng.pdf. Accessed July 11, 2015.
- 17.Diasorin. The diagnostic specialist. Available at: http://www.diasorin.com/en. Accessed July 29, 2014. [Google Scholar]
- 18.Maunsell Z, Wright DJ, Rainbow SJ. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem. 2005;51(9):1683–1690. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 19.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91(8):3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 20.Li R, Scanlon KS, Serdula MK. The validity and reliability of maternal recall of breastfeeding practice. Nutr Rev. 2005;63(4):103–110. doi: 10.1111/j.1753-4887.2005.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO child growth standards. 2006. Available at: http://www.who.int/childgrowth/publications/technical_report_pub/en. Accessed July 12, 2014.
- 22.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter TO, Herreros F, Zhang JH et al. Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. Am J Clin Nutr. 2012;95(1):137–146. doi: 10.3945/ajcn.111.018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mustard CA, Derksen S, Berthelot JM, Wolfson M. Assessing ecologic proxies for household income: a comparison of household and neighbourhood level income measures in the study of population health status. Health Place. 1999;5(2):157–171. doi: 10.1016/s1353-8292(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 26.Soobader M, LeClere FB, Hadden W, Maury B. Using aggregate geographic data to proxy individual socioeconomic status: does size matter? Am J Public Health. 2001;91(4):632–636. doi: 10.2105/ajph.91.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2001. [Google Scholar]
- 28.Ross AC, Manson JE, Abrams SA et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Infant and young child nutrition. 2002. Available at: http://apps.who.int/gb/archive/pdf_files/WHA55/ewha5525.pdf. Accessed August 19, 2014.
- 30.Leerbeck E, Søndergaard H. The total content of vitamin D in human milk and cow’s milk. Br J Nutr. 1980;44(1):7–12. doi: 10.1079/bjn19800004. [DOI] [PubMed] [Google Scholar]
- 31.Hollis BW, Roos BA, Draper HH, Lambert PW. Vitamin D and its metabolites in human and bovine milk. J Nutr. 1981;111(7):1240–1248. doi: 10.1093/jn/111.7.1240. [DOI] [PubMed] [Google Scholar]
- 32.Reeve LE, Chesney RW, DeLuca HF. Vitamin D of human milk: identification of biologically active forms. Am J Clin Nutr. 1982;36(1):122–126. doi: 10.1093/ajcn/36.1.122. [DOI] [PubMed] [Google Scholar]
- 33.Roth DE. What should I say to parents about vitamin D supplementation from infancy to adolescence? Paediatr Child Health. 2009;14(9):575–577. doi: 10.1093/pch/14.9.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 35.Braegger C, Campoy C, Colomb V et al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013;56(6):692–701. doi: 10.1097/MPG.0b013e31828f3c05. [DOI] [PubMed] [Google Scholar]
- 36.Eidelman AI, Schanler RJ, Johnston M et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]