Abstract
OBJECTIVES:
Eosinophilic esophagitis (EoE) is a chronic inflammatory condition that causes esophageal remodeling and stricture formation. We compared the clinical course of symptoms, endoscopic findings, histology, and changes in phenotype over time in EoE patients with inflammatory and fibrostenotic phenotypes.
METHODS:
Data were obtained from EoE patients from three medical centers and followed prospectively. Endoscopic features and histology from index and follow-up endoscopies were recorded. Behavior was classified as inflammatory if endoscopic findings demonstrated furrows or white plaques and as fibrostenotic if endoscopic findings included fixed rings or strictures.
RESULTS:
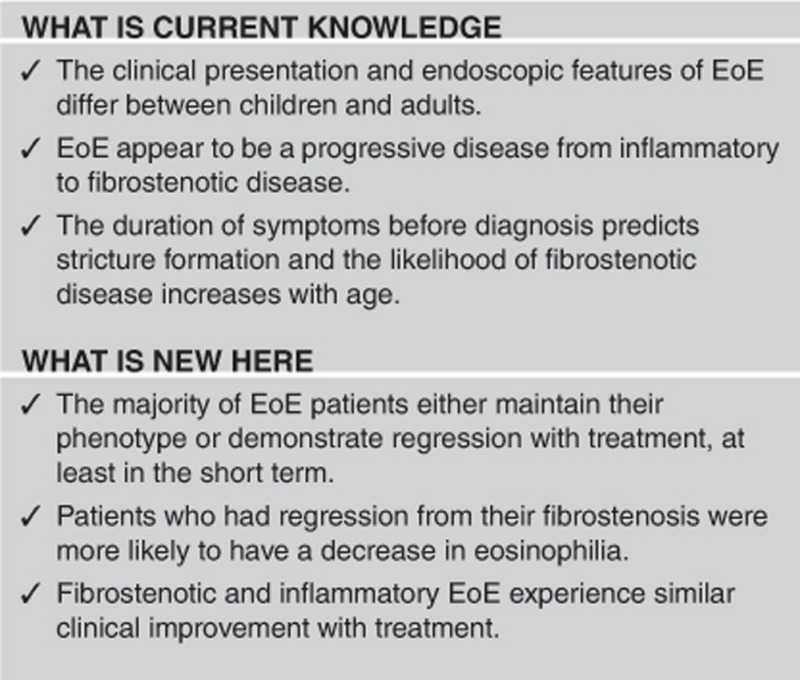
Two hundred and fifty-six EoE patients were included in the analysis. The mean age was 32±18 years, 25% of patients were <18 years, 89% of patients were Caucasians, and 74% of patients were male. The mean duration of symptoms before diagnosis was 6.8±7.2 years with a follow-up of 1.7±1.9 years (maximum follow-up of 12 years). Fifty-four percent of patients presented with fibrostenotic EoE, whereas 46% presented with inflammatory EoE. Patients with inflammatory disease were younger than those with fibrostenotic disease (24±19 vs. 39±15 years, P<0.001). Patients with fibrostenotic disease had a longer duration of symptoms than those with inflammatory disease (8.1±7.7 vs. 5.3±6.3 years, P=0.002). Over the study period, 47 (18%) had remission of inflammatory EoE, 68 (27%) continued to have inflammatory disease, 74 (29%) continued to have fibrostenotic disease, 65 (25%) fibrostenotic patients had regression of fibrosis, and 2 patients (1%) progressed from inflammatory disease to fibrostenotic disease. Patients who had regression from their fibrostenosis were more likely than patients who continued to demonstrate fibrostenosis to have a decrease in proximal (54% vs. 32%, P<0.001) and distal (70% vs. 38%, P<0.001) eosinophilia.
CONCLUSIONS:
Most EoE patients maintained their phenotype or had an improvement with <1% progressing from inflammatory to fibrostenosis. This suggests that early therapeutic strategies aimed at controlling inflammation may interrupt, decrease, or prevent the remodeling fibrosis in EoE.
INTRODUCTION
Eosinophilic esophagitis (EoE) is an increasingly recognized, immune-mediated chronic disease. Allergies trigger a proinflammatory cytokine cascade, which leads to eosinophil recruitment into the esophagus.1 Management is aimed at reducing allergen exposure and using anti-inflammatory medications, especially topical corticosteroids, to reduce the burden of inflammation.2 Many EoE patients have symptoms for years before seeking evaluation. This prolonged period of uncontrolled inflammation leads to esophageal remodeling and development of fibrostenosis.3, 4
The clinical presentation and endoscopic features of EoE differ between children and adults.2 In children, EoE typically presents with reflux-like symptoms, failure to thrive, abdominal pain, nausea, and early satiety. Although dysphagia and food impactions may occur, these symptoms are much less common in children.5 Endoscopic features include longitudinal furrows, white plaques, and loss of vascularity, whereas stricture formation is uncommon.5 In contrast, adults with EoE primarily complain of dysphagia and up to 55% of patients present with food impaction.6, 7 These symptoms can be a consequence of either stricture formation or a reduction in esophageal compliance.3, 8 On endoscopy, adults may display furrows, white plaques, fragile mucosa, and strictures. Concentric rings are the most common endoscopic finding and are described as felinization (faint rings that disappear with insufflation) or trachealization (rigid and fixed fibrotic rings that can cause a narrowing of the lumen).9, 10
These observations suggest that different phenotypes exist between children and adults with EoE. Common pediatric symptoms appear related to ongoing esophageal inflammation, and common adult symptoms appear to be secondary to fibrosis or stenosis. Furthermore, esophageal dilation is much less commonly performed in children with EoE than adults.11
Although fibrostenosis is uncommon in children, both phenotypes are frequently noted in adults.4, 12 A study of the Swiss EoE registry demonstrated that the duration of symptoms before diagnosis was the only factor that predicted stricture formation, suggesting that untreated inflammation is a major determinant of symptom development.4 A retrospective study analyzing the differences between EoE phenotypes in 379 patients showed that the likelihood of fibrostenotic disease increased with age, with twice the odds of fibrostenotic disease for every 10-year increase in age.12
The aims of this study were to (1) compare symptoms, endoscopic findings, and histology of EoE patients with inflammatory and fibrostenotic phenotypes and (2) to examine changes in phenotype over time in a multicenter study.
METHODS
Patient population
The data for this study was extracted from ongoing prospective registries of three medical centers (Walter Reed National Military Medical Center, Bethesda, MD, Mayo Clinic, Jacksonville, FL, and Icahn School of Medicine at Mount Sinai, New York, NY). EoE patients were prospectively recruited and enrolled into their institutions' respective clinical databases. All three registries include clinical, endoscopic, and histologic data on patients followed longitudinally. The inclusion criteria consisted of EoE patients in each respective registry who had more than one follow-up visit and repeat endoscopy with esophageal biopsies. The index endoscopy was at diagnosis for all patients. EoE patients with only one endoscopy were excluded. All EoE patients were diagnosed per recent consensus guidelines: clinical symptoms of esophageal dysfunction and histopathology demonstrating at least 15 eosinophils per high-powered field (eos/hpf) in at least one biopsy.5 In all cases, diagnosis was established when dense eosinophilia persisted after at least an 8-week trial of proton pump inhibitors (PPI). There were no patients on EoE-specific treatment at the time of diagnosis. Informed consent and assent, when applicable, was obtained from patients or their families for having their data collected. This study was approved by the institutional review boards of each institution.
Data collection for clinical symptoms, endoscopic features, and histology
Endoscopic findings, histologic features, and duration of disease before diagnosis were collected at index and at each subsequent clinic visit. Data were collected on all follow-ups while patients were on the same therapy. Patients completed symptom surveys assessing change in predominant symptoms (e.g. dysphagia in adults) described as “improved,” “worse,” or “remained the same” compared with their previous visit. For the analysis of symptom improvement over time, all endoscopies were performed while patients were on treatment. During upper endoscopy, a minimum of eight biopsies were taken, four from the mid-proximal esophagus (10–15 cm above the gastroesophageal junction) and four from the distal esophagus (3–5 cm above the gastroesophageal junction) to improve diagnostic accuracy.13 Five endoscopic features were recorded during the procedure to include concentric rings, longitudinal furrows, white plaques/exudates, edema, and strictures. Endoscopic features were assessed based on the recently validated Endoscopic Reference Score (EREFS) classification, which was developed to ensure good interobserver agreement.14 In this classification, the five endoscopic findings were scored (edema: 0–1; rings: 0–3; exudates: 0–1; furrows: 0–2; strictures: 0–3) and a summative EREFS score ranging from 0 to 10 was then calculated for each patient before and after treatment. Concentric rings were classified as felinization if the rings were faint or disappeared with air insufflation (EREFS grade 1) and trachealization if the rings were fixed and did not disappear with insufflation (EREFS grade 2 or 3). Dilation was performed with bougie-type dilators (Savary or Maloney) or through-the-scope balloons at the discretion of the endoscopist. All tissue was fixed in formalin and embedded in paraffin for histological examination. Esophageal biopsy slides from index and follow-up endoscopies were read by gastrointestinal pathologists at each institution and were then graded from 1 to 3 depending on the maximum peak eosinophil count (1=≤5 eos/hpf, 2=6–14 eos/hpf, 3=≥15 eos/hpf). Response to treatment was defined as achieving ≤5 eos/hpf on follow-up endoscopy after medical treatment.
Classification of disease behavior
Disease behavior was classified endoscopically. At index endoscopy, phenotype was classified as inflammatory if findings were limited to longitudinal furrows, white plaques, or felinization (rings were faint or disappeared with insufflation) and as fibrostenotic if findings included trachealization or any type of esophageal stricture (diffuse narrowing or dominant stricture). Those with mixed features of inflammatory and fibrostenotic phenotypes were classified as fibrostenotic phenotype. Change in behavior from index to last follow-up was classified into 1 of 5 categories based on endoscopic findings and histology after repeat endoscopy: remission of inflammatory (based on achieving ≤5 eos/hpf), remained inflammatory (≥15 eos/hpf), remained fibrostenotic (persistence of strictures or fixed rings and continued to have ≥15 eos/hpf), progressed from inflammatory to fibrostenosis (developed new fixed rings or strictures and had ≥15 eos/hpf), or had regression of fibrostenosis (based on regression of fixed rings/strictures and ≤5 eos/hpf). Medical treatment consisted of continuation of PPI, topical steroids (aerosolized swallowed fluticasone or oral viscous budesonide with dosage based on patient age), or specialized diets (empiric elimination, or allergy-testing directed). Endoscopic treatment consisted of dilation with size and type based on the discretion of the endoscopist.
Statistical analysis
Patients with inflammatory disease were compared against those with fibrostenotic disease by examining presenting symptoms, clinical history, and grade of eosinophilia in the proximal and distal esophagus collected at the time of index visit. Changes in phenotype and histology were based on comparing data from the final follow-up visit to index visit. Symptom improvement over time was based on the final follow-up visit assessment. Data were collated and analyzed using statistical software package IBM SPSS Statistics 21.0 (IBM, Armonk, NY, USA). Demographic and clinical characteristics of the study cohort were presented descriptively using means and standard deviations for continuous variables and counts with proportions for categorical variables. Fisher's exact test and χ2 test were used for statistical analysis between group comparisons of categorical data. Independent-sample t-test and one-way analysis of variance were used for comparisons of continuous data. Levene's test was used to assess equality of variances among groups. Bonferroni or Tamhane's T2 were used for post hoc pairwise comparisons. Logistic regression was used to identify patients who were likely to report symptom improvement vs. patients who had no change or worsened symptoms on last follow-up visit. Logistic regression was also used to identify baseline characteristics that may predict resolution of the inflammatory phenotype of EoE. A probability value of <0.05 was considered statistically significant.
RESULTS
Characteristics of study cohort
A total of 256 EoE patients from three institutions (Walter Reed: n=83; Mount Sinai: n=91; Mayo Clinic: n=82) were identified and included in the analysis. The mean follow-up time was 1.7±1.9 years, with a maximum follow-up of 12 years (Table 1).
Table 1. Characteristics of study population.
| All patients (n=256) | Children (n=64) | Adults (n=191) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age (years±s.d.) | 32±18 | 8±4.5 | 40±14 | — |
| % Male | 74 | 86 | 70 | 0.013 |
| % Caucasian | 89 | 88 | 89 | 0.820 |
| Clinical characteristics | ||||
| Duration of symptoms before diagnosis (mean years±s.d.) | 6.8±7.2 | 3.5±3.2 | 8.0±7.8 | <0.001 |
| Mean follow-up time (years±s.d.) | 1.7±1.9 | 0.90±1.2 | 1.90±2.1 | <0.001 |
| Symptom improvement over time (%) | 65 | 72 | 63 | 0.085 |
| Disease phenotype | ||||
| Fibrostenotic disease (%) | 54 | 16 | 67 | <0.001 |
| Inflammatory disease (%) | 46 | 84 | 33 | <0.001 |
Among the study cohort, 64 (25%) were pediatric (age <18 years) and 191 (75%) were adults. Pediatric patients were more likely to present with inflammatory than fibrostenotic disease when compared with adults (84% vs. 33%, P<0.001). Adults had a longer duration of symptoms before diagnosis than pediatric patients (8.0±7.8 vs. 3.5±3.2 years, P<0.001). Medical treatment of EoE included PPI alone in 14% of the cohort, topical steroids in 65%, of which 43% were intermittently dosed and 22% were on continuous steroids, and 21% were managed with diet (dietary restriction followed by sequential food challenges if restriction was effective). The majority of pediatric patients (58%) were treated with diet, whereas the majority of adults (73%) were treated with topical steroids.
Endoscopic features using EREFS classification
Changes in endoscopic features as assessed by the EREFS score revealed a significant decrease in score from index to follow-up (2.4±1.3 vs. 1.4±1.3, P<0.001). This decrease was also significant when stratified by age group and institution. Patients treated with continuous steroids or diet had a greater decrease in EREFS score compared with patients treated with PPI only or intermittent steroids (−1.3±1.7, −1.3±1.7, −0.4±1.6, −0.7±1.4, respectively, P=0.004).
Dilation and EoE
Of the 256 patients, 70 (27%) underwent dilation. EoE patients with fibrostenotic disease were more likely to undergo dilation than those with inflammatory disease (44% vs. 9%, P<0.001). On assessment at last follow-up, patients who were dilated had similar rates of overall symptom improvement compared with those who were not dilated (51% vs. 56%, P=0.735).
Comparisons of inflammatory to fibrostenosis phenotypes
Patients with inflammatory disease presented at a younger age than those with fibrostenotic disease (24±19 vs. 39±15 years old, P<0.001). Duration of symptoms before diagnosis was greater in patients with fibrostenotic than inflammatory phenotype (8.1±7.7 vs. 5.3±6.3 years, P=0.002). Patients with fibrostenotic disease were more likely to present with dysphagia (92% vs. 67%, P<0.001) and food impactions (46% vs. 30%, P=0.010). Patients with inflammatory disease were more likely than those with fibrostenosis to have coexisting atopic conditions (Table 2).
Table 2. Comparison of inflammatory vs. fibrostenotic phenotype.
| All patients (n=256) | P-value | Pediatric (n=64) | P-value | Adults (n=191) | P-value | |
|---|---|---|---|---|---|---|
| Inflammatory vs. fibrostenosis | Inflammatory vs. fibrostenosis | Inflammatory vs. fibrostenosis | ||||
| Demographics | ||||||
| Age (years±s.d.) | 24±19 vs. 39±15 | <0.001 | 8±5 vs. 11±4 | 0.046 | 38±15 vs. 41±14 | 0.179 |
| % Male | 74 vs. 75 (%) | 0.774 | 83 vs. 100 (%) | 0.333 | 65 vs. 83 (%) | 0.241 |
| % Caucasian | 87 vs. 90 (%) | 0.556 | 87 vs. 90 (%) | 1.000 | 87 vs. 90 (%) | 0.627 |
| Atopic conditions | ||||||
| Asthma | 33 vs. 23 (%) | 0.121 | 46 vs. 30 (%) | 0.492 | 21 vs. 23 (%) | 0.853 |
| Food allergies | 41 vs. 25 (%) | 0.007 | 69 vs. 40 (%) | 0.148 | 18 vs. 24 (%) | 0.356 |
| Eczema | 22 vs. 11 (%) | 0.017 | 43 vs. 20 (%) | 0.292 | 5 vs. 10 (%) | 0.272 |
| Allergic rhinitis | 52 vs. 38 (%) | 0.032 | 65 vs. 80 (%) | 0.476 | 41 vs. 35 (%) | 0.430 |
| Clinical presentation | ||||||
| Dysphagia | 67 vs. 92 (%) | <0.001 | 48 vs. 70 (%) | 0.305 | 84 vs. 94 (%) | 0.037 |
| Food impaction | 30 vs. 46 (%) | 0.010 | 19 vs. 80 (%) | <0.001 | 40 vs. 44 (%) | 0.755 |
| Heartburn | 30 vs. 40 (%) | 0.115 | 13 vs. 30 (%) | 0.189 | 45 vs. 41 (%) | 0.639 |
| Regurgitation | 36 vs. 27 (%) | 0.248 | 40 vs. 40 (%) | 1.000 | 30 vs. 25 (%) | 0.654 |
| Symptom characteristics | ||||||
| Symptom improvement over time | 60 vs. 69 (%) | 0.256 | 70 vs. 80 (%) | 0.784 | 52 vs. 68 (%) | 0.024 |
| Duration of symptoms (years±s.d.) | 5.3±6.3 vs. 8.1±7.7 | 0.002 | 3.5±3.4 vs. 3.7±2.4 | 0.818 | 6.9±7.7 vs. 8.5±7.8 | 0.201 |
| Follow-up time (years±s.d.) | 1.6±2.0 vs. 1.7±1.9 | 0.442 | 1.0±1.2 vs. 0.7±0.9 | 0.466 | 2.0±2.4 vs. 1.8±1.9 | 0.497 |
| Disease characteristics | ||||||
| Grade 3 mid-proximal eosinophilia (≥15 eos/hpf) | 82 vs. 79 (%) | 0.769 | 87 vs. 90 (%) | 0.726 | 77 vs. 78 (%) | 0.606 |
| Grade 3 distal eosinophilia (≥15 eos/hpf) | 89 vs. 88 (%) | 0.359 | 91 vs. 100 (%) | 0.636 | 86 vs. 88 (%) | 0.441 |
| % Patients underwent dilation | 9 vs. 44 (%) | <0.001 | ||||
When stratified by age category, a greater proportion of pediatric patients with fibrostenotic compared with inflammatory EoE presented with food impaction (80% vs. 19%, P<0.001). In adults, dysphagia was more prevalent in fibrostenotic vs. inflammatory disease (94% vs. 84%, P=0.037), whereas in pediatric patients, dysphagia was similar (70% vs. 48%, P=0.305). Within the pediatric cohort, patients with fibrostenosis were significantly older than those with inflammatory EoE (11±4 vs. 8±5 years, P=0.046). In adults, age was similar between the two phenotypes (41±14 vs. 38±15 years, P=0.179).
We also performed a subgroup analysis after categorizing patients with fibrostenotic features into two groups, those with only fibrostenotic features and those with mixed phenotype (inflammatory and fibrostenosis). We found that patients with exclusive fibrostenotic features compared with those with mixed features were similar in dysphagia (93% vs. 92%, P=1.000), food impaction (41% vs. 50%, P=0.359), rate of dilation (48% vs. 41%, P=0.576), treatment with steroids (88% vs. 73%, P=0.075), treatment with diet (0.0% vs. 9.3%, P=0.057), grade of proximal eosinophilia (2.6±0.79 vs. 2.7±0.56, P=0.269) and distal eosinophilia (2.9±0.3 vs. 2.9±041, P=0.796). Patients with fibrostenosis alone compared with those with mixed features had a longer duration of symptoms (10.2±8.8 vs. 7.2±6.9, P=0.038). We also compared fibrostenotic and mixed phenotypes to inflammatory EoE (Supplementary Table 1 online).
Phenotypic course of EoE
More than half of patients (56%) maintained their phenotype over the mean study follow-up time of 1.7 years. Of the cohort, 47 patients (18%) had remission of inflammatory EoE, 68 patients (27%) remained inflammatory, 74 patients (29%) remained fibrostenotic, 65 patients (25%) had regression of fibrostenosis, and 2 patients (1%) progressed from inflammatory to fibrostenotic. Patients who had resolution of inflammatory phenotype or remained inflammatory were more commonly treated with topical steroids compared with the other groups, P<0.001 (Table 3). The two patients who progressed to fibrostenosis by developing strictures or fixed rings were young adults who did not follow their prescribed dietary restriction therapy. Patients who remained fibrostenotic or had regression of fibrostenosis were significantly older than the other patient categories (Table 3) (P<0.001). Duration of symptoms was significantly different between groups with patients having persistent fibrostenosis or regression of fibrostenosis having the longest duration (P=0.013). Follow-up time was similar across all categories (P=0.672). No patients developed esophageal malignancies or other gastrointestinal conditions over the duration of study with a maximum follow-up of 12 years. Logistic regression did not identify any baseline characteristics that would predict remission of EoE.
Table 3. Characteristics of disease stratified by behavior.
| Remission of inflammatory | Persistence of inflammatory | Progression to fibrostenosis | Persistence of fibrostenosis | Regression of fibrostenosis | P-value | |
|---|---|---|---|---|---|---|
| N (%) 256 EoE patients | 47 (18%) | 68 (27%) | 2 (1%) | 74 (29%) | 65 (25%) | — |
| Mean age (years±s.d.) | 26±20 | 23±18 | 18±0.3 | 38±14 | 40±17 | <0.001 |
| Histology grade 3 (>15 eos/hpf) | ||||||
| Index proximal eosinophilia (%) | 72 | 75 | 100 | 84 | 74 | 0.833 |
| Index distal eosinophilia (%) | 74 | 93 | 100 | 83 | 95 | 0.366 |
| Last visit proximal eosinophilia (%) | 0 | 62 | 50 | 62 | 31 | 0.006 |
| Last visit distal eosinophilia (%) | 0 | 81 | 50 | 65 | 38 | 0.004 |
| Clinical symptoms | ||||||
| Duration of symptoms (years±s.d.) | 6.5±7.6 | 4.2±5.0 | 2.6±0.8 | 8.0±7.1 | 8.2±8.3 | 0.013 |
| Follow-up time (years±s.d.) | 1.5±2.0 | 1.6±2.1 | 2.2±2.6 | 1.7±1.9 | 1.8±1.8 | 0.465 |
| Change in symptoms at follow-up | <0.001 | |||||
| % Patients improved symptoms | 72 | 54 | 0 | 69 | 69 | |
| % Patients unchanged symptoms | 19 | 35 | 50 | 25 | 26 | |
| % Patients worsened symptoms | 9 | 10 | 50 | 7 | 5 | |
| Treatment | <0.001 | |||||
| PPI only (%) | 15 | 12 | 0 | 22 | 9% | |
| Intermittent topical steroid (%) | 23 | 31 | 0 | 54 | 58 | |
| Continuous topical steroid (%) | 34 | 16 | 0 | 23 | 20 | |
| Diet (%) | 28 | 41 | 100 | 1 | 12 | |
Histopathology and EoE phenotypes
There was no significant difference between inflammatory and fibrostenotic disease in proximal (82% vs. 79%, P=0.769) or distal (89% vs. 88%, P=0.359) grade 3 eosinophilia (>15 eos/hpf) at index endoscopy. Among those with fibrostenosis at index, more patients who remained fibrostenosis compared with those who had regression of fibrosis had grade 3 eosinophilia in the proximal (62% vs. 31%, P=0.005) and distal (65% vs. 38%, P=0.003) esophagus. Patients who had regression from their fibrostenosis were more likely to have a decrease in proximal (54% vs. 32%, P<0.001) and distal (70% vs. 38%, P<0.001) eosinophilia. On follow-up, 46% of patients with inflammatory EoE compared with 28% of patients with fibrostenosis had response to medical treatment (≤5 eos/hpf, P<0.008). Among patients treated with steroids, 40% had treatment response and among patients treated with diet, response was 42% (P=0.872).
Clinical symptoms over time
During follow-up assessment, a similar proportion of patients who presented with fibrostenotic and inflammatory EoE reported improvement in symptoms (69% vs. 60%, P=0.256). Symptom improvement was similar among patients treated with PPI only, steroids, or diet (58%, 62%, 76%, P=0.212). Dilation was more common in patients treated with PPI alone and topical steroids vs. patients treated with diet (27%, 35%, 2%, P<0.001).
DISCUSSION
In this multicenter study, EoE patients with inflammatory disease were younger and had a shorter duration of symptoms compared with those with fibrostenotic disease. Additionally, the majority of patients maintained their phenotype over a 1.7-year follow-up, and, more importantly, did not progress to fibrosis. This implies that either a longer duration of untreated disease is required for stricture formation, or that early medical intervention may delay progression to fibrostenosis. The majority of our patients who had fibrostenotic disease was older, had a longer duration of symptoms before diagnosis, and presented more frequently with dysphagia and food impactions. This supports recently published data demonstrating that continued untreated inflammation may lead to fibrosis and is associated with longer duration of symptoms.4, 12 Furthermore, fibrostenotic disease was rare in children, likely due to their shorter duration of symptoms before diagnosis. This observation suggests that early recognition and treatment of EoE could affect the natural course of endoscopic findings and reduce stricture formation.
Progression from inflammatory to fibrostenotic disease was rare in our cohort and only noted in two young adults during the study follow-up period. This observation, along with the finding that regression of fibrostenotic disease was associated with a decrease in esophageal eosinophilia, suggests that early intervention in inflammatory EoE may delay or even prevent fibrostenosis, although studies with a longer duration of follow-up are needed to confirm this finding. A previous study demonstrated that overall esophageal diameter increased after only 6 weeks of topical steroids in a small number of EoE patients,15 but it is unclear if such a short duration of treatment reversed fibrostenotic features or treated edematous changes. In another study of 26 children with EoE, both topical steroids and dietary restriction reversed esophageal fibrosis after 8 weeks of treatment.16
In addition to inflammatory and fibrostenosis, previous studies have described a third EoE phenotype. This third phenotype included patients with mixed features of fixed rings or strictures and white plaques or furrows.4, 12 In our study, we chose to limit our phenotypes to only inflammatory and fibrostenosis, similar to the manner in which Crohn's disease, another chronic immune-mediated condition, is characterized.17 We performed a subgroup analysis comparing fibrostenotic patients to a mixed phenotype (inflammatory and fibrostenosis), and found no significant clinical or histologic difference between the two; however, there was a significantly longer duration of symptoms in the fibrostenosis group. There are two small studies, to date, which have demonstrated reversal in esophageal remodeling and fibrosis with treatment in EoE children,16, 18 yet in a study examining the effects of topical steroids on collagen deposition in adult EoE patients, a significant reduction in collagen was not observed.19
Approximately half of the patients with EoE in our study achieved histologic response to either topical steroids or dietary treatment. One important factor to be considered is the definition of therapeutic response. In our study, we used stringent criteria of achieving (≤5 eos/hpf). Although our study was not controlled, our results are consistent with data from clinical trials where response to swallowed fluticasone has ranged from 50 to 62%.20, 21, 22 Response to budesonide is more variable in clinical trials (64–94%) and is dependent on several factors to include delivery mode (nebulizer vs. swallowed viscous), outcomes measured, dosage of drug, and duration of treatment.23, 24, 25, 26 A recent study found response to budesonide to be 50% when using a compound symptomatic and histologic primary end point.27 Histologic response to specialized diets ranges from nearly 50% for allergy-testing-directed elimination diet and ~70% for empiric food elimination diets.28 No studies to date have examined the role of combination steroid and diet treatment for the management of EoE.
In our multicenter study, we examined characteristics associated with different EoE phenotypes and prospectively assessed endoscopic findings and clinical symptoms in each patient. Similar to other studies, our data confirm that the longitudinal history of EoE is benign.29, 30, 31 Regardless of phenotype, our EoE patients had favorable clinical outcomes with medical or dietary treatment and none developed malignancy. This may be related to a better understanding in the pathogenesis and treatments of EoE during the past decade.32 Controlled studies have reported histologic remission with the use of higher doses of topical steroids or dietary intervention.21, 33, 34, 35 Furthermore, esophageal dilation has been reported to be a safe and effective therapy in EoE, particularly in patients who have fixed rings or strictures, but is unlikely to have an impact on the inflammatory process.36
Our study has several strengths. First, it included a large cohort of pediatric and adult EoE patients from three referral institutions and no heterogeneity was observed among study sites. Second, all EoE patients were followed longitudinally and data points were collected prospectively, eliminating recall bias. Third, all three centers maintained a comprehensive registry in which EoE patients were followed closely and data points were uniformly collected. There are some limitations to our study. Our cohort had a short follow-up period; however, our study was performed in an effort to gain preliminary insight into the longitudinal course of EoE. Symptom assessment was performed using surveys that assessed symptoms over a short period of time. These were not validated for EoE and therefore were only used to assess relative symptom improvement. Additionally, symptoms in EoE may be intermittent and the intensity may fluctuate over time. The intent of this simple survey was to assess the patient's general symptom status at follow-up. Treatment trials were not controlled and there may have been limited information regarding the number and types of treatments patients received over time. We did not assess adherence to treatment, which may have affected our treatment outcomes. Finally, it is important to note that the results of this study represent the experience from three tertiary medical centers where EoE patients are aggressively followed and treated and this may not represent that of general community practices.
EoE is a condition with a wide range of clinical presentations, depending on age of diagnosis and duration of symptoms. Our study findings reveal that inflammatory and fibrostenotic patients have different clinical and endoscopic characteristics. In addition, only two patients progressed from inflammatory to fibrostenosis with the majority of EoE patients either maintaining their phenotype or demonstrated regression. Therefore, early therapeutic strategies aimed at controlling inflammation may interrupt or prevent remodeling fibrosis in EoE. Further long-term longitudinal studies are needed to amplify the findings of this study.
Study Highlights
Guarantor of the article: Fouad J. Moawad, MD.
Specific author contributions: Manish B. Singla: study concept, data collection, analysis and interpretation of data, and drafting of manuscript; Mirna Chehade: study concept, data collection, drafting of manuscript, and critical revision of manuscript for important intellectual content; Diana Brizuela: data collection and drafting of manuscript; Corinne Maydonovitch: statistical analysis and drafting of manuscript; Yen-Ju Chen: data collection; Mary Ellen Riffle: data collection; Sami R. Achem: study concept, data collection, drafting of manuscript, and critical revision of manuscript for important intellectual content. Fouad J. Moawad: study concept, data collection, drafting of manuscript, and critical revision of manuscript for important intellectual content.
Financial support: None.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009; 137: 1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon ES. Diagnosis and management of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2012; 10: 1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatek MA, Hirano I, Kahrilas PJ et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology 2011; 140: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer AM, Safroneeva E, Bussmann C et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013; 145: 1230-6e1–1230-6e2. [DOI] [PubMed] [Google Scholar]
- Liacouras CA, Furuta GT, Hirano I et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128: 3–20. [DOI] [PubMed] [Google Scholar]
- Kerlin P, Jones D, Remedios M et al. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol 2007; 41: 356–361. [DOI] [PubMed] [Google Scholar]
- Sperry SL, Crockett SD, Miller CB et al. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc 2011; 74: 985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodeme F, Hirano I, Chen J et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2013; 11: 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano I, Moy N, Heckman MG et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2012; 62: 489–495. [DOI] [PubMed] [Google Scholar]
- Kim HP, Vance RB, Shaheen NJ et al. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liacouras CA. Clinical presentation and treatment of pediatric patients with eosinophilic esophagitis. Gastroenterol Hepatol (NY) 2011; 7: 264–267. [PMC free article] [PubMed] [Google Scholar]
- Dellon ES, Kim HP, Sperry SL et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014; 79: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JA, Lager DJ, Lewin M et al. The optimal number of biopsy fragments to establish a morphologic diagnosis of eosinophilic esophagitis. Am J Gastroenterol 2014; 109: 515–520. [DOI] [PubMed] [Google Scholar]
- Hirano I, Moy N, Heckman MG et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2013; 62: 489–495. [DOI] [PubMed] [Google Scholar]
- Lee J, Huprich J, Kujath C et al. Esophageal diameter is decreased in some patients with eosinophilic esophagitis and might increase with topical corticosteroid therapy. Clin Gastroenterol Hepatol 2012; 10: 481–486. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Morotti RA, Konstantinou GN et al. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy 2012; 67: 1299–1307. [DOI] [PubMed] [Google Scholar]
- Satsangi J, Silverberg MS, Vermeire S et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceves SS, Newbury RO, Chen D et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy 2009; 65: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucendo AJ, Arias A, De Rezende LC et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. J Allergy Clin Immunol 2011; 128: 1037–1046. [DOI] [PubMed] [Google Scholar]
- Butz BK, Wen T, Gleich GJ et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology 2014; 147: 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JA, Jung KW, Arora AS et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2012; 10: 742–749. [DOI] [PubMed] [Google Scholar]
- Konikoff MR, Noel RJ, Blanchard C et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 2006; 131: 1381–1391. [DOI] [PubMed] [Google Scholar]
- Aceves SS, Bastian JF, Newbury RO et al. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol 2007; 102: 2271–2279. [DOI] [PubMed] [Google Scholar]
- Straumann A, Conus S, Degen L et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139: 1526–1537. [DOI] [PubMed] [Google Scholar]
- Straumann A, Conus S, Degen L et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2011; 9: 400–409. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Sheikh A, Speck O et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology 2012; 143: 321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2015; 13: 66–76. [DOI] [PubMed] [Google Scholar]
- Arias A, Gonzalez-Cervera J, Tenias JM et al. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology 2014; 146: 1639–1648. [DOI] [PubMed] [Google Scholar]
- Straumann A, Spichtin HP, Grize L et al. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125: 1660–1669. [DOI] [PubMed] [Google Scholar]
- Menard-Katcher P, Marks KL, Liacouras CA et al. The natural history of eosinophilic oesophagitis in the transition from childhood to adulthood. Aliment Pharmacol Ther 2013; 37: 114–121. [DOI] [PubMed] [Google Scholar]
- Lipka S, Keshishian J, Boyce HW et al. The natural history of steroid-naive eosinophilic esophagitis in adults treated with endoscopic dilation and proton pump inhibitor therapy over a mean duration of nearly 14 years. Gastrointest Endosc 2014; 80: 592–598. [DOI] [PubMed] [Google Scholar]
- Moawad FJ, Veerappan GR, Dias JA et al. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am J Gastroenterol 2013; 108: 366–372. [DOI] [PubMed] [Google Scholar]
- Lucendo AJ, Arias A, Gonzalez-Cervera J et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131: 797–804. [DOI] [PubMed] [Google Scholar]
- Molina-Infante J, Arias A, Barrio J et al. Four-food group elimination diet for adult eosinophilic esophagitis: a prospective multicenter study. J Allergy Clin Immunol 2014; 134: 1093–1099. [DOI] [PubMed] [Google Scholar]
- Gonsalves N, Yang GY, Doerfler B et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142: 1451–1459. [DOI] [PubMed] [Google Scholar]
- Moawad FJ, Cheatham JG, DeZee KJ. Meta-analysis: the safety and efficacy of dilation in eosinophilic oesophagitis. Aliment Pharmacol Ther 2013; 38: 713–720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


