Abstract
Objectives. We determined whether public funding for contraception was associated with long-acting reversible contraceptive (LARC) use when providers received training on these methods.
Methods. We evaluated the impact of a clinic training intervention and public funding on LARC use in a cluster randomized trial at 40 randomly assigned clinics across the United States (2011–2013). Twenty intervention clinics received a 4-hour training. Women aged 18 to 25 were enrolled and followed for 1 year (n = 1500: 802 intervention, 698 control). We estimated the effects of the intervention and funding sources on LARC initiation with Cox proportional hazards models with shared frailty.
Results. Women at intervention sites had higher LARC initiation than those at control (22 vs 18 per 100 person-years; adjusted hazard ratio [AHR] = 1.43; 95% confidence interval [CI] = 1.04, 1.98). Participants receiving care at clinics with Medicaid family planning expansion programs had almost twice the initiation rate as those at clinics without (25 vs 13 per 100 person-years; AHR = 2.26; 95% CI = 1.59, 3.19). LARC initiation also increased among participants with public (AHR = 1.56; 95% CI = 1.09, 2.22) but not private health insurance.
Conclusions. Public funding and provider training substantially improve LARC access.
Unintended pregnancy remains a public health concern in the United States, with growing disparities by income.1 A large observational study showed an association between increased use of long-acting reversible contraception (LARC) and declines in unintended pregnancy.2 LARC includes intrauterine devices (IUDs) and implants, which have the highest efficacy among reversible methods.3 Although LARC use is increasing among US women, it remains less prevalent than in other developed countries.4,5 Systemic barriers to LARC in the United States include too few providers offering the methods and the high costs of the devices.6
Low knowledge and misinformation about LARC are common among both providers and patients. A minority of contraceptive care providers offer IUDs to patients at high risk for unintended pregnancy, including young and nulliparous women and women who have just had an abortion or given birth.7–9 Yet these women and most others may safely use IUDs and implants.10 The majority of young women lack accurate information about LARC methods and cite health care providers as their most trusted source of contraceptive information.11
For uninsured women, the up-front cost of LARC can be over $1000.12 Although the Affordable Care Act (ACA; Pub L No. 111–148) now requires some private health insurance plans to cover contraceptives without cost sharing,13 this requirement was not in place at the time this study was conducted. The largest sources of public funding for contraception are Medicaid and state Medicaid family planning expansion programs.14 Most expansion programs broaden eligibility for contraceptive care based on women’s income, matching or exceeding expanded Medicaid eligibility for pregnancy care—typically, 200% of the federal poverty level (FPL).15 Qualifying women who receive care from participating providers may obtain contraception without out-of-pocket costs. In an ecological study, these programs were associated with increased contraceptive use.16 The CHOICE study showed increased LARC use when no-cost contraceptives were offered, but the design did not include a comparison group.2 It remains unknown whether public funding for contraception, together with provider training, affects LARC use.
To address low LARC knowledge among clinicians and support staff, we designed a 4-hour clinic-wide training curriculum about LARC methods. We tested the impact of the training intervention on women’s LARC use in a cluster randomized trial at clinics with a variety of public funding sources for contraception. The impact of funding sources on LARC use was a prespecified subanalysis of our randomized trial.17 We hypothesized that LARC use would be higher at intervention clinics and at clinics with public funding for contraception.
METHODS
We conducted this trial with 40 Planned Parenthood health centers (40 clusters) in 15 US states from February 2011 to May 2013 (Figure A, available as a supplement to the online version of this article at http://www.ajph.org). Detailed methods have been described elsewhere.17 Briefly, intervention sites received a clinic-wide training designed to reduce barriers to LARC provision. Control clinics offered standard care. We measured the impact of the clinic training intervention on individual-level contraceptive outcomes. We used a cluster design to avoid the contamination that might occur with individual randomization after a clinic-wide training intervention.18
Eligible clinics had at least 400 female patients of reproductive age annually (fewer than 20% of whom were receiving LARC at baseline), no concurrent LARC interventions, and no shared staff with other study clinics. Patient recruitment, which began after completion of the training at intervention sites, occurred during visits for family planning or abortion care. Eligible patients
were aged 18 to 25 years,
spoke English or Spanish,
were at risk for unintended pregnancy (sexually active within 3 months and not pregnant),
did not desire pregnancy within 12 months,
received contraceptive counseling, and
were willing to be contacted for follow-up.
An independent third party randomized clinics using a computerized number generator, which stratified by clinic size (≤ 4000 vs > 4000 annual patients). We concealed allocation until study initiation. Clinics were not blinded after study initiation, as it was apparent whether a clinic received the intervention. Participants were not made aware of the intervention, but were not formally blinded. The data analyst was blinded to group assignment.
The study was registered with ClinicalTrials.gov (NCT01360216). Participants could receive remuneration of up to $160 during the 1-year follow-up. There were no adverse events in this behavioral study.
Intervention
The intervention was a 4-hour continuing medical education–accredited training for all clinic staff. We designed the training using formative research that identified knowledge deficits among US providers.7–9,19,20 The curriculum included a didactic session with updated evidence on intrauterine devices and the implant, a hands-on IUD insertion practicum for clinicians, and counseling role play for health educators. The session demonstrated the use of the US Medical Eligibility Criteria for Contraceptive Use and the tiers-of-effectiveness approach for contraceptive counseling.10,21 The training emphasized LARC ethical issues, including the importance of patient-centered counseling and removal of a LARC method upon a patient’s request. A video featured other providers who had successfully integrated LARC into practice, including same-day provision. We also asked intervention clinics to play an educational patient video in the waiting room that showed peer experiences with LARC. For interested clinicians, we facilitated implant training. For clinic managers, we provided technical assistance for billing and reimbursement.
Measures and Procedures
The primary outcome was LARC initiation (yes or no) during 12 months of follow-up. Women reported LARC initiation via questionnaires at baseline and at 3, 6, 9, and 12 months, and we collected data on LARC insertions from medical records review at 12 months. To better understand the factors influencing women’s LARC use, we also assessed participants’ decision to use LARC (yes or no) during the enrollment visit by funding variables.
We assessed sources of funding for contraception using clinic- and individual-level data. Managers reported whether the clinic had a Medicaid family planning expansion program based on income (185%–200% of the FPL; yes or no) or provided reduced-cost care through the federal Title X family planning program (yes or no). Participants reported what kind of health insurance they had (none, private, public, don’t know).
We collected baseline questionnaire data on factors associated with contraceptive use,22 including age, race/ethnicity, parity, LARC use within 3 months of baseline, primary sexual partner, and receipt of public assistance (i.e., welfare, Special Supplemental Nutrition Program for Women, Infants, and Children [WIC], food stamps, unemployment). We assessed attitude toward pregnancy with a question on whether the participant would feel happy or unhappy if she became pregnant in the next year. Visit type was a clinic-level variable determined by the services received by participants recruited at the site (abortion, family planning).
To monitor fidelity to the concepts taught in the training, clinic staff noted their counseling practices on a visit summary form for each participant.
Analyses
The sample size was based on the primary outcome, the proportion of women initiating LARC during follow-up. Using a comparison of 2 group estimates, and assuming 4% LARC in control sites and 10% in intervention sites, an α of 0.05 and β of 0.20, an intracluster correlation of 0.02 with an average cluster size of 30, and 20% loss to follow-up, we calculated that a sample size of 1248 participants was required. We set a recruitment goal of 1600 in case study sites recruited fewer than anticipated participants.
We conducted analyses at the individual level; analyses followed an intent-to-treat approach and accounted for clinic-level clustering.23 Using life table analysis, we assessed LARC initiation rates overall, by study arm, and by funding source (Medicaid family planning expansion program, Title X, health insurance). In multivariable models, we included the funding source variables significant in bivariable models and other covariables (age, race/ethnicity, parity, public assistance, primary sexual partner, LARC use in 3 months prior, pregnancy attitude, visit type). To estimate the effects of the intervention and funding sources on time to LARC initiation, we used Cox proportional hazards models with shared frailty. Participants contributed observation time to the analysis until they initiated LARC, became pregnant, or exited the study at 12 months; there was no loss to follow-up. Analyses excluded women already using LARC at enrollment. We repeated proportional hazards analyses, including covariables selected a priori at the individual level (age, race/ethnicity, parity, recent LARC use, primary sexual partner, public assistance, pregnancy attitude) and site level (visit type), as well as funding source variables (Medicaid family planning expansion, Title X, health insurance). We used an interaction term to assess whether Medicaid family planning expansions modified the effect of the provider training. Because primary trial analyses found significant interaction between the intervention and visit type,17 we also tested an interaction term with the intervention and visit type. To test whether the proportional hazards assumption was met, we used Schoenfeld residuals and log-log plots against time. We repeated analyses, including LARC initiated after a pregnancy occurred, using logistic regression with generalized estimated equations (GEE) and robust standard errors for clustering.
To explore how funding sources affected LARC initiation, we also examined their relationship with women’s decision to use LARC using multivariable GEE logistic regression. We then examined whether women who decided to use LARC at baseline were more likely to actually initiate the method if they received care at a clinic with Medicaid family planning expansion or Title X programs, using multivariable proportional hazards. We used Stata version 13.0 (StataCorp LP, College Station, TX) for analyses. We used multiple imputation for missing data, which was less than 1% for any variable. We report results of 2-sided tests at the P < .05 level.
RESULTS
Forty clinics participated in the study. In total, 1500 participants enrolled (802 intervention, 698 control) from May 2011 to April 2012, with follow-up until May 2013. For the LARC initiation analysis, we excluded the 1.5% of participants using LARC at enrollment (n = 22; 10 intervention, 12 control), leaving 1478 (792 intervention, 686 control) in survival analyses. For analyses on decision to use LARC, we included all participants (802 intervention, 698 control). For analyses among women who decided to use LARC at baseline, we included 325 women (216 intervention, 109 control).
Characteristics of participants and clinics were similar by study arm (Table 1; Table A, available as a supplement to the online version of this article at http://www.ajph.org). We also assessed characteristics by Medicaid family planning expansion and found no differences. One quarter (26%) of participants reported that they received some form of public assistance; 38% reported having no health insurance, 30% had private insurance, and 28% had public insurance. Overall, 63% of clinics had a Medicaid expansion program and 58% received some Title X funding. Compared with other public funding, however, Title X funding was rarely used to cover contraceptive visits, as shown by clinics’ primary payers for contraceptive visits (Medicaid family planning expansion, 43%; Medicaid, 20%; self-pay, 20%; private insurance, 12%; Title X, 5%).
TABLE 1—
Characteristics of Study Participants and Clinics: United States, February 2011–May 2013
| Participants, No. (%) |
Clinic Sites, No. (%) |
|||||
| Characteristic | Intervention (n = 802) | Control (n = 698) | Total (n = 1500) | Intervention (n = 20) | Control (n = 20) | Total (n = 40) |
| Parity (n = 1489) | ||||||
| Nulliparous | 585 (73.4) | 467 (67.5) | 1052 (70.7) | |||
| 1 | 137 (17.2) | 141 (20.4) | 278 (18.7) | |||
| ≥ 2 | 75 (9.4) | 84 (12.1) | 159 (10.7) | |||
| Has primary sexual partner (n = 1474) | 639 (80.7) | 561 (82.3) | 1200 (81.4) | |||
| Pregnancy attitude (n = 1484) | ||||||
| Happy | 136 (17.2) | 157 (22.7) | 293 (19.7) | |||
| Unhappy | 657 (82.9) | 534 (77.3) | 1191 (80.3) | |||
| Medicaid family planning expansion | ||||||
| Yes | 12 (60.0) | 13 (65.0) | 25 (62.5) | |||
| No | 8 (40.0) | 7 (35.0) | 15 (37.5) | |||
| Title X funding | ||||||
| Yes | 13 (65.0) | 10 (50.0) | 23 (57.5) | |||
| No | 7 (35.0) | 10 (50.0) | 17 (42.5) | |||
| Visit type | ||||||
| Family planning | 12 (60.0) | 11 (55.0) | 23 (57.5) | |||
| Abortion | 8 (40.0) | 9 (45.0) | 17 (42.5) | |||
Note. Some rows do not sum to total because of missing responses.
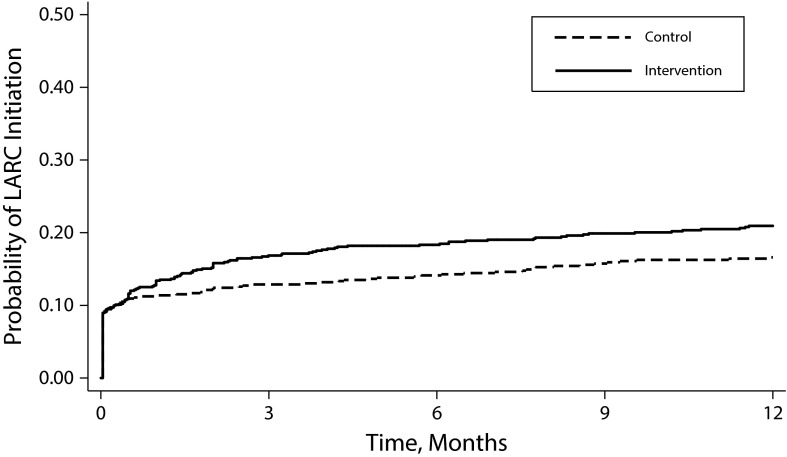
Eighteen percent (273 of 1478) of participants initiated LARC; 13% (n = 188) used IUDs and 6% (n = 85) used implants. Among women using LARC, 81% (221 of 273) initiated within 3 months following the enrollment visit. The 1-year initiation rate was 20 per 100 person-years (PY). LARC initiation was significantly higher in intervention clinics than in control clinics (22 vs 18 per 100 PY; adjusted hazard ratio [AHR] = 1.43; 95% confidence interval [CI] = 1.04, 1.98; Figure 1). Analyses including LARC initiated after a pregnancy during the study yielded similar rates (23 vs 19 per 100 PY).
FIGURE 1—
Initiation of Use of Long-Acting Reversible Contraceptive (LARC), by Study Arm: United States, February 2011–May 2013
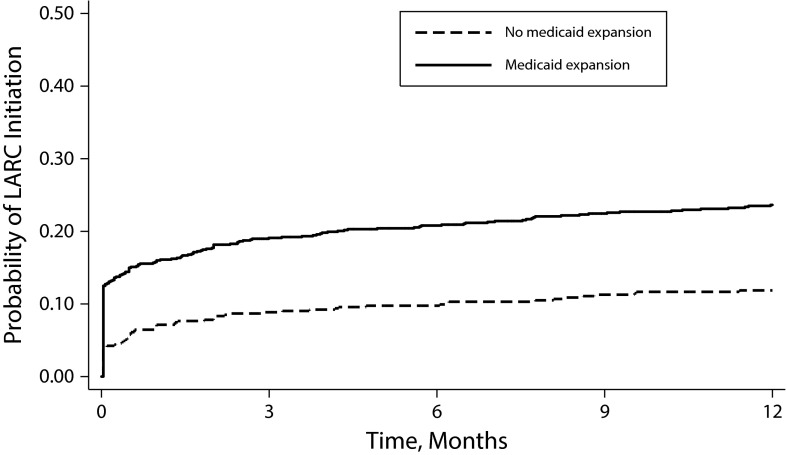
LARC initiation was significantly higher at clinics with Medicaid expansion program than in clinics without Medicaid expansion (25 vs 13 per 100 PY; AHR = 2.26; 95% CI = 1.59, 3.19; Figure 2). Clinic receipt of any Title X funding was not associated with LARC initiation in bivariable analysis (HR = 0.96; 95% CI = 0.61, 1.52) and was excluded from multivariable models. Interaction terms between the intervention and Medicaid expansion (P = .10) and visit type (P = .15) did not reach significance and were not included.
FIGURE 2—
Initiation of Use of Long-Acting Reversible Contraceptive (LARC), by Medicaid Family Planning Expansion Status: United States, February 2011–May 2013
When we controlled for sites’ Medicaid expansion programs, women with public health insurance had higher LARC initiation than uninsured women (AHR = 1.56; 95% CI = 1.09, 2.22) (Table 2), indicating that Medicaid was associated with increased LARC initiation. Women with private health insurance initiated LARC at rates similar to those of uninsured women (AHR = 1.06; 95% CI = 0.75, 1.49). Study arm met proportionality assumptions in the multivariable model, but Medicaid family planning expansion did not. Log-log plots indicated that nonproportionality by expansion was limited to the first 2 weeks after enrollment. Results from GEE models for LARC use were similar to results from proportional hazards models.
TABLE 2—
Initiation of Use of Long-Acting Reversible Contraceptive (LARC), by Study Arm and Funding Source (n = 1478): United States, February 2011–May 2013
| Hazard Ratio (95% CI) |
|||
| Variable | No. (Rate/100 PY) | Model 1 | Model 2 |
| Study arm | |||
| Control | 111 (18.1) | 1 (Ref) | 1 (Ref) |
| Intervention | 162 (21.9) | 1.43 (1.04, 1.98) | 1.44 (1.03, 2.01) |
| Medicaid family planning expansion | |||
| No | 68 (13.5) | 1 (Ref) | 1 (Ref) |
| Yes | 205 (24.6) | 2.26 (1.59, 3.19) | 2.21 (1.55, 3.16) |
| Health insurance | |||
| None | 86 (16.7) | … | 1 (Ref) |
| Private | 64 (15.8) | … | 1.06 (0.75, 1.49) |
| Public | 110 (30.2) | … | 1.56 (1.09, 2.22) |
| Don’t know | 10 (15.8) | … | 1.03 (0.53, 2.01) |
Note. CI = confidence interval; PY = person-years. Models included age, race/ethnicity, parity, public assistance, primary sexual partner, LARC use in 3 months prior to enrollment, pregnancy attitude, and visit type.
Relationships between funding sources and decision to use LARC followed the same patterns as in the main outcome: women receiving care at sites with Medicaid family planning expansions were more likely to decide to use LARC than women at sites without Medicaid expansion (25% vs 19%; adjusted odds ratio [AOR] = 1.59; 95% CI = 1.17, 2.15), but there was no difference by site Title X funding (22% vs 24%; AOR = 0.83; 95% CI = 0.61, 1.11). Compared with uninsured women, women with public insurance were more likely to decide to use LARC (AOR = 1.80; 95% CI = 1.27, 2.53) but women with private insurance were no more likely (AOR = 1.25; 95% CI = 0.87, 1.78). Among those who decided to use LARC at the enrollment visit, 61% (197 of 325) initiated the method within 1 year; however, those at clinics with Medicaid family planning expansions had higher LARC initiation rates than women at clinics without these programs (AHR = 2.04; 95% CI = 1.32, 3.16).
Among intervention site providers, fidelity to the skills included in the training was high: providers reported that they discussed the efficacy of IUDs and implants with 87% of patients and used the tiers-of-effectiveness approach to counseling with 81%.
DISCUSSION
Our results show that young women at risk for unintended pregnancy were interested in using LARC methods, and that Medicaid family planning expansion programs addressing the high costs of these methods were critical to initiation. Women were more likely to initiate LARC in clinics where providers had received an evidence-based training designed to increase their ability to offer these methods.
This study did not provide free LARC devices, and participants paid for contraceptives with cash, insurance, and a variety of public programs. Over 40% of contraceptive care visits at study sites were paid primarily by Medicaid family planning expansion programs, and these programs helped to fill a key funding gap for women wanting to use LARC. As of October 2015, 11 of the 21 states with Medicaid family planning eligibility expansions based on eligibility at or above 185% of the FPL are established as permanent state plan amendments; the remainder will expire without action from the states.24
Public health insurance was also associated with greater LARC initiation. The role of public insurance will likely grow as some states offer Medicaid to more residents under the ACA. As of October 2015, 31 states had expanded Medicaid eligibility to adults earning up to 138% of the FPL.25 Among the other states that have not expanded Medicaid, 10 also lacked family planning expansion programs. One in 5 US women aged 18 to 25 years—the group at highest risk for unintended pregnancy—live in these states.26 Regardless of who is eligible for Medicaid, meaningful LARC access will require that the methods be adequately reimbursed and that removal of the methods be a covered benefit.
Clinics’ Title X funding was not associated with LARC use. This program is important, but it has limited funds and is often used to support clinic services more broadly rather than cover individual visits.14 Likewise, women’s private health insurance was not associated with LARC use. Because this study was conducted before ACA provisions related to contraceptive coverage came into effect, some plans probably did not cover IUDs or implants. In 2014, an estimated 26% of employees with private health insurance had grandfathered plans, which are exempt from the requirement to cover contraception without cost sharing.27 As a result of the Burwell v Hobby Lobby (573 U.S. 13-354 (2014)) ruling, some “closely held corporations” will also be exempt. It remains unclear how many women’s health insurance plans will not cover contraception.
Because of nonuniform provision of Medicaid and challenges to the ACA’s contraceptive coverage provisions, health care reform will not obviate the need for public funding for contraception. Furthermore, not all women eligible for expanded public and private insurance will obtain coverage. Massachusetts learned after implementing its health care reform that young women are particularly susceptible to gaps in coverage and may have concerns about the confidentiality of services.28 Several states’ Medicaid family planning expansion programs provide models for how to address lack of coverage, gaps in coverage, inadequate coverage, and confidentiality issues.15 Recent legislative attempts to cut funding for Planned Parenthood and other Title X recipients threaten patients’ coverage for contraceptive care.
This study has limitations. The findings may not be generalizable beyond family planning clinics like Planned Parenthood health centers. The effects of the intervention may diminish with time. Some clinics participating in this study had Medicaid family planning expansion programs that determined eligibility based on income at 185% to 200% of the FPL; programs with more limited eligibility criteria are likely to have less impact on women’s LARC use. Furthermore, unmeasured state traits may have affected both expansion programs and LARC use. The landscape of private health insurance coverage is changing as new guidance for the ACA is introduced. More private health insurance plans may include LARC than did during this study period.
This study also has strengths. The cluster randomized design provides strong evidence of an intervention effect. The impact of this replicable intervention may be larger in other practice settings with lower baseline staff knowledge about LARC29; staff at our study clinics started with relatively high LARC knowledge.30 Clinic staff at intervention sites had high fidelity to the counseling techniques included in the training, and the study had low loss to follow-up.
Ultimately, reaching the national goal of reducing unintended pregnancy will require the dissemination of effective interventions to improve clinical care for contraception.31 The training intervention we tested here was designed to be scalable for community clinics serving women throughout the United States. Future efforts to reduce unintended pregnancy should address both the skills of clinic staff and the high up-front costs of contraception.
ACKNOWLEDGMENTS
This study was funded by the William and Flora Hewlett Foundation. The National Campaign to Prevent Teen and Unplanned Pregnancy provided a small grant to produce the patient education video. Units for intervention training in intrauterine device insertion were provided by Teva Pharmaceuticals Industries and Bayer HealthCare.
C. C. Harper and J. J. Speidel have served as consultants to Medicines360, a non-profit organization. J. J. Speidel has also served as a consultant to WomanCare Global. P. D. Darney served as a research consultant to Merck. The other authors declare no competing interests.
Preliminary findings from this study were presented at the Academy Health Annual Research Meeting; June 8–10, 2014; San Diego, CA.
We thank Susannah Gibbs, Laura Stratton, Lily Loew, Cait Quinlivan, Jen Grand, Racquel Enad, Helen Helfand, and Laura Elena Gomez, UCSF Bixby Center for Global Reproductive Health, for data management and collection. We thank Johanna Morfesis and Planned Parenthood investigators and research coordinators at these affiliates: Central and Greater Northern New Jersey; Columbia Willamette; Great Northwest; Greater Ohio; Greater Washington and North Idaho; Mar Monte; Mid & South Michigan; Minnesota, North Dakota, South Dakota; Mount Baker; Northern California; Pacific Southwest; Pasadena and San Gabriel Valley; Rocky Mountains; South Atlantic; Southeastern Pennsylvania; Southern New England; and Southwest and Central Florida. We thank Marsha Gelt, Cardea Services, for delivering the intervention training. All these contributors received compensation. We thank Tina Raine-Bennett and Charles McCulloch for clinical and design expertise.
Note. The findings and conclusions in this article are those of the authors and do not necessarily represent views of Planned Parenthood Federation of America, Inc. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the article.
HUMAN PARTICIPANT PROTECTION
The study protocol was approved by the University of California, San Francisco Committee on Human Research and the Allendale Investigational Review Board. Before participant enrollment, research coordinators obtained written informed consent.
REFERENCES
- 1.Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):S43–S48. doi: 10.2105/AJPH.2013.301416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peipert JF, Madden T, Allsworth JE, Secura GM. Preventing unintended pregnancies by providing no-cost contraception. Obstet Gynecol. 2012;120(6):1291–1297. doi: 10.1097/aog.0b013e318273eb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trussell J. Contraceptive efficacy. In: Hatcher R, Trussell J, Nelson A, Cates W, Kowal D, Policar M, editors. Contraceptive Technology. 20th ed. New York, NY: Ardent Media; 2011. pp. 779–863. [Google Scholar]
- 4.Daniels K, Daugherty J, Jones J. Current Contraceptive Status Among Women Aged 15–44: United States, 2011–2013. Hyattsville, MD: National Center for Health Statistics; 2014. NCHS data brief no. 173. [Google Scholar]
- 5.United Nations Department of Economic and Social Affairs. Population Division. World contraceptive patterns 2013. Available at. http://www.un.org/en/development/desa/population/publications/family/contraceptive-wallchart-2013.shtml . Accessed January 14, 2015.
- 6.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion no. 450: increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114(6):1434–1438. doi: 10.1097/AOG.0b013e3181c6f965. [DOI] [PubMed] [Google Scholar]
- 7.Tyler CP, Whiteman MK, Zapata LB et al. Health care provider attitudes and practices related to intrauterine devices for nulliparous women. Obstet Gynecol. 2012;119(4):762–771. doi: 10.1097/AOG.0b013e31824aca39. [DOI] [PubMed] [Google Scholar]
- 8.Harper CC, Henderson JT, Raine TR et al. Evidence-based IUD practice: family physicians and obstretrician–gynecologists. Fam Med. 2012;44(9):637–645. [PMC free article] [PubMed] [Google Scholar]
- 9.Harper CC, Stratton L, Raine TR et al. Counseling and provision of long-acting reversible contraception in the US: national survey of nurse practitioners. Prev Med. 2013;57(6):883–888. doi: 10.1016/j.ypmed.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. United States medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010(59):1–6. [PubMed] [Google Scholar]
- 11.Kaye K, Suellentrop K, Sloup C. The Fog Zone: How Misperceptions, Magical Thinking, and Ambivalence Put Young Adults at Risk for Unplanned Pregnancy. Washington, DC: National Campaign to Prevent Teen and Unplanned Pregnancy; 2009. [Google Scholar]
- 12.Trussell J. Update on and correction to the cost effectiveness of contraceptives in the United States. Contraception. 2012;85(2):218. doi: 10.1016/j.contraception.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 13.US Dept of Health and Human Services, Health Resources and Services Administration. Women’s preventive services guidelines. Available at: http://www.hrsa.gov/womensguidelines. Accessed March 27, 2014.
- 14.Frost JJ, Zolna MR, Frohwirth L. Contraceptive Needs and Services, 2010. New York, NY: Guttmacher Institute; 2013. [Google Scholar]
- 15.Sonfield A, Gold RB. Medicaid Family Planning Expansions: Lessons Learned and Implications for the Future. New York, NY: Guttmacher Institute; 2011. [Google Scholar]
- 16.Kearney MS, Levine PB. Subsidized contraception, fertility, and sexual behavior. Rev Econ Stat. 2009;91(1):137. doi: 10.1162/rest.91.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harper CC, Rocca CH, Thompson KM et al. Reduced pregnancy in the US from long-acting reversible contraception: a cluster randomized trial. Lancet. 2015;386(9993):562–568. doi: 10.1016/S0140-6736(14)62460-0. [DOI] [PubMed] [Google Scholar]
- 18.Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 19.Harper CC, Blum M, de Bocanegra HT et al. Challenges in translating evidence to practice: the provision of intrauterine contraception. Obstet Gynecol. 2008;111(6):1359–1369. doi: 10.1097/AOG.0b013e318173fd83. [DOI] [PubMed] [Google Scholar]
- 20.Morse J, Freedman L, Speidel JJ, Thompson KMJ, Stratton L, Harper CC. Post-abortion contraception: qualitative interviews on counseling and provision of long-acting reversible contraceptive methods. Perspect Sex Reprod Health. 2012;44(2):100–106. doi: 10.1363/4410012. [DOI] [PubMed] [Google Scholar]
- 21.Family Planning: A Global Handbook for Providers. Baltimore, MD and Geneva, Switzerland: Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs and World Health Organization; 2007. [Google Scholar]
- 22.Raine TR, Foster-Rosales A, Upadhyay UD et al. One-year contraceptive continuation and pregnancy in adolescent girls and women initiating hormonal contraceptives. Obstet Gynecol. 2011;117(2 pt 1):363–371. doi: 10.1097/AOG.0b013e31820563d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes RJ, Moulton LH. Cluster Randomised Trials. New York, NY: CRC Press, Taylor & Francis Group; 2009. [Google Scholar]
- 24.Guttmacher Institute. State Policies in Brief. Medicaid family planning eligibility expansions. Available at: http://www.guttmacher.org/statecenter/spibs/spib_SMFPE.pdf. Accessed October 23, 2014.
- 25.Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. Available at: http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act. Accessed October 23, 2014.
- 26.US Census Bureau. State characteristics datasets: annual estimates of the civilian population by single year of age and sex for the United States and states. April 1, 2010 to July 1, 2012. Available at: http://www.census.gov/popest/data/state/asrh/2012/SC-EST2012-AGESEX-CIV.html. Accessed March 26, 2014.
- 27.Kaiser Family Foundation and the Health Research & Educational Trust. Employer health benefits: 2014 annual survey. Available at: http://kaiserfamilyfoundation.files.wordpress.com/2014/09/8625-employer-health-benefits-2014-annual-survey6.pdf. Accessed January 14, 2014.
- 28.Low-Income Women’s Access to Contraception After Massachusetts Health Care Reform. Boston, MA: Ibis Reproductive Health and Massachusetts Dept of Public Health Family Planning Program; 2009. [Google Scholar]
- 29.Langston AM, Rosario L, Westhoff CL. Structured contraceptive counseling—a randomized controlled trial. Patient Educ Couns. 2010;81(3):362–367. doi: 10.1016/j.pec.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Thompson KM, Stern L, Gelt M, Speidel JJ, Harper CC. Counseling for IUDs and implants: are health educators and clinicians on the same page? Perspect Sex Reprod Health. 2013;45(4):191–195. doi: 10.1363/4519113. [DOI] [PubMed] [Google Scholar]
- 31.US Dept of Health and Human Services. Healthy People 2020. Available at: http://www.healthypeople.gov/2020/topicsobjectives2020. Accessed May 1, 2013.